Table of Contents
The behavior of fine mineral particles in a slurry is difficult to control, presenting a serious problem in many processes. At fine particle sizes, gravitational force is weak. Electrostatic force, although strong in dry conditions, can rarely be applied for particles in an aqueous slurry. The applications for using magnetic force are limited because most materials are diamagnetic to weakly paramagnetic. Without being able to use these forces effectively, the movement of fine particles is hard to control.
Magnetic Reagent and its Functions
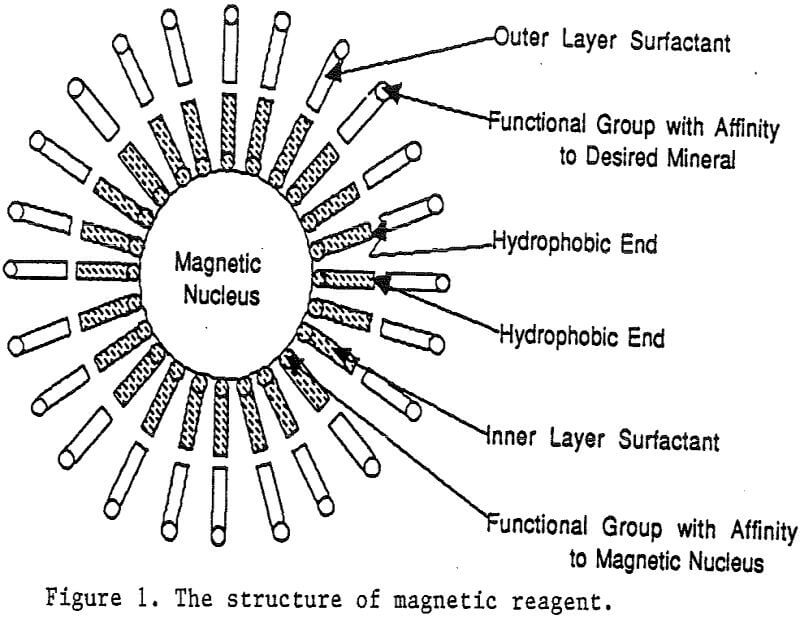
A magnetic reagent is a composite of magnetic materials and surface active agents. The surface active agents contain functional groups which can react with magnetic materials and other materials. Under appropriate conditions, the agents can serve as a bridge to couple magnetic materials to other materials. Thus, the composite of magnetic materials and surface active agents with the capabilities to couple other materials is a magnetic reagent.
Adsorption of Magnetic Reagent
The adsorption of magnetic reagents on fine particles is similar to the adsorption of surfactants in many aspects. Common mechanisms for the adsorption of surfactants on particles include chemical bonding, electrokinetic force, van der Waals force, and hydrogen bonding. These principles can be applied to control the adsorption of magnetic reagents, although the extent of these forces may vary from the adsorption of surfactant alone. Experiments to verify the adsorption mechanisms have been conducted. Some examples are discussed here.
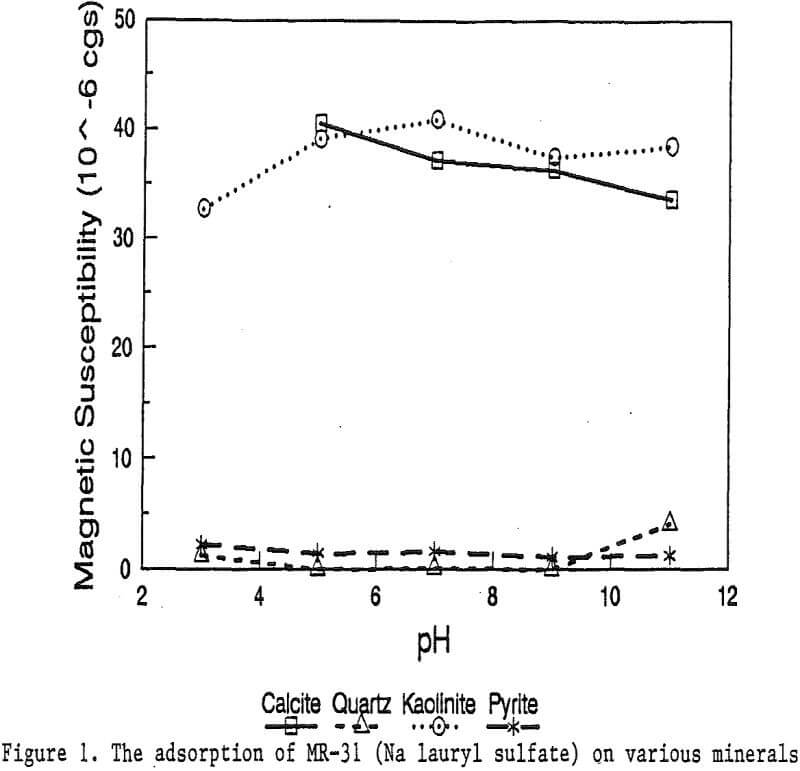
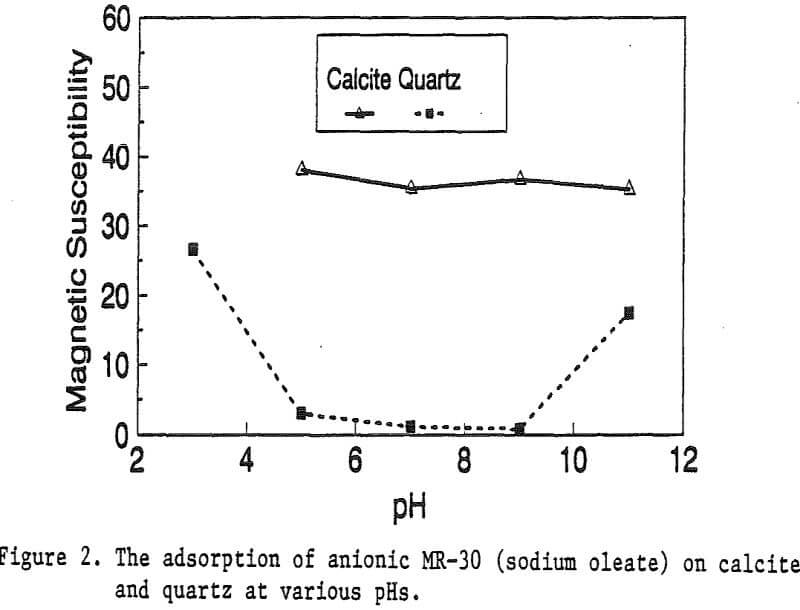
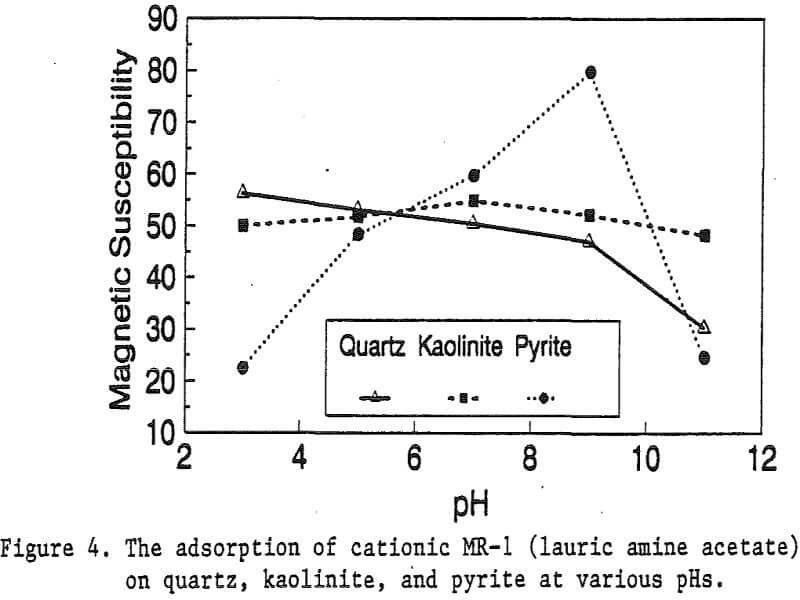
The magnetic susceptibility of each mineral was measured with a Johnson & Matthey magnetic susceptibility balance at 2 kilogauss. The results were: 0.44 x 10 -6 cgs/g for quartz, 4.84 x 10 -6 cgs/g for kaolinite, 3.94 x 10 -6 cgs/g for calcite, and 1.46 x 10 -6 cgs/g for pyrite.
The adsorption gradually decreased with the increase of pH. At pH 11, magnetic enhancement is at 30.3 x 10 -6 cgs/g. Kaolinite adsorbed MR-1 quite constantly throughout the whole pH range of investigation. Magnetic susceptibility was increased from 4.84 x 10 -6 cgs/g to 54.9 x 10 -6 cgs/g at pH 7. The adsorption of MR-1 strongly depended on the pH. At pH 3, the magnetic susceptibility of pyrite increased from 1.46 x 10 -6 cgs/g to
22.4 x 10 -6 cgs/g at 0.002 g/g magnetic reagent dosage. At pH 9, the magnetic susceptibility increased to 79.8 x 10 -6 cgs/g at the same reagent dosage. The surface charge on pyrite may be responsible for this change.
Potential Applications to Minerals Processing
The magnetic reagents can be used to enhance direct separations, much in the same manner as flotation reagents. Fine tuning the magnetic reagent to attach to a certain mineral in the slurry and then performing a magnetic separation to remove that mineral will be possible.



Coal Cleaning with Magnetic Reagent
Coal is an important energy resource. To be efficiently and acceptably utilized, most coals require cleaning to remove inorganic impurities. Many advanced physical separation technologies have been investigated; Magnetic separation is one of them. However, even high gradient magnetic separation technology has obtained only limited success.
Coal is a diamagnetic material. The minerals associated with coal, mostly clays, quartz, calcite and pyrite, are also diamagnetic to weakly paramagnetic. Thus, the magnetic properties of most minerals associated with coal are too low to allow a clean separation using the existing magnetic separators. To selectively increase the magnetic property of the minerals, while not changing the coal, would be necessary for an efficient magnetic separation.
Properties of Magnetic Reagent
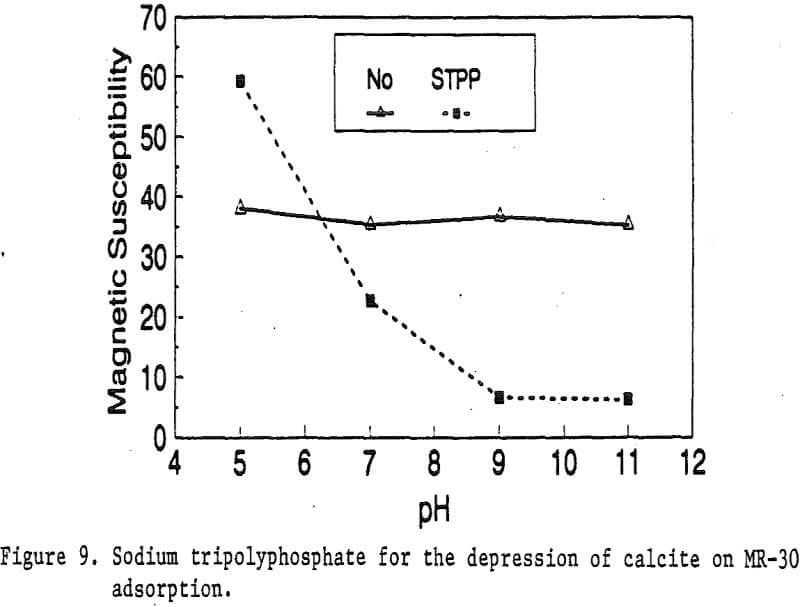
Magnetic reagents are magnetic materials with surfactants built on their surface to serve as bridges connecting magnetic materials and other minerals. The functional group of the bridging surfactant can be tailored for an individual magnetic reagent to result in selective adsorption on desired nonmagnetic minerals. When a mineral adsorbs the magnetic reagent, it becomes magnetic. Basically, the reagent behaves in a similar fashion as a flotation collector. Its adsorption on mineral particles depends on the functional group of the reagent and the surface properties of the minerals. Depressants and activators for the adsorption of flotation collectors on minerals have similar effects on the adsorption of magnetic reagents as well.
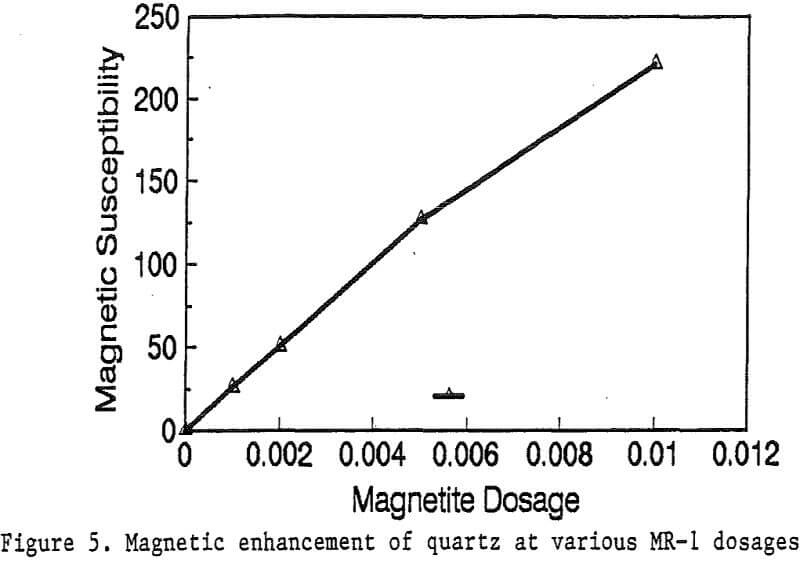
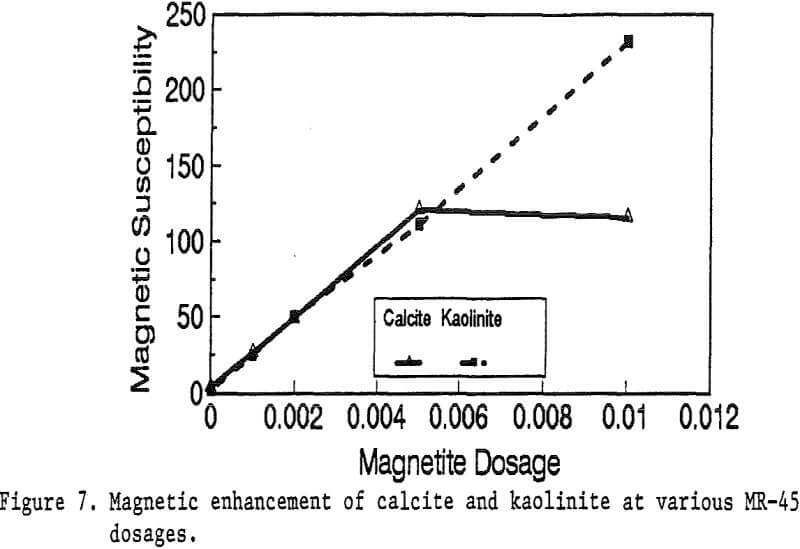
Primary amines such as lauric amine acetate have been known as good flotation collectors for many minerals, including the four minerals mentioned above. The magnetic reagent MR-1, which utilizes lauric amine acetate as the bridging surfactant, can also be strongly adsorbed by the four minerals. As shown in Figure 2, the magnetic susceptibilities of these minerals continuously increases with the increasing MR-1 dosage, until the saturation state of adsorption has been reached for each mineral.
Coal Cleaning
Reagent System: To apply magnetic reagent technology to coal cleaning, the preference is to enhance the magnetic properties of all the minerals associated with the coal but not the coal. A cationic magnetic reagent has the capability to be adsorbed by all minerals, therefore, nonionic surfactants have to be added to depress the coal.
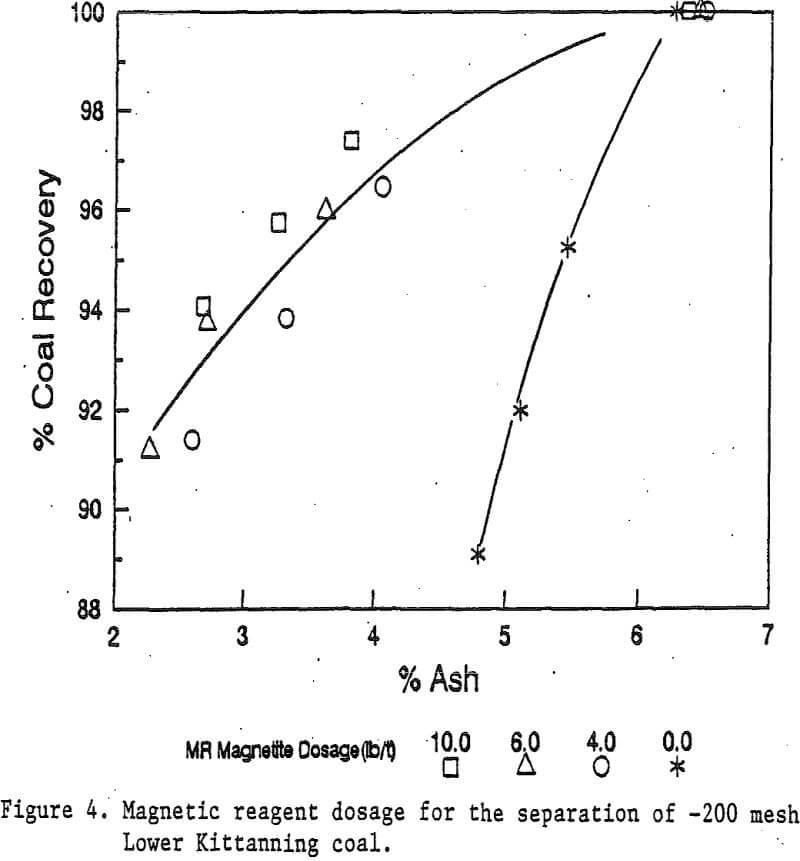
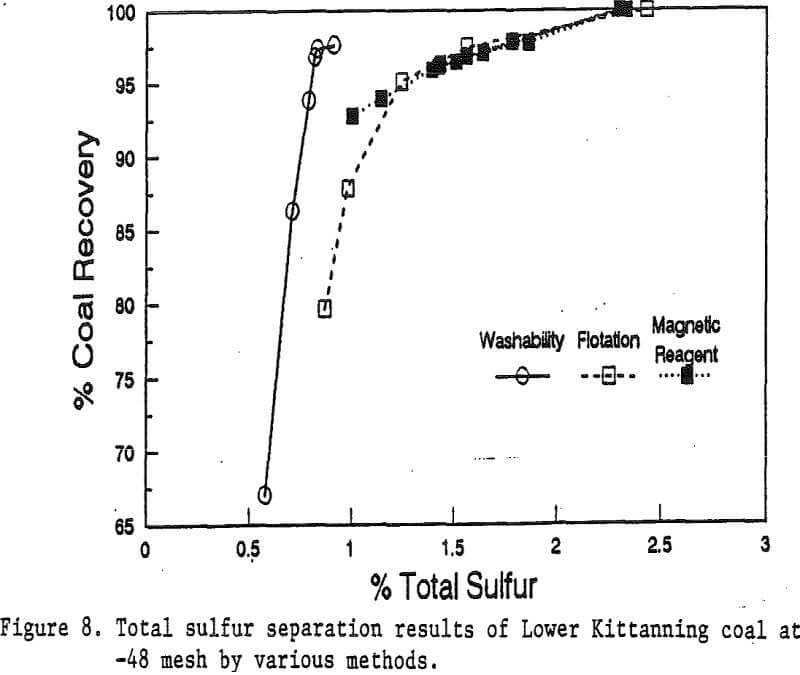
Reagent Dosage: Four dosages of magnetic reagents were studied for the Illinois No. 6 coal at the -48 mesh particle size. These dosages were 0, 3, 5, and 10 pounds of magnetite per ton of coal. The diamine dosage for the magnetic reagent was equivalent to about one third of the magnetite dosage. Magnetic separation was carried out with a stainless steel ball matrix.
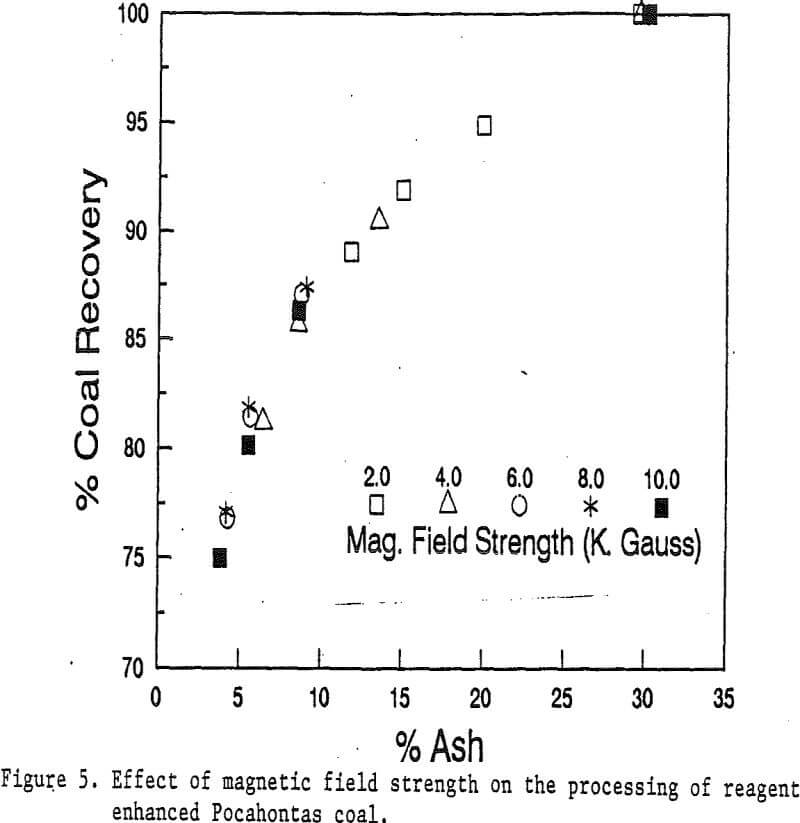
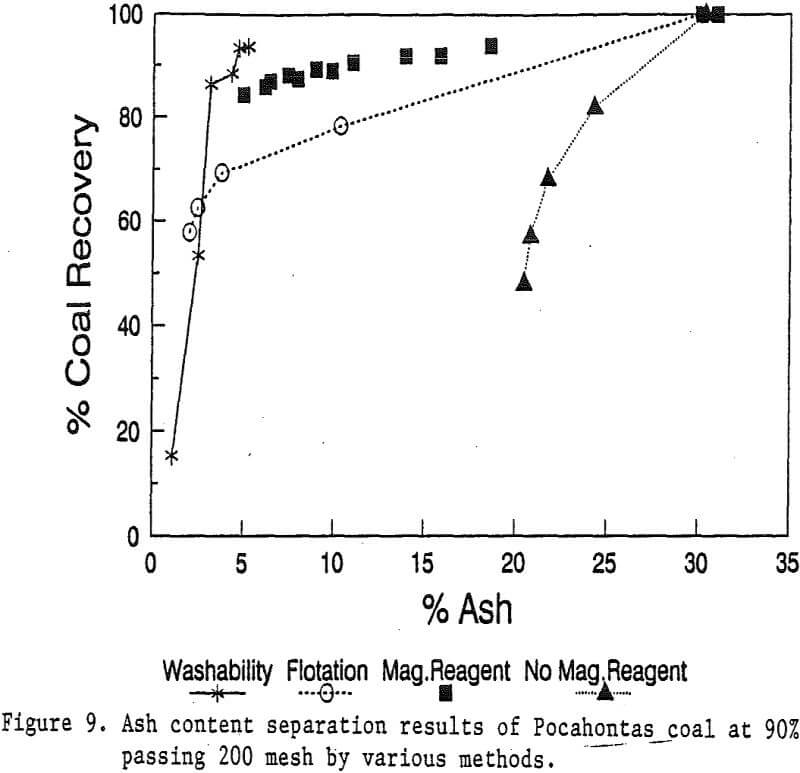
In this study, the effects of magnetic field strength were investigated in a range of up to 10 kilogauss. The Pocahontas coal at 90% passing 200 mesh was processed at 5 different magnetic field strengths: 2, 4, 6, 8, and 10 kilogauss, using a coarse screen matrix.
In a high intensity magnetic separator, the magnetic force on a particle is proportional to the magnetization of the matrix material and the magnetization of the particles. The magnetization of the matrix materials is usually proportional to the applied magnetic field strength up to approximately 6 kilogauss (varies with materials). At 6 kilogauss, the magnetization is nearly saturated. There is little gain in magnetization for matrix materials from 6 to 20 kilogauss.