Table of Contents
A temperature of 250° to 260° C was found to be satisfactory for simultaneous removal of iron and manganese from the pregnant leach liquor resulting from leaching of low-grade manganese ore. A holding time of about 15 minutes at this temperature was sufficient for maximum iron removal under oxidizing conditions. Using optimum conditions with oxygen overpressure, precipitation of 98 percent of both iron and manganese was obtained.
Satisfactory separation of iron from manganese in the autoclave residue was shown to be possible by use of a simple water leach that dissolved the ammonium-manganese sulfate. A relatively pure cell feed, containing about 30 to 45 gpl manganese, was obtained at the concentration required for manganese electrolysis.
Above a certain temperature the oxygen atmosphere accelerated the precipitation of ferric oxide from leach liquor. For an oxygen over-pressure of 50 pounds this temperature was approximately 150° C. This acceleration was apparently due to ferrous ion oxidation in solution. Precipitation was accelerated with temperature much more markedly with an oxygen atmosphere.
Oxygen atmosphere had no apparent effect on manganese removal. The activation energy for the precipitation of ammonium-manganese sulfate is greater than zero at temperatures less than approximately 175° C and less than zero at temperatures above 175° C. A simple rate expression for the precipitation of ammonium-manganese sulfate was found to be
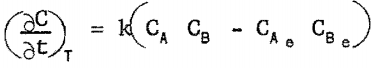
Industrial manganese requirements in this country are directly related to steel production. Almost 14 pounds of manganese is consumed as ferroalloys, metal, and direct-charged ore for each short ton of open-hearth, Bessemer, basic oxygen process, and electric steel produced. Ferromanganese is the form most commonly used for manganese addition in steelmaking. Annual consumption of this alloy exceeds 800,000 tons, while approximately 20,000 tons of metallic manganese is consumed. Manganese production depends almost exclusively on imported ores, which are supplied mostly from South America, Africa, and India, The small deposits of high-grade domestic ores have been all but depleted and the low-grade domestic ores, with the exception of small tonnages of manganiferous ores charged directly to the furnaces, cannot compete with the high-grade imported ores.
Low-grade manganese deposits in the United States are extensive. Because of the strategic importance of the metal, a vast amount of research has been carried out in an effort to develop processes that could be feasibly employed in event foreign ores become unavailable.
One process that has been studied by the Bureau of Mines involves the leaching of Georgia umber, a manganiferous oxide ore found in the Cartersville district of Georgia. This ore is considered representative of the umber deposits. The process utilizes a waste material from steel mill operations, spent pickle liquor, which results from the removal of oxide scale from steel products that are treated by immersion in dilute sulfuric acid. The spent liquor presents a costly and difficult disposal problem in most steelmaking areas, and any solution involving commercial use would be welcomed by the mill operators. Pilot-plant studies in utilizing the liquor in an umber leaching process are described in a Bureau of Mines report. In this process, the waste pickle liquor or acidified ferrous sulfate solution is used to extract the manganese, along with minute amounts of other metal impurities, from the umber ore. The resulting solution contains, after filtration, about 8 to 15 grams per liter (gpl) Mix and 30 to 40 gpl Fe as soluble sulfates. One phase of the research involved the development of a cyclic process whereby anolyte from manganese electrolysis cells was brought to leaching composition by addition of pickle liquor and used to leach fresh ore. The recycled anolyte contained manganese sulfate and ammonium sulfate. After leaching the ore, the manganese sulfate content and impurities in the cyclic leach liquor had been increased by the additional manganese extraction. The impurities were essentially iron in the form of ferric sulfate plus a small amount of ferrous sulfate. It was necessary to treat the pregnant liquor to remove iron and to concentrate the manganese to about 30 gpl before return to the manganese electrolysis cells.
The inverse solubility of manganese sulfate in aqueous solutions has been studied by a number of investigators, and the solubilities have been published. Fuller arranged the available data to give a curve showing the values in grams per liter, which varied from 164 gpl at -10° C to 2.7 gpl at 170° C, This phenomenon offers a means of separating manganese sulfate in pure form from its aqueous solutions. However, investigations have shown that the presence of free sulfuric acid has a marked effect in increasing solubility of manganese sulfate in aqueous solutions, even at elevated temperatures.
In a Bureau of Mines Fellowship study, Clendenen demonstrated that with aqueous ferric sulfate-manganese sulfate solutions, the iron can be separated by precipitation as ferric oxide at elevated temperatures, but that the free acid (formed by the conversion of the iron to the oxide) increases the solubility of the manganese sulfate and prevents effective precipitation of this compound. Conditions were established to obtain maximum iron precipitation during the study, but these same conditions were not considered practical for precipitation of manganese. In a manganese leach-electrolysis circuit, ammonium sulfate would be present in the solution, and additional tests showed that under the circumstances both manganese and iron could be precipitated, regardless of the presence of free sulfuric acid.
In preliminary tests at the Tuscaloosa laboratory, autoclaving cyclic leach liquor containing ammonium sulfate, manganese sulfate, and iron sulfates under oxidizing conditions resulted in precipitation of over 90 percent of both iron and manganese. If filtration was carried out under pressure, a filtrate was obtained containing some ammonium sulfate but essentially free of iron and manganese, depending on the autoclaving conditions. It was further demonstrated that the iron residue identified as ferric oxide, could be separated from the manganese by simple water washing, resulting in a concentrated solution of ammonium-manganese sulfate, (NH4)2Mn2(SO4)3.
The object of the investigation reported here was to establish optimum conditions, using high temperature-pressure methods, for the removal and separation of iron and manganese from pregnant leach liquor obtained by leaching low-grade umber in a cyclic leaching-electrolysis process. Kinetic analyses of some of the chemical reactions involved are also presented.
Experiment
Preparation of Leach Liquor
The solution used in this investigation was the liquid overflow product obtained from a cyclic leach-electrolysis circuit operated for the extraction of manganese from a Georgia umber. A synthetic anolyte, similar to a tank-house effluent from the. electrolysis of manganese that had been renewed with ferrous sulfate and sulfuric acid to give the extraction efficiency of fresh pickle liquor, was used as the leaching agent to remove 87 percent of the manganese from the fresh umber ore as soluble manganese sulfate. The filtered cyclic leach liquor contained, by chemical analysis in grams per liter, Fe++, 7.65; Fe+++, 7.44; Mn, 27.9; (NH4)2 SO4, 121.0; and free H2SO4, 21.3. This liquor was used for all tests listed in this report.
Test Equipment and Procedure
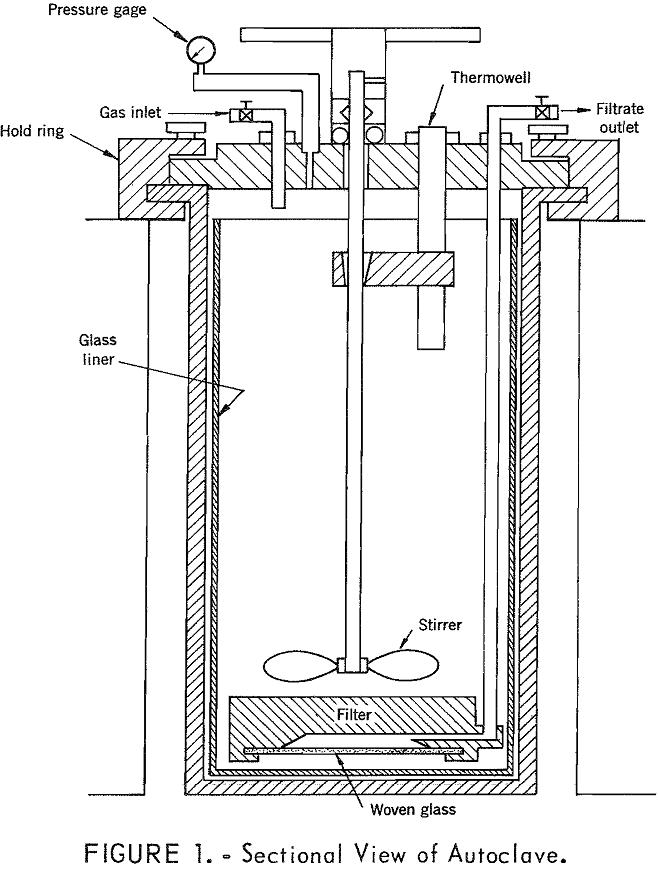
Laboratory experiments were made in a standard 1,000-psi stirrer-type Parr pressure apparatus of 2-liter capacity. A view of the autoclave is shown in figure 1. It was constructed of type 316 stainless steel and heated externally by strip heaters. Temperature was maintained by means of a manually operated variable voltage transformer and indicated by a Chromel-Alumel thermocouple and an indicating potentiometer. A glass liner was provided and metal parts contacting the leach solution under pressure were fabricated of titanium to prevent corrosion. An internal filter with a glass-fiber filter cloth was designed and constructed of Teflon to allow filtration of the hot acidified solution under pressure. A standard water-jacketed glass condenser was used to cool the liquid and prevent vaporization after it was withdrawn under pressure. Oxygen and helium were supplied as required from tanks through suitable reducing and control valves. Normally the charge consisted of 1,000 ml of solution, which was placed in the autoclave and the apparatus closed except for the bleed valve. Heat was applied and when steam appeared at the bleed valve, which indicated removal of air, this valve was closed. Overpressure was then established with oxygen or helium, through the gas inlet valve, as desired to meet the test conditions. After the proper temperature and pressure were obtained and held for a predetermined length of time, filtrate samples were taken by carefully opening the filtrate outlet valve and allowing the clear liquor to flow through the pressure filter into the water condenser. Measured volumes of filtrate were taken and chemically analyzed and at the conclusion of the test the remainder of the filtrate was withdrawn prior to releasing the pressure and disassembling the equipment.
Tests were made on leaching manganese from the residues with water. Residue remaining after withdrawal of the filtrate was weighed, analyzed, and the soluble manganese ammonium sulfate separated from the relatively insoluble ferric oxide by leaching and washing with limited amounts of water.
Results
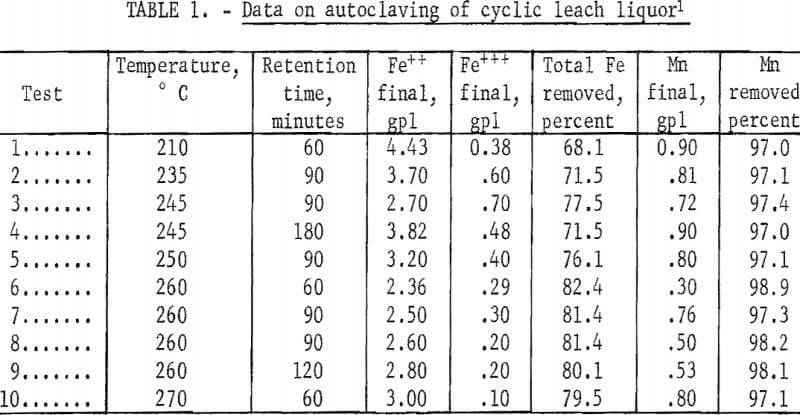
Tests made in previous studies of synthetic solutions containing manganese and iron sulfates but no ammonium sulfate showed that a temperature of at least 200° C and a holding time of 60 minutes were necessary for satisfactory precipitation of the iron from solution. Initial autoclaving tests made to determine the applicability of these conditions to the cyclic leach liquor containing ammonium sulfate are shown in table 1. They illustrate that removal of at least 80 percent of the iron and 98 percent of the manganese can be obtained at a temperature of 260° C and a holding time of 60 minutes, with removal of iron definitely increasing with increasing temperature and removal of manganese slightly increasing with increasing temperature. Tests made with holding time greater than 60 minutes did not increase removal of the metals. The tests also showed that the iron remaining in solution was in the ferrous state.
To compare precipitations under atmospheres of steam plus oxygen and atmospheres of steam plus inert gas, several tests were made in which oxygen and helium were added to the autoclave. Typical results of several tests given in table 2 show that removal of total iron was increased to 98 percent by the addition of oxygen; this increase at these high temperatures was probably due to the increased oxidation of the ferrous iron. Table 3 shows the changes occurring during a typical single autoclave test with oxygen added after 250° C was reached. Conversion of the ferrous iron was rapid.
The results of varying temperature and time under helium and oxygen overpressures are shown in table 4. These tests show that inert overpressure did not increase removal of the iron, and that oxygen overpressure increased iron removal. In these experiments with oxygen, oxygen overpressure was maintained at 40 to 50 pounds above steam pressure.
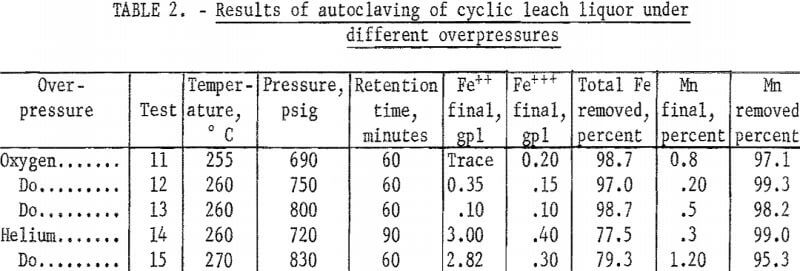
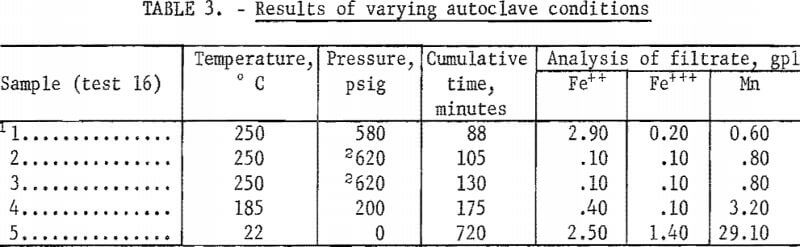
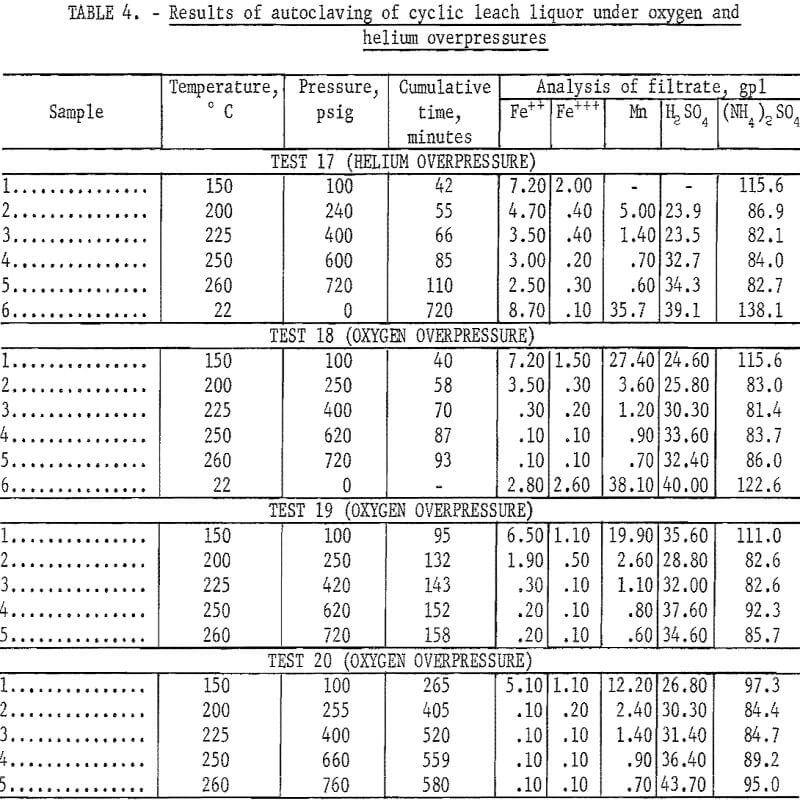
Evaluation of the data in tables 2, 3, and 4 shows that in an oxygen atmosphere and at 250° C or above, more than 97 percent of the iron was precipitated. The reaction and precipitation was complete and occurred with either fast or slow heating rates as shown in table 4. The last lines in tests 17 and 18 of table 4 indicate that the iron is redissolved differently when the temperature is allowed to drop to 22° C under helium overpressure and under oxygen overpressure. Essentially all of the iron is redissolved as ferrous ion under helium overpressure. Not all of the iron is redissolved under oxygen overpressure; the quantity of ferrous ion redissolved is less than that dissolved originally by 4.40 gpl and the ferric ion in solution is more than that originally present by 1.10 gpl.
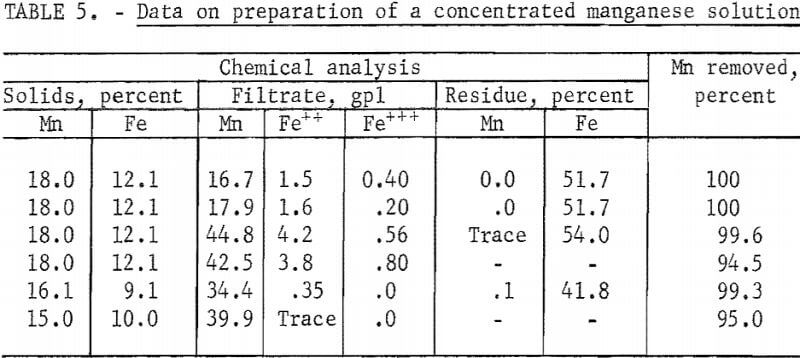
Filtration of the pregnant liquor from optimum autoclave conditions (oxygen overpressure and 250° C) resulted in a residue containing iron and manganese in typical amounts indicated in columns 1 and 2 of table 5. The residue may contain many solid phases. Under optimum autoclave conditions, however, a large part of the iron precipitate is ferric oxide and essentially all of the manganese precipitate is ammonium manganese sulfate. The residue can be washed with a limited quantity of water to give a filtrate containing about 30 to 45 gpl manganese and a ferric oxide residue. Typical results of washing and recoveries obtained are shown in table 5.
Kinetic Analysis
The experimental data were not adequate for a complete kinetic analysis. The sulfuric acid iron solution was a very complex system. Many chemical reactions were involved and even though under optimum conditions final analysis revealed most of the iron precipitated as ferric oxide, considerable quantities of iron precipitate probably existed in several other solid phases at earlier times in the experiment and at lower temperatures. Because the data were not adequate, no further attempt is made here to analyze the iron precipitation. Since the manganese precipitation was apparently through a simpler mechanism, however, and appeared to be independent of overpressure, some additional analysis of this precipitation in the autoclave experiment is presented.
Kinetic Analysis of Ammonium-Manganese Sulfate Precipitation
The experimental data were not of the usual variety for kinetic studies. Time-concentration curves at a set temperature are the usual type experimental data for kinetic studies. In these experiments both temperature and concentration varied with time, and the experimental data were simultaneous time-concentration curves and time-temperature curves. These data were converted into the usual type data for testing kinetic models by measuring the slopes of experimental data, and making a series of cross plots. This procedure reduced the accuracy of the data, but slopes were measured as carefully as possible.
Concentration is a property that, for the particular system in these experiments, is a function only of time and temperature. Therefore,

where
C = concentration,
t = time, and
T = temperature.
Or, rearranging,

The quantity (∂c/∂t)T as a function of concentration may be used to verify chemical rate equations.
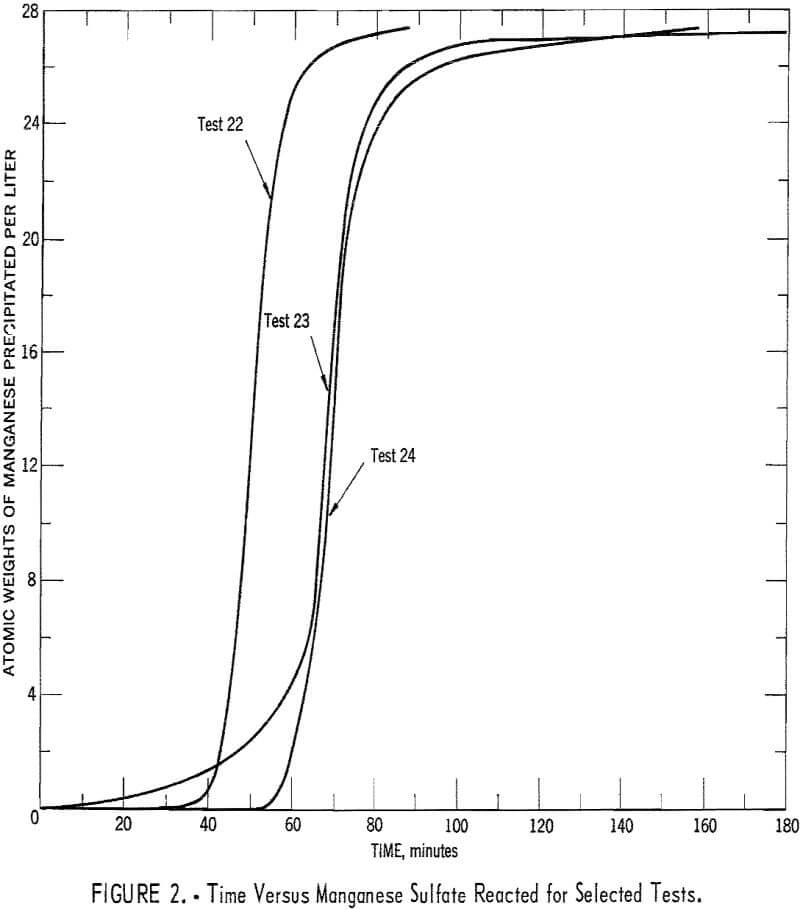
Experimental data were used to calculate values of (∂c/∂t)T, at various concentrations from equation 2. Referring to equation 2: Values of dc/dt were obtained by measuring slopes of the time-concentration curves, values of dT/dt were obtained by measuring slopes of the time-temperature curves, and values of (∂c/∂t)T were estimated by measuring slopes of concentration-temperature curves that were obtained from cross plots of several time-concentration and time-temperature curves obtained from experiments with different heating rates. Time-concentration curves for three selected experiments are shown in figure 2. Time-temperature curves were of generally similar shape. This serves to illustrate that there were some variations in concentration and temperature increases among experiments, which made the cross plots mentioned above possible. The estimated value of (∂c/∂t)T along with concentration measurements were
used to calculate values of rate constants and test rate equations. Agreement of rate constants, among experiments, at each temperature was used as a test of rate equations and to estimate the values of the rate constants.
In testing rate equations for manganese precipitation, several assumptions were made. It was assumed that manganese precipitated only as ammonium-manganese sulfate; that the rate of precipitation was a function only of manganese sulfate concentration in solution, ammonium sulfate concentration in solution, and temperature; and that the rate equation was of the form
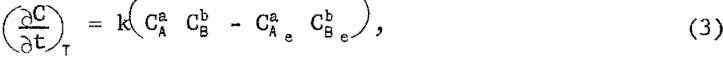
where
(∂c/∂t)T = rate of precipitation of ammonium-manganese sulfate at a given temperature,
k = specific reaction rate constant for the precipitation.
CA = concentration of manganese sulfate in solution,
Ca = concentration of ammonium sulfate in solution,
a = a constant,
b = a constant,
CAe = equilibrium concentration of manganese sulfate in solution, and
CBe = equilibrium concentration of ammonium sulfate in solution.
The most successful rate equation was found to be

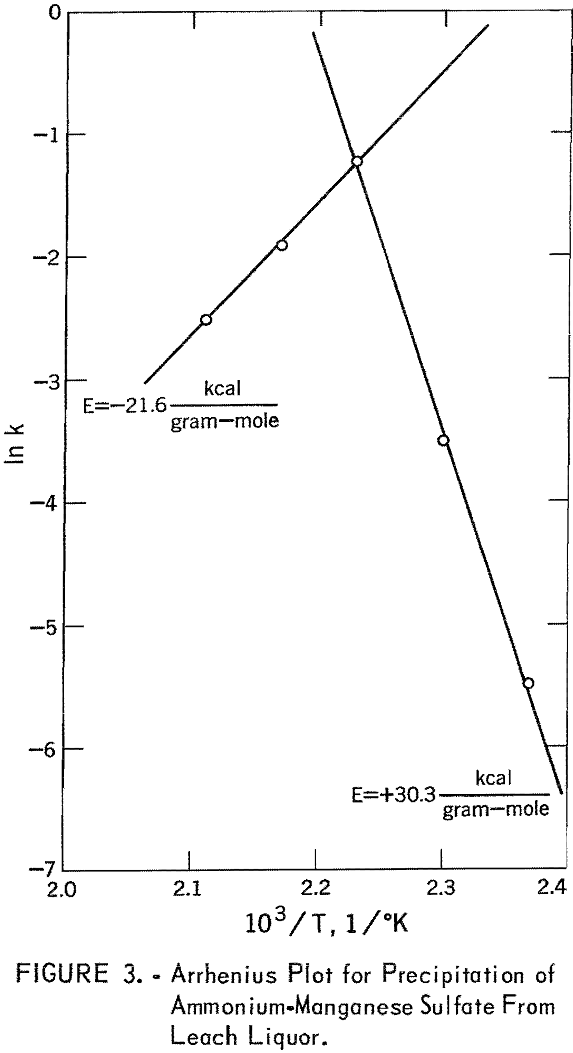
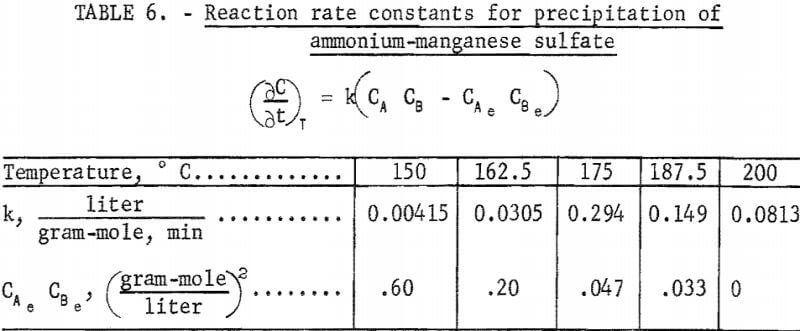
The best values of the product CAe CBe for fitting this equation and the k values determined for this rate equation at five temperatures are given in table 6. These results show that the rate of the precipitation of ammonium-manganese sulfate increased with temperature to approximately 175° C and then decreased with temperature. These rate constants are plotted by the Arrhenius equation in figure 3. The activation energy below approximately 175° C was calculated in this way to be 30.3 kcal/gram-mole and above 175° C was calculated to be -21.6 kcal/gram-mole. It has been reported by Fuller that the solubility of manganese sulfate exhibits inverse dependence on temperature up to 170° C. The temperature of 175° C indicated in figure 3 may represent the temperature above which the solubility of manganese sulfate exhibits normal dependence on temperature.
Although considerable care was taken in measuring slopes for these calculations, the possible error from these operations was relatively large. It is believed, however, that the values of the rate constants are of the correct order of magnitude and that the form of equation 4 is appropriately simple.