Preparation of ZrO2 from ZrCl4: The products of chlorinated zircon which consist mainly of zirconium tetrachloride and silicon tetrachloride are collected under the afore-mentioned conditions. This collected ZrCl4 is calcined at 900°C for 1 hour to produce commercial grade of ZrO2. Fig. (5) shows that a maximum recovery of ZrO2 of – 92% is obtained from the collected ZrCl4 resulting from the chlorination of zircon charge at 1100°C for 30 minutes. The produced ZrO2 with purity of 99% from chemical analysis, is in the form of monoclinic and cubic as revealed by XRD, Fig. (6).
Another process for the preparation of highly pure ZrO2 – 99.8% from the collected ZrCl4 is also performed by dissolving the zirconium tetrachloride in water, forming the zirconyl chloride which crystallize in the form of ZrOCl2.8H2O, by either evaporation of the solution or by increasing the acidity by additions of hot hydrochloric acid. The produced zirconyl chloride is calcined at 900°C for 1 hr.
Kinetics And Mechanism of Chlorination Process
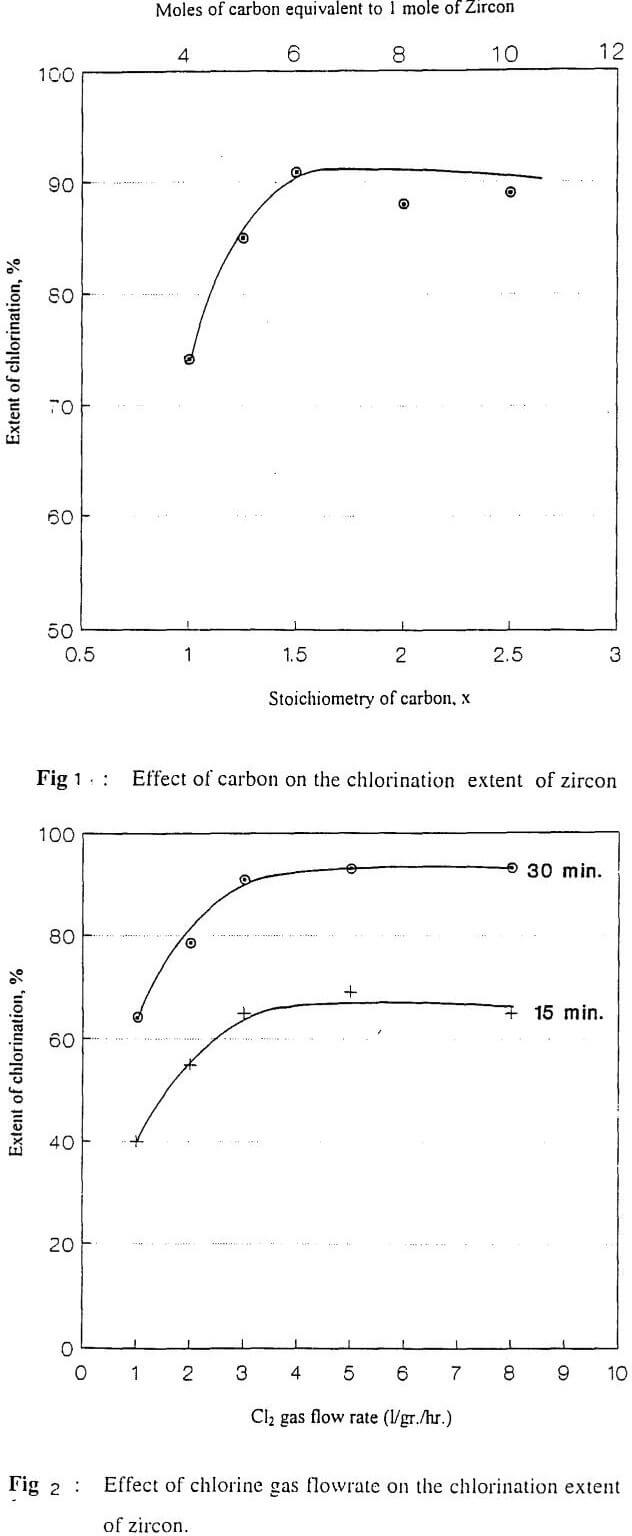
The results of zircon chlorination obtained within the temperature range 800-1100°C for different periods up to 90 minutes are found to satisfy the following kinetic equation based on chemical reaction model up to 80% reaction extent, Fig. (13).
l – (l-x)1/3 = kt
where k is the rate constant and x is the fraction of zircon chlorinated in time (t) in minute. Arrhenius equation is applied to calculate the apparent activation energy of the process by plotting Log k against l/T Fig. (14). From the slope of the straight line, the activation energy is calculated as equals 36 kJ/mole.
The following mechanism can be considered and suggested for the chlorination of zircon.
1. activation of chlorine gas on carbon surface
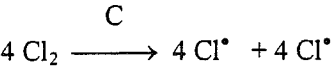
2. Transfer of the activated chlorine gas to zircon/carbon interface, followed by chemical reaction to produce ZrCl4, SiCl4 and oxygen
8 Cl° + ZrSiO4 → ZrCl4 + SiCl4 + 2 O2
3. Transfer of oxygen gas to carbon surface and formation of CO
2 O2 + 4 C → 4 CO
The overall reaction is as follows:
ZrSiO4 + 4 Cl2 + 4 C → ZrCl4 + SiCl4 + 4 CO
It may be proposed also that if the carbon is physically poisoned the chlorination of zircon is subsequently inhibited. Also the recombination of activated chlorine atoms by its collision to form deactivated chlorine molecules at certain conditions may retard the chlorination process of zircon.
2 Cl° + 2 Cl° + 2 Cl2 → 2 Cl2 +4 Cl2
The chlorination process of zircon is a wasteless route in metallurgical industries. Each one of the collected products can be used in its own special field. The remaining non-chlorinating zircon can be sold as a premium grade of white colour in some applications which needs a high purity zircon. Both collected products of zirconium and silicon tetrachloride are obtained from the chlorination of zircon at optimum conditions, 1100°C for 30 minutes. The Zirconia produced by calcination of ZrCl4 at 900°C is partially stabilized in form of mixture of cubic and monoclinic. On other hand, Zirconia produced by hydrolysis followed by crystallization in presence of HCl and calcination is purely monoclinic form. The purity of obtained Zirconia is ≥ 99%.