Table of Contents
There are two basic problems when one attempts to separate a solution of metal ions utilizing a chelation-solvent extraction cascade (commonly called liquid ion exchange). First, it is impossible to produce any great degree of separation unless the separation factor (also called selectivity, which is the ratio of the distribution coefficients) is very large. Second, the richest possible extracted product, using an infinite number of equilibrium stages, is at best only in equilibrium with the feed solution.
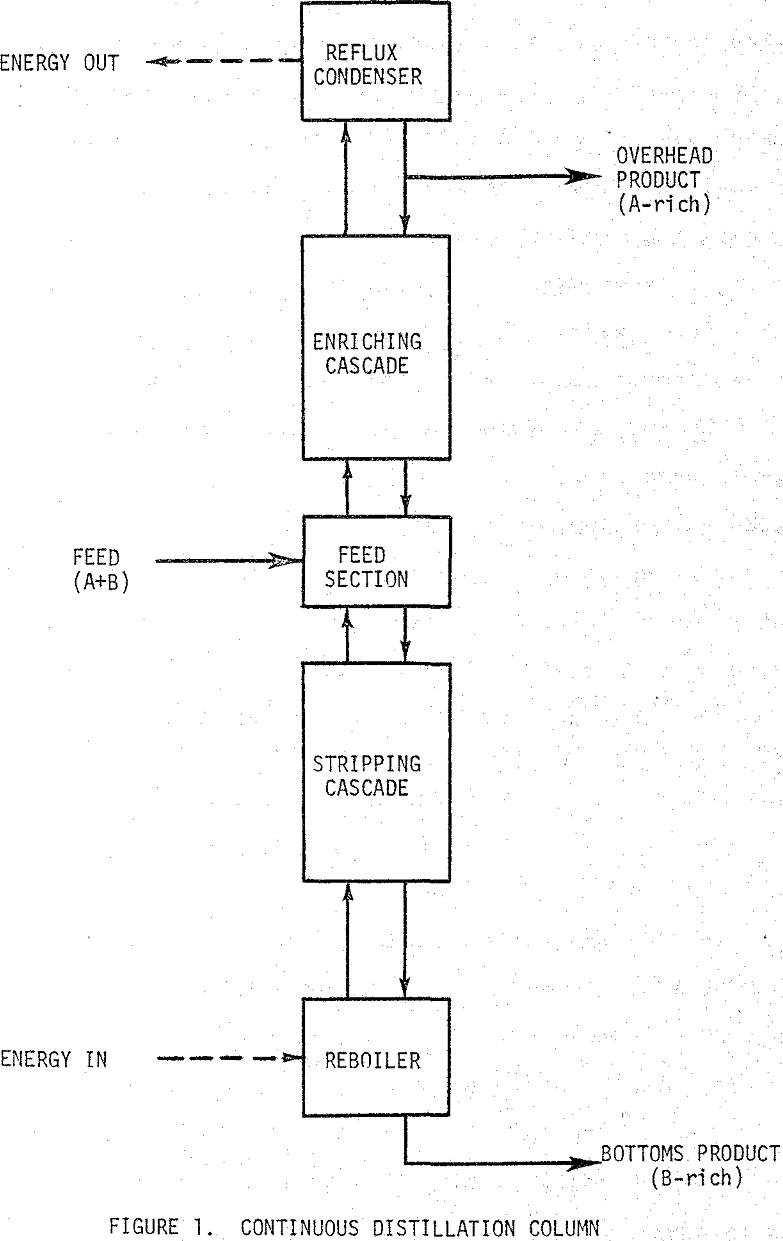
Distillation
Distillation is a method of separating the components of a solution by supplying thermal energy which causes each component to distribute itself between a vapor and a liquid phase. Each compound distributes differently resulting in a preferential migration of the more volatile components to the vapor phase. If the vapor phase is removed and condensed, a liquid solution is obtained that is richer in the more volatile components than the feed solution.
If the vapor rising in the cascade is washed with a liquid, the less volatile component is removed from the vapor resulting in a concentration of the more volatile material in the vapor at the top of the cascade. The less volatile material is concentrated in the liquid phase at the bottom of the cascade. This procedure is called supplying reflux to the cascade or simply refluxing. For a fixed number of stages the purity of the product being removed is a function of the amount of reflux being supplied.
Metal Ion Fractionation
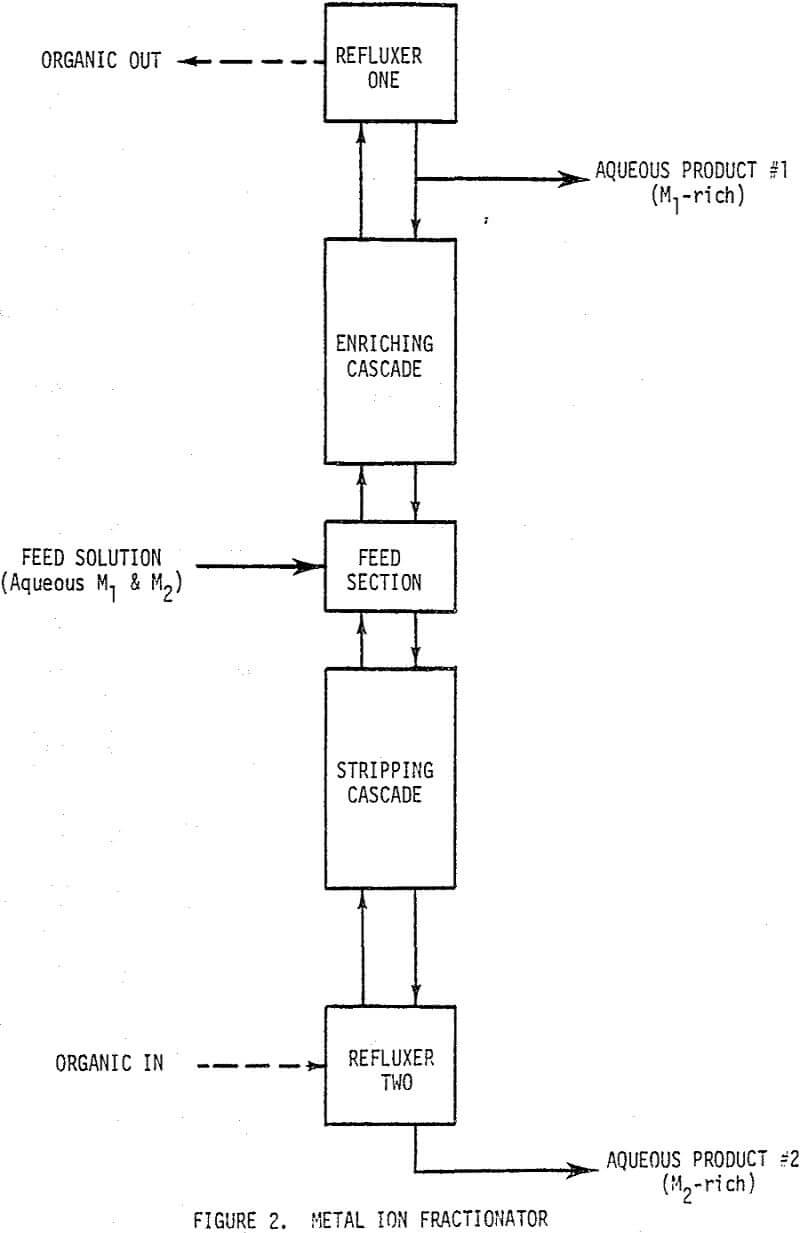
The metal ion fractionation unit operation will be analogous to the continuous rectification unit operation. An aqueous solution of metal ions will be fed to the feed section of the fractionator and aqueous products will be taken from both ends of the unit. The separation will be accomplished by supplying the organic phase to one end of the unit and removing it at the other in a condition ready for recycle to the organic feed end.
The enriching cascade functions to assure that the organic phase (assumed lighter than the aqueous) leaving the top of the cascade is loaded with the more strongly complexed metal ion, M1. The multistage cascade is fed organic from the stripping cascade., which contains all the M1 to be recovered from the feed, and aqueous reflux from refluxer one, which contains relatively pure M1. The cascade produces the M1 loaded organic and the aqueous phase containing the M2 that has been rejected in the enriching cascade. It is essential that hydrogen ion concentration, [H+], be noted in all stages, as all of the chelating reactions are pH dependent.
Flow Sheets
Two different flow sheets will be presented in this section. The first represents a process in which the recovery of a specific metal ion from a solution is accomplished with a relatively selective chelating agent. The second is a case in which two metal ions are to be separated into two solutions each containing a specific ion.
The chelating agent must be more selective for the metal being recovered than for any of the other metals in solution. The circuit consumes only acid to produce a concentrated metal ion solution. The overall material balance indicates that hydrogen ion replaces the metal ion in solution. Therefore, care must be taken in the disposal of the barren solution.
The enriching and stripping cascades may require a number (10 to 20 possibly) of equilibrium stages to accomplish the desired separation. This rules out the use of mixer-settlers because of the inventory of organic necessary to fill the system. Multistage extractors must then be used. These extractors require light hydrocarbon carriers for the chelating agent for efficient operation.