Table of Contents
Because an economic method for preparing pure boron has not been developed, a program was started by the Bureau of Mines to evaluate existing techniques or to develop new methods for preparing the pure element. This report describes investigations directed toward the development of techniques for the preparation of pure boron by fused-salt electrolysis.
Only two of the several fused-salt baths tested were satisfactory for the preparation of boron. These baths contained (1) potassium fluoride, lithium fluoride, potassium fluoborate and boron trioxide; and (2) potassium fluoride, potassium chloride, and boron trioxide. Of these two mixtures, the latter was more satisfactory.
Elemental boron of from 90- to 95-percent purity and, in isolated cases, as high as 98-percent purity, was prepared from these two types of fused-salt baths. There appeared to be no distinct differences either in the yields or the purity of boron prepared at current densities ranging from 795 to 1,000 amperes per square foot. Current efficiencies were as high as 85 percent.
Although a number of different cathode materials were used, including molybdenum, tantalum, titanium, zirconium, and tungsten, low-carbon iron was the moat satisfactory cathode material. A thin protective boride layer formed on the iron cathode and not only protected it against corrosion but also prevented excessive iron contamination in the boron deposits. In most of the electrolytes the optimum bath temperature from the standpoints of crucible attack and loss of bath salts by volatilization was found to be between 800° and 850° C. Efforts were made to develop baths which could be operated at lower temperatures, but this work was not. successful. Although many attempts were made to electrodeposit boron from baths which did not contain boron tri-oxide, the anode effect invariably precluded satisfactory deposition.
Many methods and techniques were used to determine the impurity levels in boron, but completely reliable chemical and spectrographic analyses were not achieved.
Introduction
The work described in this report is part of a program conducted during the past 3 years by the Bureau of Mines at Albany, Oreg. The purpose of this program was to develop new methods and to evaluate existing methods for preparing pure boron. The problem of producing pure boron is complicated by the active nature of this element and the readiness with which it forms borides. High-purity boric acid, boron trioxide, and borax are abundant and commonly used for the preparation of boron halides and elemental boron. Boron halides can be prepared by a variety of techniques, and several reducing agents have been used to prepare elemental boron from these compounds. These methods are useful for the small-scale laboratory preparation of elemental boron, but they have not been adopted to commercial practice. In general, these methods are expensive and in most instances they do not yield boron of greater than 99-percent purity.
During the past 60 years many researchers have described fused-salt baths which would dissolve boric anhydride (boron trioxide) and from which elemental boron could be electrodeposited. A number of salts which dissolve boron trioxide including NaCl, KCl, CaCl2, KF, K2CO3 , and K2SO4 were studied by H. H. Kahlenberg who attempted to electrodeposit the element. Although small amounts of impure boron were obtained in some tests, he was not able to develop a practical electrolytic process.
Little is known about the physical properties of boron because of the many difficulties encountered in obtaining the pure element. At the high temperatures required for the reduction of boron compounds, boron is very reactive with other elements and forms stable borides. These boride compounds are difficult to separate from elemental boron.
High-purity boron can be obtained by hydrogen reduction or by thermal decomposition of boron halides or diboranes. The yields in most of these cases usually are very low and because of the high temperatures required and slow reaction rates, costs are so high that such methods are useful only for small-scale laboratory preparations or in special instances where cost is a negligible factor. G. H. Fetterly produced boron on a pilot plant scale by hydrogen reduction of boron trichloride. The boron was deposited on carbon resistor rods, Henri Moissan prepared elemental boron by the magnesium reduction of boron trioxide. This process yielded only impure boron containing boron suboxide. Impure material analyzing 84 percent boron and 14.5 percent oxygen was prepared by Weintraub using similar reduction techniques.
J. L. Andrieux and W. J. Deiss studied the electrolysis of fused borates and mixtures of borates and fluorides of the alkali and alkaline earth metals. Andrieux electrolyzed borax at 1,000° C. and obtained a product containing 70 percent boron and 20 percent oxygen. Andrieux was able to prepare boron of 92-percent purity by electrolysis of magnesium borate-magnesium fluoride baths. The boron contained 1 percent oxygen and 6 percent magnesium.
Theoretical Considerations
The deposition mechanism occurring during the electrolysis of fused salts cannot be fully explained. In systems containing KCl, KBF4 , and B2O3 , the alkali-chloride (KCl) is considered the solvent. Based on the behavior of aluminum oxide-cryolite baths, described by Doucet, and on the principles of complex formation by Kortum and Bockris, the following mechanism is proposed to account for the solubility of boron trioxide (B2O3) and potassium fluoborate (KBF4) :
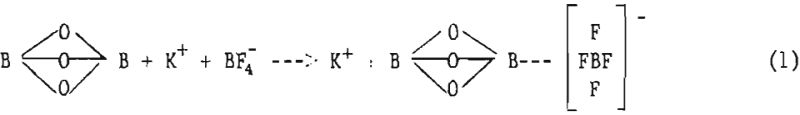
Boron ions for deposition are supplied by the boron tiroxide (B2O3) according to Murphy, Tensley, and Meenaghan:
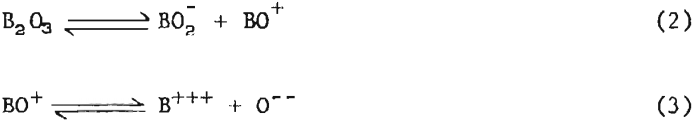
where the net reaction is that boron deposits at the cathode and oxygen is evolved at the anode. The overall net reaction is still uncertain inasmuch as one does not know whether the potassium fluoborate is depleted in the bath.
Miller used a reducing atmosphere of hydrogen over the surface of the electrolyte and found that at approximately 800° C., elemental fluorine and chlorine were evolved and that the potassium fluoride concentration increased during the electrolysis. This reaction was shown as follows:
4 KBF4 + 2KCl → 4B + 6KF + 5F2 + Cl2…………………………………………………….(4)
Previous work by the Bureau of Mines on the same system used by Miller failed to give a deposit of boron because of the anode effect. However, in this work an inert atmosphere of argon was employed which gives rise to a somewhat different mechanism than that presented by Miller. It is possible that the use of hydrogen could give rise to a catalytic effect or an intermediate mechanism whereby boron would be deposited. Such an effect, of course, would not be observed with the use of argon.
N. P. Nies found that suitable molten-salt baths could be prepared from simple salts of the type tested unsuccessfully by Kahlenberg. Nies found that substantially any desired amount of boron trioxide could be dissolved in potassium chloride by the addition of a predetermined amount of potassium fluoride. Baths used by Nies conducted current freely and were satisfactorily fluid with boron trioxide concentrations up to 40 percent. H. S. Cooper stated that pure crystalline aggregates of boron were prepared by electrolyzing a fused-salt bath of potassium chloride and potassium fluoborate at temperatures in the range of 650° to 1,000° C. Cooper noted that there was a decided current drop as electrolysis progressed. Cooper attributed this current drop to the change in resistivity of the bath which he said was characteristic of baths containing fluorides. W. J. Kroll, however, states that fused-salt electrolytes cannot be operated exclusively with fluoride baths containing only fluoride salts and that oxides must be added to avoid the anode effect, because only the oxide is decomposed by the current. Cooper minimized the anode effect by the use of high current which caused sustained agitation of the bath. Presumably agitation introduced oxygen into the bath by absorption from the atmosphere.
Experimental Procedures and Results
Cell Design
A number of cell designs were considered for the electrodeposition of boron from fused-salt baths. A simple, small, laboratory-scale , fused-salt electrolytic cell was used during most of this investigation. This cell, shown in figure 1, was constructed to provide for the periodic addition of fresh salts and for the periodic removal of cathodes.
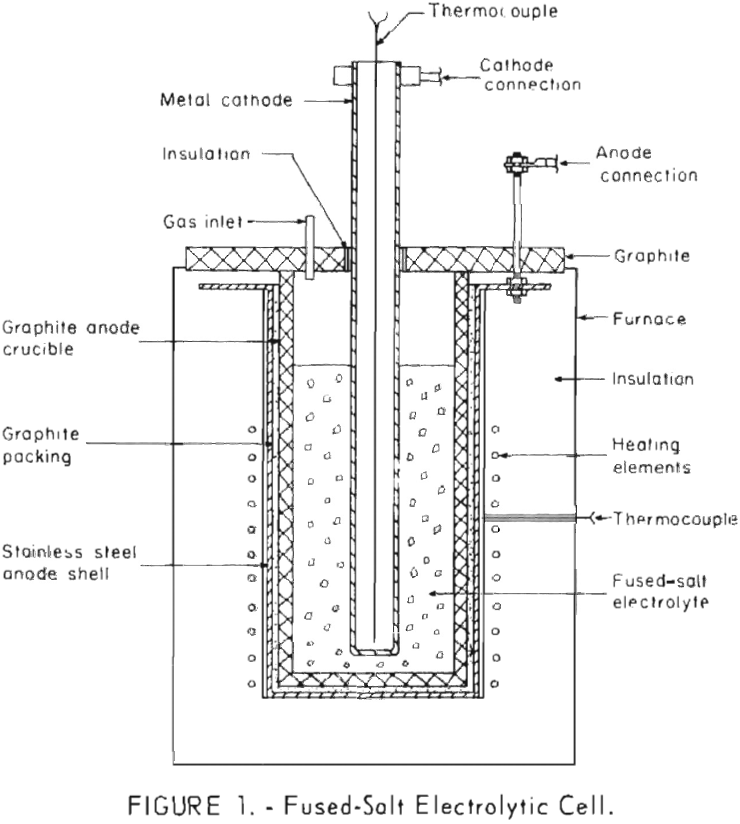
The crucible, which also served as the anode, was made of National Carbon Co. A.T.J, graphite. The crucible was 5 inches in outside diameter and 9 inches long with a 3/8-inch wall and a ½-inch thick bottom. It was encased in a 16-gage type 430 stainless steel shell. An electric pot furnace was used to heat the lower 6 inches of the crucible. The graphite crucible and stainless steel shell were made anodic by connecting the anode lead from the rectifier directly to the metal shell. The graphite crucible was inserted into the shell and powdered graphite was packed between the crucible and the shell to insure good contact.
The hollow cylindrical cathode, 1-5/16 inches in outside diameter by 14 inches long with the lower end welded shut, was suspended to within one-half inch of the bottom of the crucible. The electrical connection was made with a heavy copper clamp attached to the top of the cathode. During the later stages of the work, the cathode was internally cooled. A step-controlled 12-volt, 1 ,000-ampere direct-current copper oxide rectifier was used to supply current.
Anode Materials
Initial tests were performed in graphite crucibles fabricated from ordinary arc-furnace electrodes. However, these crucibles were attacked by the fused-salt baths, and both the deposited boron and the fused-salt electrolytes were seriously contaminated by carbon. During several of the tests, portions of the crucible walls were completely decomposed by the electrolytes at the high operating temperatures. Crucibles fabricated from National Carbon Co. A.U.C. extruded graphite withstood the severe conditions much better than crucibles fabricated from arc-furnace electrodes. The ash content and density of four grades of graphite used as crucibles are shown in table 1.
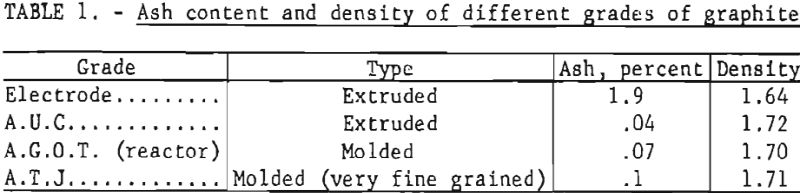
The A.T.J. very fine grained molded graphite was the most satisfactory crucible material.
Cathode Materials
The proper choice of a cathode material for the preparation of high-purity boron had to be carefully considered because boron reacts with most metals to form borides. A review of the literature showed that only a few metals do not form borides. These metals are gold, bismuth, cadmium, copper, lead, antimony, tellurium, and zinc. Of these, only copper and gold have melting-points above 700° C. Despite their tendency to form borides, carbon and some of the higher melting-point metals that could possibly be used arc: Silver, beryllium, cobalt, chromium, iron, molybdenum, niobium, palladium, platinum, tantalum, thorium, titanium, vanadium, tungsten, and zirconium. A number of these metals were tested as cathodes. Molybdenum tended to become brittle; it flaked off and contaminated both the electrolyte and the boron product. Tantalum, titanium, zirconium, and tungsten all had the same tendency to become brittle and in each case severe attack occurred on the cathode at the surface of the electrolyte. Inconel (80 percent nickel, 14 percent chromium, and 6 percent iron) was used; however, the deposited boron contained excessive amounts of nickel. In this investigation no method was found which would separate the nickel from the boron.
Low-carbon iron was used as a cathode material in spite of its alloying characteristics and reactivity with boron. The bulk of the boron deposit was easily removed from the cathode by prolonged leaching with water. A thin adherent boride layer formed and remained fast to the iron cathode. A second boron deposit could be made directly on top of this boride layer and iron contamination in the second boron deposit was much less than that found in the first deposit.
Most of the cathodes used in these investigations were fabricated from low-carbon iron tubing with the lower ends welded shut. By inserting a thermocouple into the hollow cathode it was possible to check the bath temperature during cell operation. Air was circulated through the hollow cathode when cooling was desired.
Operation of the Electrolytic Cell
In the first few test runs the open top of the graphite crucible was covered with transite to prevent excessive heat loss; however, because of the corrosive gases and high temperatures involved it was necessary to replace the transite with a graphite lid. When new graphite crucibles were used, the quality and yield of the deposits immediately varied.
A thick black finely divided carbon scum formed on the surface of the molten bath during operation of the cell. This finely divided carbon was a product of the erosive action of the hot bath on the graphite crucible. At first the amount was small, and no effort was made to identify or remove it, but as electrolysis was continued with any given bath, the accumulation of scum became a serious problem. The purity of the boron product was greatly improved when this finely divided carbon was carefully removed before inserting or removing the cathodes from the electrolyte. When this material was not removed, the deposited boron contained approximately 5 percent carbon. Under identical conditions the carbon content was reduced to 0.5 percent simply by skimming the carbon from the bath.
In earlier work, when sufficient boron was deposited, the cathode was withdrawn from the molten electrolyte and quickly covered with dry sodium chloride. Fresh electrolyte was added to adjust the bath level and to replenish the depleted boron. A new cathode was inserted into the molten bath, and the electrolysis was continued. After a cathode had cooled to approximately 500° C. the cathode and deposit were immersed in water for about 1 hour. At the end of this time most of the boron deposit had fallen from or could easily be scraped from the cathode.
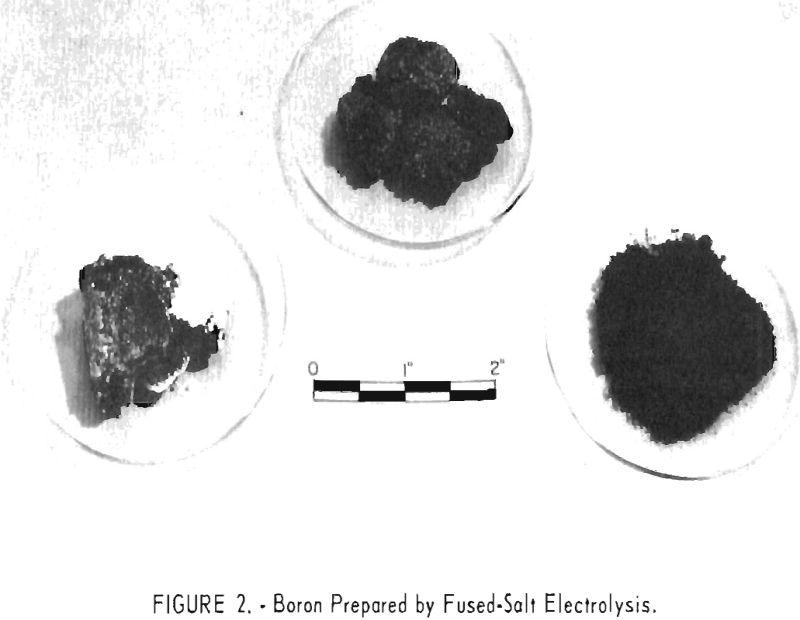
Figure 2 shows boron prepared by fused-salt electrolysis. At the left is boron which has been removed from the cathode but still retains its sodium chloride coat. The middle sample is water-washed boron, and the right sample is boron which has been thoroughly leached with acids and dried.
During the later stages of this investigation a different technique was used to prevent excessive oxidation of the boron. The principle was simply to form a solid layer or coating of solidified electrolyte over the boron deposit before the cathode and deposit were removed from the electrolyte. At the completion of electrolysis, the cathode was cooled internally, either by circulating a cooling liquid or more commonly by circulating air through a copper tube inserted into the hollow cathode. After the cathode was cooled for 5 to 10 minutes, it was removed from the molten bath, allowed to cool slightly, and then immersed in water. Quenching the deposit in water while the cathode was still hot facilitated rapid separation of the deposit from the cathode by thermal shock and also aided dissolution of the water-soluble salts.
During electrolysis, a stream of argon was directed over the surface of the electrolyte to prevent excessive oxidation of the cathodes and anodes.
Temperature
Boron deposited from baths operated below 750° C. was generally more amorphous and less pure than boron deposited at higher temperatures. The highest purity boron obtained during this study was from baths which were operated at temperatures ranging from 800° to 850° C.
Operating temperatures were measured at several points in the electrolytic cell. One point was generally on the outer surface of the anode or anode shell with a second in the electrolyte itself; however, because of the high temperatures involved and the corrosive nature of the baths, this point was used only for making periodic observations of bath temperatures. At a third point of measurement and control, located inside of the cathode itself, it was observed that the temperature was somewhat higher than that of the surrounding bath. This difference in temperature was also noted and studied by G. T. Miller. He found that the cathode temperature was from 30° to 100° C. higher than that of the surrounding electrolyte. This increase indicates that the formation of boron may be a secondary reaction occurring during electrolysis in the fused bath. Miller postulated that the exothermic cathode process involves the reduction of BF3 or BF4 ions with elemental potassium. If this is correct, elemental boron is produced by the reaction of potassium with the boron compound. There was evidence that potassium was deposited at the cathode when the potassium fluoborate and boron trioxide were expended. It is also possible that the temperature increase may have been caused by resistance heating of the cathode.
Most of the salts used for the electrodeposition of boron have relatively high melting points which necessitated operating temperatures ranging from 700° to 1,000° C. Several attempts were made to prepare and operate salt mixtures with a lower melting point. Lithium fluoride and lithium chloride salts were added to some of the electrolytes. A 1:1 weight ratio of lithium and potassium fluorides forms a eutectic mixture that has a melting point of 495° C. When an equal weight of potassium fluoborate was added to the eutectic mixture, the melting point of the resulting bath was near 600° C.; however, this bath would not conduct current freely, and electrolysis was not possible. When boron trioxide was added to the bath it was necessary tn increase the temperature to 700° C., to maintain a fluid melt. A satisfactory boron deposit was obtained by electrolysis of this bath. A similar effort was made to develop chloride baths that could be operated at lower temperatures. A eutectic mixture of lithium and potassium chlorides (weight ratio 2:3 respectively) which melts at 360° C. was prepared. An equal weight of potassium fluoborate was added to this eutectic mixture and the temperature was increased to 650° C. to obtain a fluid bath. When electrolysis was started, severe fuming occurred, and a satisfactory boron deposit was not obtained.
Baths containing lithium fluoride or chloride did not appear to have any advantages over conventional baths and the purity of the boron (78 to 83 percent) was not improved. Current efficiencies ranged from 50 to 85 percent. The main disadvantage was the severe fuming which occurred during electrolysis
Cathode Surface and Length of Electrolysis
Considerable difficulty was encountered because of the tendency of the boron deposit to slough off the cathode during electrolysis, especially when the bottom of the cathode was round. When the bottom of the cathode was flat or rough, the boron had a greater tendency to adhere to it. To promote better adherence of the boron deposits, tests were performed with cathodes having the following surfaces:
- Smooth-semipolished surface, round bottom.
- Sand-blasted surface, round bottom.
- Sand-blasted surface, round bottom, and a series of 1/32-inch grooves cut into the surface of the cathode.
- Sand-blasted surface, flat bottom.
- Sand-blasted surface, flat bottom, and a spiral weld bead on the surface of the cathode. The beads were approximately 1/8 inch thick and ½ inch apart.
The boron deposits consistently fell from the first and second cathodes during electrolysis and some of the deposits fell from the third cathode. The boron deposits on the fourth and fifth cathodes were usually exceptionally adherent. As a result, cathodes used during the later stages of this work had a sand-blasted surface and a flat bottom.
Calcium contamination, which originated either as an impurity constituent from the feed materials or was introduced inadvertently from Transite or other insulating materials, adversely affected bonding of the boron deposit to the cathode.
The duration of deposition and the thickness of the deposits affected the adherence. As the time of electrolysis was increased there was a greater tendency for the deposit to slough off of the cathode. Deposits ¼ to 3/8 inch thick were formed in 1-½ hours, while 3/8- to ½-inch deposits were formed in 2 hours. If electrolysis was continued for longer than 2 hours, either the entire deposit or portions of it fell from the cathode; however, this boron could easily be recovered from the electrolyte. This sloughing characteristic of the deposit might be used in a continuous or semicontinuous cell. Boron could be deposited continuously on a cathode until it sloughed from the cathode and boron deposits could be recovered later from the bottom of the cell.
Composition of Fused-Salt Baths
Based on the experiments reported by Andrieux and Deiss four exploratory runs were made using baths composed of sodium salts and boron trioxide. Pertinent data on these runs are shown in table 2. The deposits were thin, and although some of the boron had fallen from the cathode, the quantity and quality of the boron was discouragingly low. The average current efficiency of these runs was not over 25 percent. Chemical analyses of the deposits showed they contained 70 to 74 percent elemental boron, 5 to 6 percent sodium and 0.4 to 2.0 percent iron.
Cooper found that potassium salts were far superior to sodium salts for the electrodeposition of boron. The use of potassium as the positive radical in either chloride- or fluoride-type baths appeared to be essential. Cooper found that if sodium salts were substituted, even In part, for the potassium salts, current efficiencies and purity were adversely affected.
A series of experiments, patterned on Cooper’s work, were performed with potassium fluoride baths. Efforts were made, to lower the operating temperatures of these baths by the addition of lithium fluoride. The bath compositions and operating conditions used in these runs are shown in table 3. In all instances the baths were fluid at temperatures between 700° and 750° C.
Firm, adherent deposits were obtained in these tests. The highest current efficiency obtained (85 percent) was in run 10 with this type of bath. Although the addition of lithium fluoride allowed operation at lower temperatures than those reported by Cooper, corrosion of the anode remained a problem. The purity of the deposited boron did not exceed 91.5 percent, and it contained 3.6 percent carbon (see table 3, run 10). The boron deposited from baths containing KBF4 was consistently high in phosphorus which ranged from 1 to 10 percent, as phosphorus was an impurity in the potassium fluoborate.
Several fluoride salt baths were prepared to substantiate an observation made by Kroll that fused-salt baths containing only fluoride salts become inoperative because of the anode effect. Tests showed (see runs 7, 9, and 10, table 3 and run 11, table 4) that electrolysis of fused-salt baths did not proceed unless boron trioxide was added. In each case the anode effect occurred almost as soon as electrolysis was started in these baths. A few grams of boron trioxide added to the electrolyte would insure continued electrolysis until the oxide was exhausted. Cooper claimed that he was able to avoid the anode effect when operating fluoride baths by operating at a high current density which caused constant agitation of the electrolyte and possibly introduced oxygen by absorption from the air.
After boron trioxide was added to the fluoride baths (in runs 9 and 10, table 3) boron was deposited on low-carbon iron cathodes. A total of 288 grams of crude boron was obtained on 5 separate cathodes during run 10, which represented 75 percent of the available boron in the bath. The overall cur-rent efficiency during run 10 was 85 percent. National Carbon Co. A.U.C. graphite was used as a cathode during run 10. However, it began to decompose soon after electrolysis was started. A hollow copper cathode was also tested in this bath. The boron that was deposited on the copper cathode was relatively free of contamination, but the surface of the cathode above the electrolyte was severely oxidized and the oxide scale contaminated the electrolyte.
Electrolyte Alkalinity
Almost identical reports by N. P. Nies and Borax Consolidated Ltd. state that as electrolysis of baths containing potassium chloride, potassium fluoride, and boron trioxide progresses there is a change in the characteristics of the baths and that the purity of the boron product is impaired. This change is characterized by the increased alkalinity of the bath. A brief study of the effects of changing alkalinity on the boron deposition was made. It was necessary to utilize the pH of an aqueous solution of a solidified sample of the electrolyte to determine the alkalinity of the baths. A 0.33-gram sample of the solidified electrolyte was dissolved in 100 milliliters of distilled water and the pH of this solution was determined. The pH of the aqueous solution at the beginning of a run was approximately 6.9; however, after 2 hours of electrolysis the pH of the electrolyte sample was 7.3. This increase in alkalinity was effectively controlled either by the periodic addition of anhydrous hydrogen chloride directly to the melt in gaseous form or by the addition of solid ammonium chloride.
Baths were operated in which the alkalinity was varied from pH 4.4 to 7.3. Bath compositions and operating conditions during these tests are shown in table 4. Chemical analysis of the boron deposits varied from 89.0 to 90.0 percent boron while the spectrographic analysis did not reveal any distinct differences between the runs. The only difference noted was that the boron deposited from baths where the pH varied from 4.4 to 5.2 were finer grained than the boron deposited at the higher alkalinity.
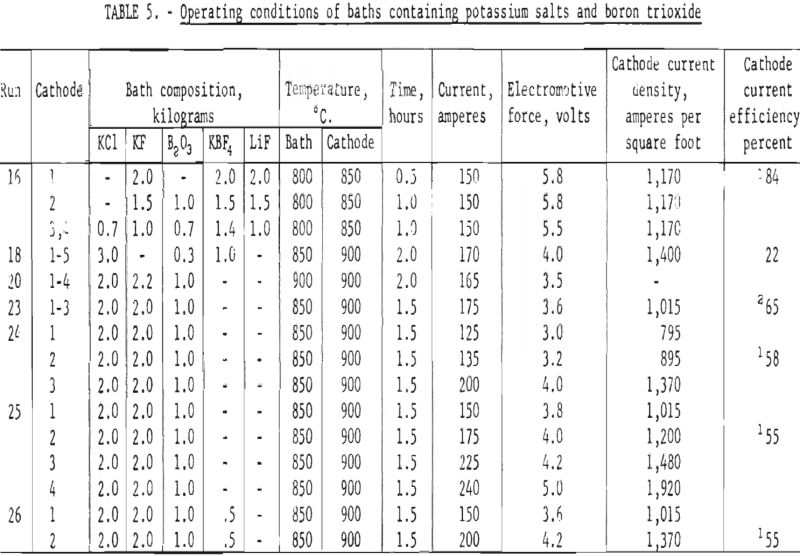
The work of Nies indicated that a very efficient bath for the electrodeposition of boron could be made from potassium fluoride, potassium chloride, and boron trioxide with weight-percent ratios of 40:40:20, respectively. Table 5 lists a series of runs made using similar bath conditions. Both current and voltages were varied to observe the effects of current density on the deposition of boron. The deposits from these runs contained an average of from 90 to 91 percent elemental boron. There were no appreciable differences in the boron deposited at cathode current densities ranging from 795 to 1,920 amperes per square foot.
The highest current efficiencies (84 percent) obtained from this type of bath was in run 16. Chemical analysis of this deposit showed it contained 90 percent elemental boron.
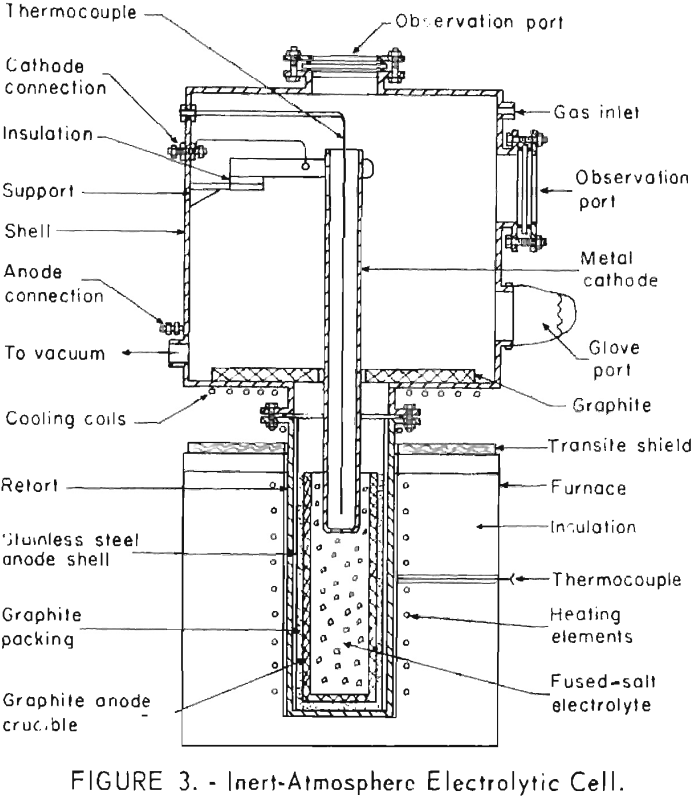
Inert-Atmosphere Cell
A controlled-atmosphere cell was constructed to as-certain the feasibility of electrodepositing boron from its salts in the absence of air. This cell is shown in figure 3. The cell consisted of an externally heated stainless steel retort which housed a graphite crucible containing the electrolyte. The inert-atmosphere chamber above the crucible was constructed to contain the electrodes, fresh electrolyte, and necessary electrical connections. Means were provided for necessary cooling, evacuation of the system, observation, and manipulation of the equipment inside the chamber.
Many exploratory runs were made with this apparatus. The fused-salt baths were composed of potassium fluoride, potassium chloride, and potassium fluoborate with weight-percent ratios of 40:30:30, respectively. The electrodeposition of boron was not attained with these baths both because of excessive fuming and because of the anode effect. Bath mixtures containing weight-percent ratios 35:25:20:20, respectively, of potassium fluoride, potassium chloride, potassium fluoborate, and boron trioxide were operated successfully.
The boron deposited during these runs was similar in every way to that prepared in cells open to the atmosphere. Physical, chemical, and spectrographic examinations of the boron product indicate no advantage was gained by conducting electrolysis in an inert-atmosphere cell, at least in these particular baths.
Analytical Results
Although many methods and techniques were used to determine impurity levels in boron, completely reliable analyses were not achieved. In general, analytical methods developed by Columbia University were used for the chemical analysis of boron Most of these methods follow a conventional procedure in which boron is converted by digestion with acids or by fusion to boric acid which is then determined volumetrically by titration with sodium hydroxide in the presence of mannitol. One of the many problems involved in the analysis of boron is the difficulty of dissolving or digesting elemental boron. Chemical analysis for total elemental boron has been found to vary as much as ± 10 percent on the same sample. It has been suggested that oxygen might account for these variations. However, no reliable quantitative method is known for the determination of oxygen in elemental boron.
Chemical analyses of as-deposited boron are shown in table 6. When the black carbon scum was carefully removed from the surface of the electrolyte, a decided decrease in carbon contamination of the boron was noted as can be seen by comparing the carbon content of the boron from runs 23 to 26 with that from runs 9 and 14. Higher purity boron was recovered from baths which contained boron trioxide but did not contain potassium fluoborate.
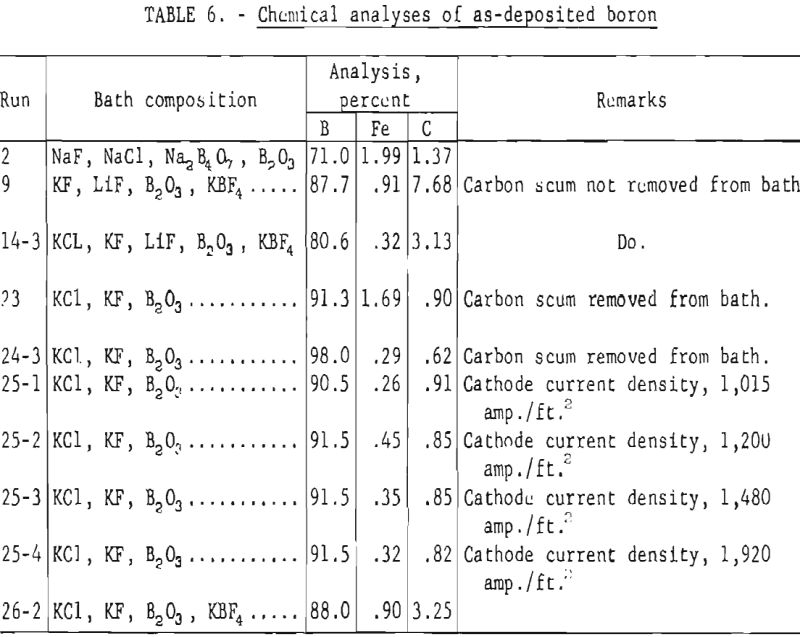
Run 25 of table 6 shows that wide variations in cathode current density had no effect on the purity of the boron. The low purity of the boron (run 2) prepared from a bath composed of sodium salts is considered typical.
During the course of the investigation two major classes of electrolytes were used:
- Those containing potassium fluoborate and boron trioxide.
- Those containing boron trioxide.
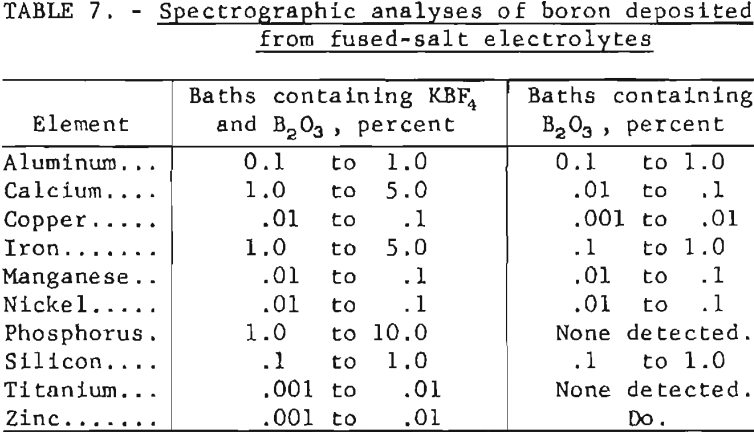
Spectrographic analyses of boron prepared by the electrodeposition of boron from the two classes of electrolytes are shown in table 7. The data shown are based on estimates from qualitative spectrographic analysis of boron samples. The potassium fluoborate contained 0.05 percent phosphorus which had a tendency to concentrate and codeposit with the boron at the cathode. As much as 10 percent phosphorus was found in some of the boron deposits.
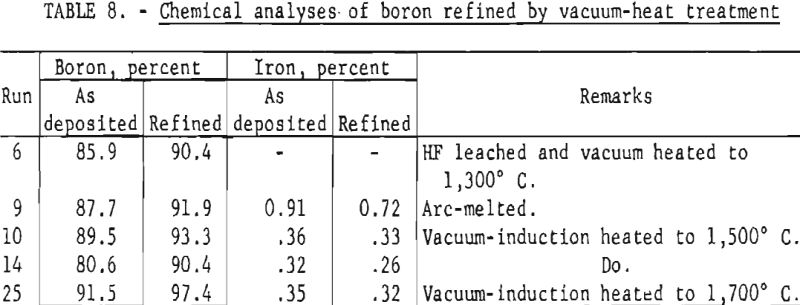
The as-deposited boron was purified by treatment with hydrofluoric acid to remove silicon, by high-temperature (-1,700° C.), high-vacuum sintering, and by arc melting under reduced pressure. The purity of the refined boron ranged from 90 to 97 percent. Typical analyses of refined boron are shown in table 8.
Conclusions
In general it was not possible to electrodeposit high-purity boron from any of the fused-salt baths studied; however, deposits containing up to 98 percent elemental boron were obtained.
The boron purity was improved by leaching with hydrofluoric acid and by applying a high-temperature , high-vacuum treatment. The purity of the refined boron ranged from 90 to 98 percent.
The major contaminant in the electrodeposited boron was carbon which came from the graphite anode. Iron, oxygen, silicon, and phosphorus were other impurities usually present in the boron.
Boron deposited on metal cathodes contained the borides of the cathode base metal. Low-carbon iron was the most satisfactory cathode material tested. A thin adherent layer of iron boride formed on the cathode and acted as a protective coating and as a base for further deposition. Although it has been reported that copper can be used as a cathode material, it was not found to be satisfactory during these investigations.
The operating temperature of the fused-salt baths ranged from 650° to 1,000° C. From the standpoints of yield, purity, and the nature of the deposits, baths operating between 800° and 850° C. were most satisfactory. Boron deposited below this range was amorphous, and there appeared to be little advantage in operating above this temperature because of the deterioration of the graphite crucibles.
In spite of claims that lowering the alkalinity of the fused-salt bath was a prime factor in preparing high-purity boron of a high yield, it was found that there was no appreciable difference in either purity or yield when electrolysis was performed in low-alkalinity baths. The only difference observed was the decreased grain size of boron deposited at lower alkalinity. There was no significant difference in the boron deposited at cathode current densities ranging from 795 to 1,920 amperes per square foot.
Repeated attempts were made to operate fused-salt baths containing boron salts other than the oxides. All of these attempts failed, and it was necessary to have boron trioxide present before boron could be electrodeposited. The best results from the standpoint of boron purity and yield were obtained from fused-salt baths composed of potassium fluoride, potassium chloride, and boron trioxide.
In spite of many claims that boron of higher than 99-percent purity can be directly prepared by the electrolyses of fused-salt baths, it was found that a more realistic value lies between 90- and 98-percent purity. Deposits which analyzed 98.0 percent boron were prepared during several runs, but boron of this purity could not be consistently obtained. Boron of higher purity, as reported in many publications, may have been obtained through estimates based on semiquantitative spectrographs analyses. These estimates are usually sufficient for most metallic impurities; however, many of the identifying spectra of these metallic impurities are similar to those of boron. Small amounts of tungsten can not be detected spectrographically in the presence of the more volatile boron. Aluminum and silicon have many identifying properties similar to those of boron. There have been no satisfactory methods developed for the analyses of oxygen or nitrogen in boron.
While high-purity boron was not successfully prepared by electrodeposition from fused-salt baths, this investigation served to delineate many of the problems and factors affecting this method of preparing the element. Much more work is necessary to develop suitable materials of construction for the cell components and more effective bath compositions. However, these investigations have demonstrated that boron of from 95- to 98-percent purity can be prepared by electrolysis of the fused-salt baths described in the literature.
