In recent years, the interest in liquid metals as heat-transfer media for power plants has been very great. The possibility of the development of nuclear power plants has increased this interest and served as the impetus behind much research on low melting metals and alloys for such purposes. The principal reasons for consideration of liquid metals as heat-transfer media lie in their high thermal conductivity and consequent high heat-transfer coefficients, stability at high temperatures, and the high ranges of temperature possible.
The element gallium possesses some of the requisite properties for a heat-transfer liquid. It is a unique material, having a low melting point and a high boiling point. Pure gallium melts at 29.78° C, and suitable alloying will produce a metal which melts below room temperature. The boiling point is about 2000°C. As it, is a liquid metal, the heat-transfer characteristics would be good. Gallium is not now readily available, due in part to a lack of uses for the metal. Nevertheless, it is not a rare element, and a sufficient supply of gallium exists to permit its consideration for this use.
Experimental Results
As a first approximation, the development of low melting gallium alloys was based on alloying elements suitable for use in a nuclear power plant, which also lowered the melting point of gallium. Information from the literature indicates that tin, aluminum, and zinc are the only suitable elements which cause a lowering of the melting point of gallium. Indium and silver also lower the melting point of gallium, but are of little interest for use in nuclear power plants. Of the elements reported not to lower the melting point of gallium, there is some ambiguity on the behavior of copper. Weibke obtained solidus arrest temperatures of 29°C for Cu-Ga alloys from 60 to 90 pct Ga, 0.8°C lower than the generally accepted melting point. This may be the effect of a eutectic close to gallium, or, more likely, the result of impurities, or experimental error.
In all cases, the solidus temperature was the melting point of gallium. Since these elements (As, Ca, Ce, Mg, Sb, Si, and Tl) did not lower the melting point of gallium, they were not considered further as components of a eutectic-type alloy.
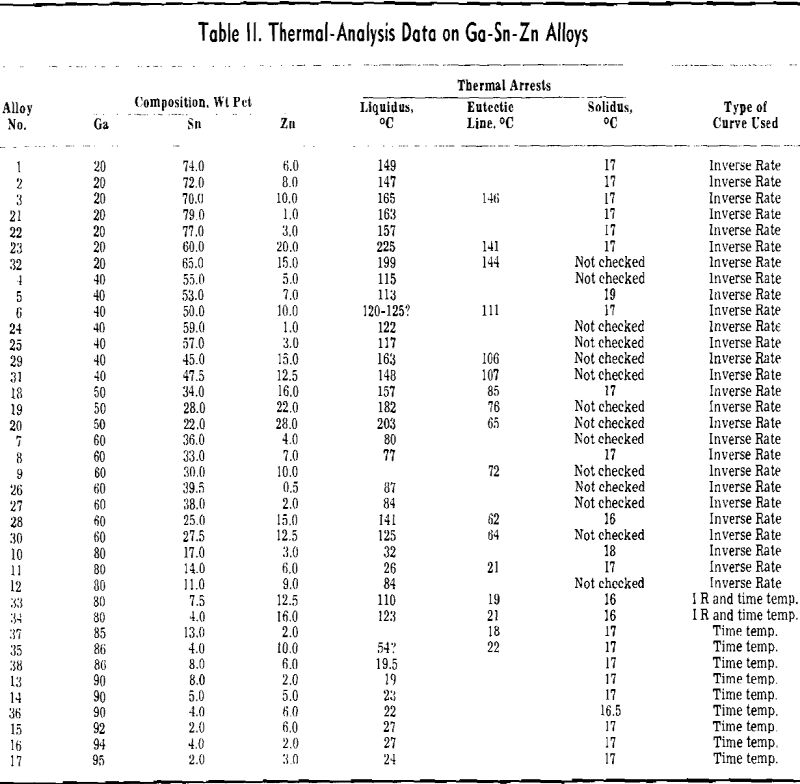
Preliminary considerations of this system for low-melting alloys were encouraging. All three binary systems were of the simple eutectic type. The composition and melting points of the eutectics were as follows: Sn-9 pct Zn (199°C), Ga-8 pct Sn (20°C), and Ga-5 pct Zn (25°C). Therefore, the probability of a ternary eutectic was high. For reasons to be discussed later, aluminum could not be used as an alloying constituent, leaving the Ga-Sn-Zn system as the only one of interest for low-melting gallium-
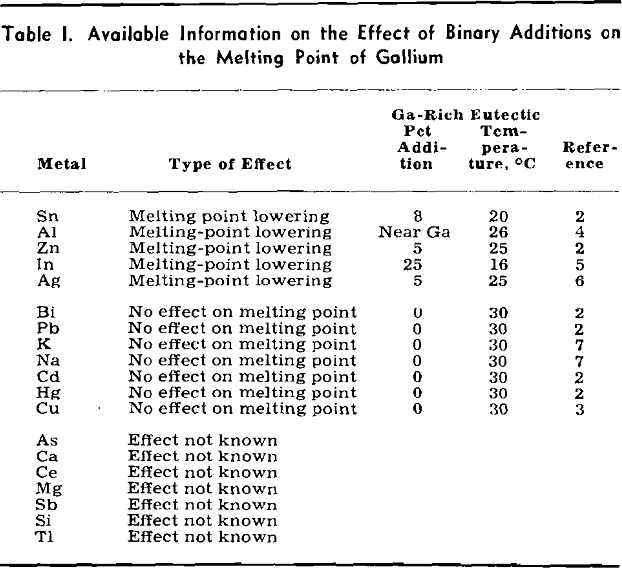
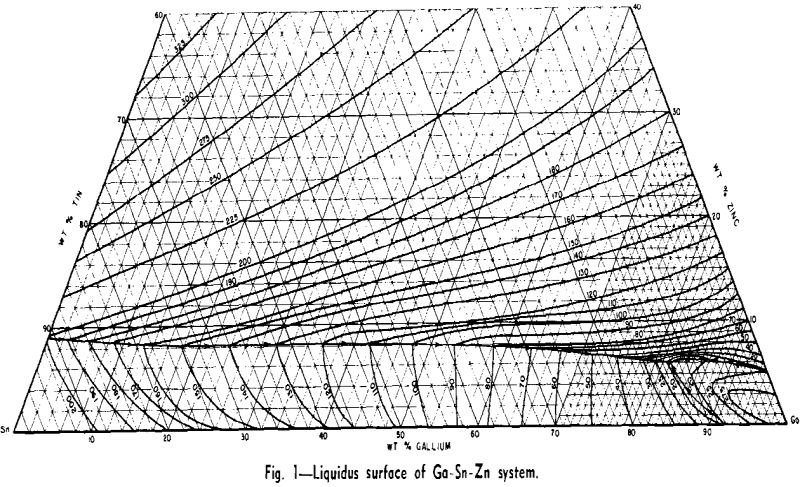
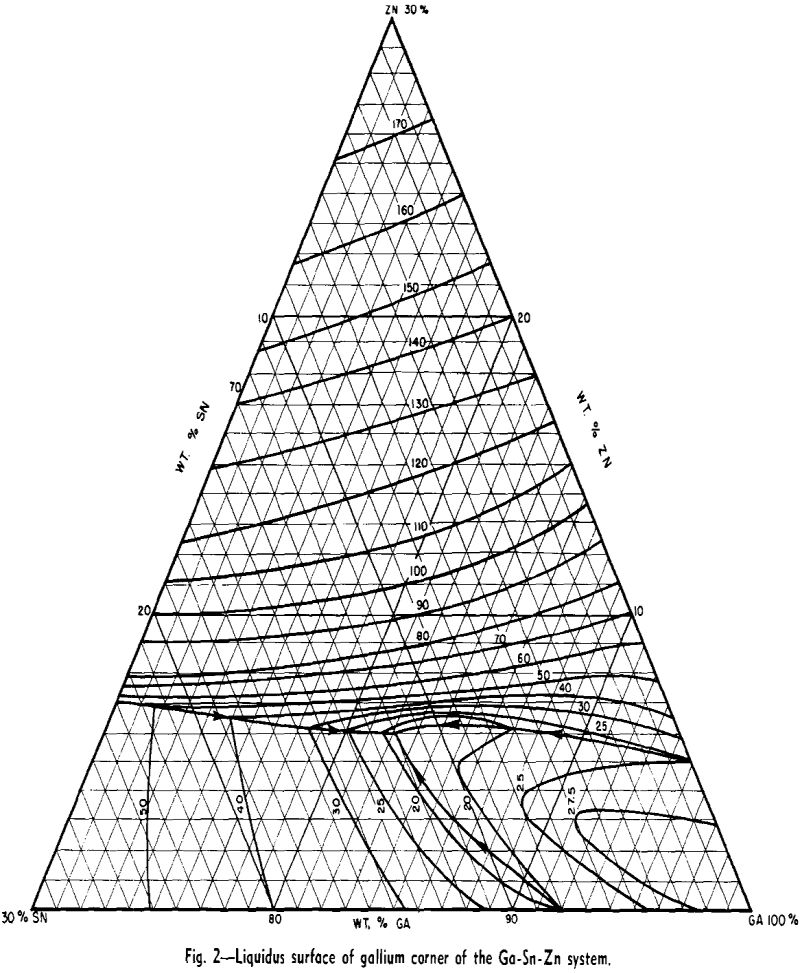
rich alloys of three or more components. Consequently considerable effort was expended in working out the liquidus surface.
Alloys Containing Aluminum
Aluminum added to Ga-Sn-Zn alloys at first appeared to be advantageous. The invariant point of gallium-rich Ga-Sn-Zn-Al alloys on a virgin run was found to be 15.5°C. However, on rerunning the same alloys, the invariant point obtained was 17°C, identical with that of the Ga-Sn-Zn eutectic.
The Ga-Sn-Zn-Al alloys were found to oxidize very rapidly in air at room temperature. Held at 50°C for 3 to 4 hr, they tended to turn to powder. To see which of the elements was promoting the oxidation, the alloys listed below were held in air at 100°C for 24 hr and the following observations were made:

Dilution of Ga-Sn-Zn Eutectic
When a material as costly as gallium is intended for heat-transfer purposes, it is of interest to dilute it as much as possible, while still retaining its low melting characteristics. With this view in mind, a study was made of methods of diluting the Ga-Sn-Zn eutectic so as to maintain its 17°C solidus temperature and minimize the increase in liquidus temperature.
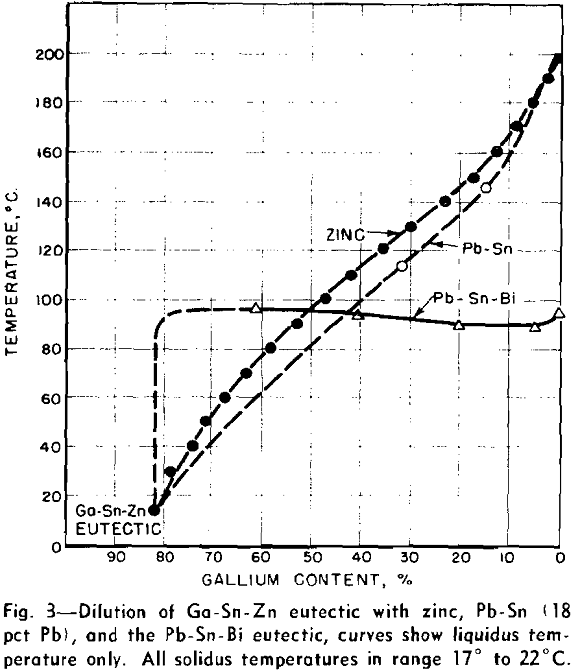
Further evidence of selective freezing of the two eutectic components comes from the appearance of these alloys at room temperature. The Pb-Sn-Bi component solidifies into a single hard lump located at the bottom of the liquid-gallium component of the alloy. This is a bad characteristic for liquid metals, which may have to be pumped through zones of variable temperature.

