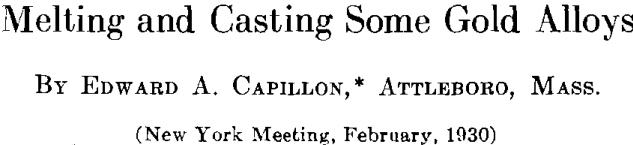The problem of scrap is probably of greater importance in the production of gold, silver and other precious metal alloys than is the case for base metals and alloys. Remelting of gold and silver scrap in the shop is always a costly process because it involves losses by oxidation of the base metals in the alloy with a consequent increase in the percentage of precious metal. This is most marked in the case of alloys containing the low-boiling and easily oxidized metals zinc and cadmium. Since it is not generally feasible to assay every shop remelt it follows that the possibilities of lost values are large. So-called rolled gold and silver plate is made by soldering or welding the precious metal alloys onto base metal. Such plate when defective must be sent to the refinery to recover the gold and silver values, thereby involving additional expense in the form of refining charges. It is obvious therefore that gold and silver alloy scrap is an important item in manufacturing cost and must be reduced to a minimum. The amount of scrap resulting from poor metal will depend on a number of factors in the manufacturing operations, not least among which is the melting and casting practice. The notes which follow are a summary of experiments performed at various times to determine what variables in melting and casting are influential in the production of sound alloys.
Metals used in Alloying
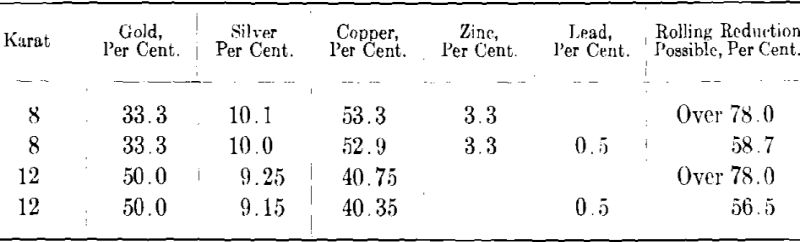
Pure gold, that is, gold of 24-kt. value is very much too soft for jewelry purposes and it is therefore always alloyed with other metals to increase its hardness and resistance to wear. For the same reason pure silver is seldom used alone but is alloyed with copper, the standard alloy being sterling silver which contains 92.5 per cent, silver and 7.5 per cent, copper. The metals commonly used for alloying gold are silver, copper, nickel and zinc. In addition to hardening the gold and producing alloys of different karat values the use of these metals makes possible the production of gold alloys of various colors such as yellow, red, green and white. The gold-silver-copper alloys vary in color from light greenish-white through green, yellow and red depending on the relative amounts of the three metals. Green or yellowish-green colored golds are also obtained by combining relatively large amounts of zinc (maximum about 18 per cent.) with gold, copper and small amounts of silver. In recent years the so-called white golds have been very popular for jewelry chiefly because of the resemblance to the color of platinum. The bluish-white color of these white golds depends on the presence in the alloy of both nickel and zinc, the former in 14 kt. (58.3 per cent, gold) white golds varying between 12 and 17 per cent, and the latter between 4 and 8 per cent. Some of the white gold alloys on the market contain palladium which increases somewhat the workability and gives a white color more nearly like that of platinum. The high cost of palladium, however, prohibits its extensive use in white gold alloys. Silver, copper, nickel and zinc are the only metals used in large quantities for the production of gold alloys in the jewelry industry. Cadmium is occasionally added to some yellow golds but there does not seem to be any logical basis for its use. Besides, it has the disadvantage of being vaporized at a comparatively low temperature so that much of it is apt to be lost on melting. Some time ago a new gold alloy containing aluminum was introduced on the market. Because of its aluminum content it was supposed to be considerably lighter than the ordinary alloyed golds, thereby enabling jewelers to produce a greater number of articles out of a given weight of alloy than had been previously possible. This alloy however was never a commercial success because of the casting difficulties occasioned by the presence of aluminum. Moreover, the aluminum oxide often present in minute inclusions made the polishing of the alloy a difficult and unsatisfactory process.
As stated in a later section of this paper small amounts of certain impurities may have a very harmful effect on the mechanical properties of gold and its alloys. Fine gold and silver are generally of a high degree of purity, gold from the U. S. Mint assaying 99.98 per cent, or better while ordinary commercial silver will assay 99.9 per cent. To avoid contamination it is important to use only the purest base metals for alloying, such as electrolytic copper, Mond process nickel pellets and Bunker Hill grade 99.99 per cent. zinc. Time and labor can be saved and the chances of error lessened by having on hand a number of different base-metal alloys which can be melted with fine gold in various proportions to produce the color and karat desired. Thus, for white gold an alloy containing copper, nickel and zinc is used while for yellow gold the base alloy is copper and silver, or, copper, silver and zinc. When high temperatures are necessary to melt the base metal and zinc in considerable amount is present, as in the case of the copper-nickel-zinc white golds, the melting should be carried on under a heavy layer of boric acid crystals so as to prevent excessive loss of zinc. For convenience in weighing, the base alloy is granulated by pouring slowly into a tank full of water.
Gas and Electric Melting
Although in most cases gas furnace melting is satisfactory the electric furnace has a distinct advantage from the standpoint of metal losses. Since there is no strong upward draft of gases and the temperature can be easily controlled it is possible to cut down loss of zinc and other volatile metals. This consideration is of prime importance where large amounts of scrap stock are returned to the furnace for remelting as occurs in the manufacture of sheet metal stampings in the jewelry industry.
The author made comparative tests of a 12-kt. white gold alloy melted in an ordinary crucible gas furnace and in an electric furnace of the nonmetallic resistor type. No temperature control or pyrometer was used for the gas furnace melt, the correct pouring heat depending on the judgment of the melter. The melts in the electric furnace were made and cast at 2300° F., this temperature being maintained automatically by an electric controller. The time required for melting is about the same for both methods. Twenty ounce melts of the alloy were made in an uncovered graphite crucible and cast to ingots 2 by 3 by 0.5 in. These ingots were then rolled to strip 0.030 in. thick, cut to small pieces about 0.5 in. wide and then remelted and recast. This procedure was repeated four times, samples being taken of the original melt and of the four remelts. The samples assayed as follows:
The electric furnace also makes it possible to melt alloys in a controlled atmosphere. As shown in another part of this paper, sterling silver and gold alloys of high silver content are very susceptible to oxygen. For the purpose of melting in vacuum or in various gases the induction furnace of the high frequency type offers interesting possibilities. The high frequency furnace has the advantage over resistance type furnaces that very high temperatures can be obtained making it possible to use metals which because of high fusing temperatures cannot be melted by any other means.
Defects in Ingots
Ingot defects which may cause trouble in the mechanical treatment of the alloy or lead to defective finished stock can be approximately classified under the following headings; (1) surface defects; (2) weak or porous metal; (3) gas inclusions; (4) metallic impurities, oxides, sulfides, etc.
A superficial inspection of the ingot will reveal surface defects but it is often impossible to determine from the exterior appearance of the ingot whether it is defective due to any of the other three causes listed above. Badly “gassed” ingots will either show no pipe at all or a convex surface at the top, but it is quite possible for an ingot to develop a fairly prominent “pipe” and yet contain enough free gas to show blisters in the final rolled and annealed stock.
Surface Defects
These are apt to result from films of oxide carried over with the stream of metal on pouring. It is therefore necessary that the surface of the metal in the crucible be entirely free of oxides before the metal is poured. This can be readily accomplished by fluxing with boric acid crystals. The acid combines with the oxides to produce an easily fusible slag which can be removed by means of a carbon rod. Copper, zinc and nickel oxides are readily fluxed by boric acid. On the other hand, the oxides of such metals as iron, aluminum, manganese, magnesium and chromium form so readily that it is extremely difficult to obtain satisfactory ingots of gold alloys containing appreciable quantities of these metals. These oxides form as a refractory skin on the melt as soon as the flux is skimmed off. Ingots cast from such melts show a wrinkled surface and deep inclusions where the metal has been unable to break through the tenacious oxide films. Possibly some improved gold alloys might result from the use of such metals as iron and chromium if this problem of oxides could be overcome. With recourse to vacuum melting and casting this would be feasible.
Ingot Proportions
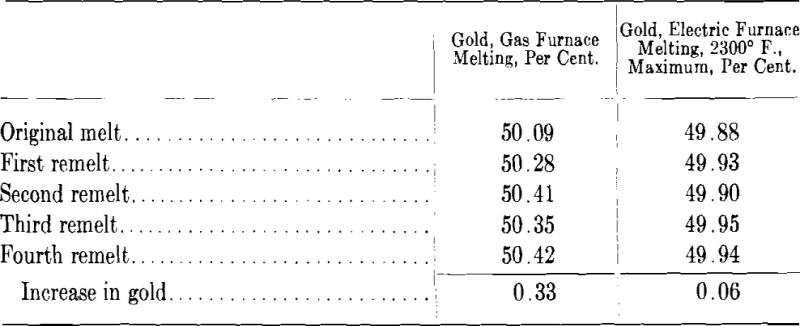
As in any metallic casting the last portion of a gold ingot to solidify contains the bulk of the metallic and gaseous impurities. The majority of the ordinary gold alloys do not form very deep pipes in casting but it has been observed that in a poorly proportioned ingot the top contains “porous” metal which generally pulls apart in the early stages of rolling. By a change in the proportions of the ingot mold, scrap from this source can often be reduced to a minimum. In Figs. 1A and B is sketched the type of broad and short ingot which is conducive to much scrap metal. Fig. 1A illustrates the condition where the metal has been poured at one end of the mold and as indicated by the dotted area the poor metal extends practically from the top to the bottom of the ingot. In Fig. 1B the conditions have been somewhat improved by pouring the metal back and forth from one end of the mold to the other but the amount of scrap will still be about 30 per cent. The proportions shown in Fig. 1C are much more satisfactory and shearing off the top of the ingot does not involve the scrapping of large amounts of metal.
In a well proportioned ingot the length should be about four times the width. Excessively long ingots, on the other hand, are to be avoided as the metal on pouring is apt to splash against the sides of the mold during the early stage of the pour. An ingot suitable for most gold alloys is 3 in. wide by 12 in. long by 0.6 in. thick and will weigh in 14-kt. white gold about 150 oz. Should it be desired to obtain sheets of large
width as is the case in preparing blanks for long tubes it is best to cross-roll to the desired width rather than cast a wide ingot.
Porous Ingots
It is well known that the solubility of most gases is very much less in the solid than in the liquid metal. Familiar examples are the systems silver-oxygen and copper-sulfur dioxide. When metals solidify in chill molds the bulk of the gas absorbed in melting is liberated but is unable to escape because of the rapidity of freezing of the metal. The result is an ingot which is porous at the center. Such porosity is not generally suspected from the exterior appearance of the casting but if the metal is rolled to thin sheet and annealed the surface will show blisters, their extent depending on the amount of gas retained in the metal when cast.
Blisters may also be caused by inclusions of oxides formed during pouring and mechanically entrapped. These can be largely avoided by clearing the melt with boric acid, as stated in a previous paragraph.
Molten silver absorbs oxygen very readily, one volume of silver at 1783° F. holding in solution 22.4 volumes of oxygen at atmospheric pressure. Solid silver at 1472° F. will only retain 0.354 volume of oxygen, the excess oxygen, about 22 volumes, being liberated on solidification of the metal. The liberation of this large amount of gas causes the well-known “spitting” of fine silver.
Because of this solubility of oxygen in silver, gold alloys containing large amounts of the metal are very apt to absorb oxygen during melting and so form blistered stock. This is not very extensive in alloys containing large amounts of gold as in 18-kt. (75 per cent.) gold, but alloys of gold, silver and copper of 12-kt. (50 per cent.) gold or less do produce blistered sheet in many cases despite the utmost effort to keep a reducing atmosphere in the furnace and to clear the surface of the metal with flux. Carter, in his work on gold-silver-copper alloys, shows that on cold-rolling there is an increase in specific gravity considerably greater than that due to the natural compression of the alloy in the rolling process. For instance, the specific gravity of a 14-kt. alloy containing 58.33 per cent, gold, 20.83 per cent, silver and 20.84 per cent, copper increased from 7.00 Troy oz. per cu. in. for the as-cast alloy to 7.11 Troy oz. per cu. in. for the hard-rolled alloy. The increase in specific gravity is attributed to the presence of gas which compresses more than the alloy itself on rolling.
Melting and casting in vacuum or in inert gases is scientifically the logical method of eliminating gas porosity in cast metals but to date such processes are practical only in the laboratory. The most convenient way of obtaining sound metal in the shop is by the use of deoxidizers added to the melt just prior to pouring. All deoxidizers do not behave alike however and they must be used with much caution as some deoxidizers will often remedy porosity at the expense of hot-short or brittle metal. Deoxidizing agents should have three characteristics, viz., (1) their oxides should form easily fusible slags with flux; (2) they should not form low-melting or brittle compounds with the metal to be deoxidized ; (3) their oxides should be insoluble in the liquid metal. Statement 2 is of much importance in considering these materials as small traces left in the metal may often seriously injure its physical properties.
In connection with gold and silver alloys the author has made a number of tests of deoxidizers. The results obtained are summarized below.
Deoxidizers
Zinc.—As pointed out by Wise the addition of small amounts of zinc does improve the surface of gold-silver-copper alloy ingots. Zinc, while not a very energetic deoxidizer, has the advantage that in small quantities it does not form brittle compounds with either gold, silver or copper. To check the effect of zinc two melts were made and cast to ingots about 2 by 2 by 0.430 in. Their compositions were:
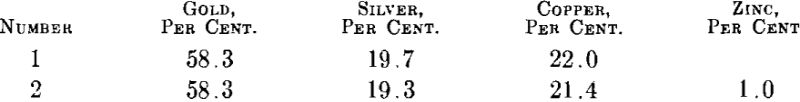
The surfaces of these ingots were machined down about 0.015 in. on each side and the ingots then rolled to 0.030 in. with reductions of 50 per cent, between annealing at 1300° F. It was found that the annealed strip from ingot 1 was badly blistered while the strip from ingot 2 was practically free of blisters.
Aluminum, Magnesium, Manganese.—These deoxidizing agents have been grouped together because they have one property in common which largely precludes their use in precious-metal alloys. This disadvantage is the fact that the oxides of the three metals form tenacious films on the melt which are not easily fluxed off. The oxides carried over in the stream of metal form deep inclusions in the casting which cannot be removed except by extensive machining of the surfaces.
Silicon.—Tests on an alloy similar to the 14-kt. alloy mentioned under zinc, showed that silicon is quite unsuitable for use as a deoxidizer of gold alloys. While it is true that silicon is a powerful deoxidizer and that it produces melts which are apparently extremely fluid and clear, its use even in small quantities, 0.1 per cent., produces appreciable hot shortness in the alloy, as the following data for an alloy of gold, 58.3 per cent., silver, 19.7 per cent., copper, 22 per cent., show:
![]()
The reason for this injurious effect is to be attributed to the formation of a very low-melting gold-silicon eutectic. According to di Capua silicon is practically insoluble in solid gold, the two elements forming a simple eutectiferous series. The eutectic melts at the remarkably low temperature of 698° F. It follows that the presence of as little as 0.1 per cent, silicon will give rise to this eutectic and so produce hot shortness in the alloy.
Phosphorus.—This nonmetal in the form of copper-phosphorus alloy containing 15 per cent, phosphorus has been found to be a very efficient deoxidizer for sterling silver. It must however be used in as small quantities as possible as any considerable amounts remaining in the alloy will develop hot shortness.
Use of Deoxidizers in Sterling Silver
As has been stated above molten silver has the property of absorbing very large amounts of oxygen, but when the metal solidifies the bulk of this gas is rejected and any silver still in the liquid state is thrown out by the gas with almost explosive force, producing the familiar phenomenon of “spitting.” This occurs only in the case of fine silver.
In sterling silver the copper, acting in the capacity of a weak deoxidizer, combines with the oxygen to form the stable oxide, Cu2O. However, not all of the oxygen absorbed by the silver is converted to Cu2O so that, according to Streicher we are dealing with a four-phase system, namely silver-dissolved oxygen-copper-cuprous oxide. When the silver-copper alloy solidifies the excess oxygen in the silver separates out to form minute pores in the center of the ingot. These pores produce blisters in annealed strip metal.
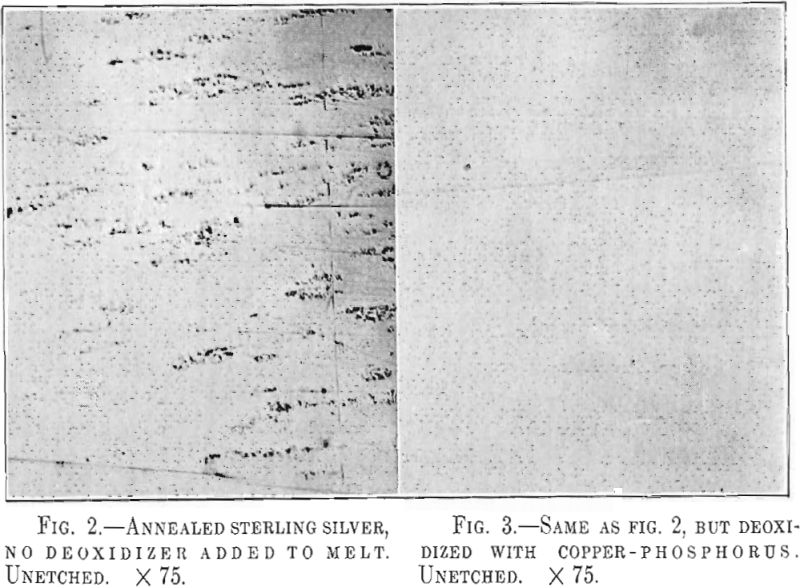
Untreated sterling silver when rolled thin, 0.015 in. or less, shows in striking manner the effect of these pores. Fig. 2 illustrates the appearance of such a strip of silver rolled from an ingot which had been melted in an ordinary gas crucible furnace in contact with air. The roughness is duo to the presence of minute blisters which have broken open in the polishing and also to hard oxide inclusions. The use of copper-phosphorus has been found very efficient in correcting this trouble. Fig. 3 shows a flawless silver strip made from silver deoxidized with phosphorus.
Cuprous oxide is readily soluble in liquid copper but the solubility is only 0.009 per cent, in the solid metal, and hence the bulk of the Cu2O separates out when the copper solidifies. A similar action occurs when sterling silver containing dissolved Cu2O is cast. The presence of this free oxide in the solid alloy produces what Streicher calls “blue” silver. Jewelers have trouble with so-called “fire” in sterling and while in most cases this is due to superficial oxidation during annealing the author has found cases in which the “fire” in the stock was the result of oxides absorbed in the melting process. Ordinary surface oxidation can be removed by pickling the alloy in acid but oxides originally in the ingot will not disappear no matter how much the alloy is pickled or polished. Sterling silver which has been treated with phosphorus is free of this latter type of “fire” and has a pure white color instead of the bluish- white color observed in oxide-containing silver.
In connection with this report the author made numerous ductility tests and chose for this work the Olsen ductility testing machine. This
ductility test has the advantage that specially shaped specimens are not necessary and that the quantity of metal required for accurate results is small, which consideration is of importance in experimenting with gold alloys.
It is interesting to note that the ductility of sterling silver is appreciably increased by the deoxidation treatment. Similar results have been obtained for copper by Webster, Christie and Pratt. In some comprehensive tests they show that the ductility of phosphorized copper, as measured by contraction of area, is greater than that of the metal in the tough pitch condition.
The results obtained by the author for sterling silver (92.5 silver- 7.5 copper) are as follows:

Use of Phosphorus in Gold Alloys
As in sterling silver, alloys of gold, silver and copper will contain pores and oxide particles if not deoxidized. Fig. 4 is a photomicrograph of an alloy containing gold, 50 per cent., silver, 10 per cent., and copper, 40 per cent.; a typical red gold of 12-kt. quality. This sample was melted in the gas furnace under oxidizing conditions and shows numerous oxide inclusions. At high power these inclusions appear as bluish-colored
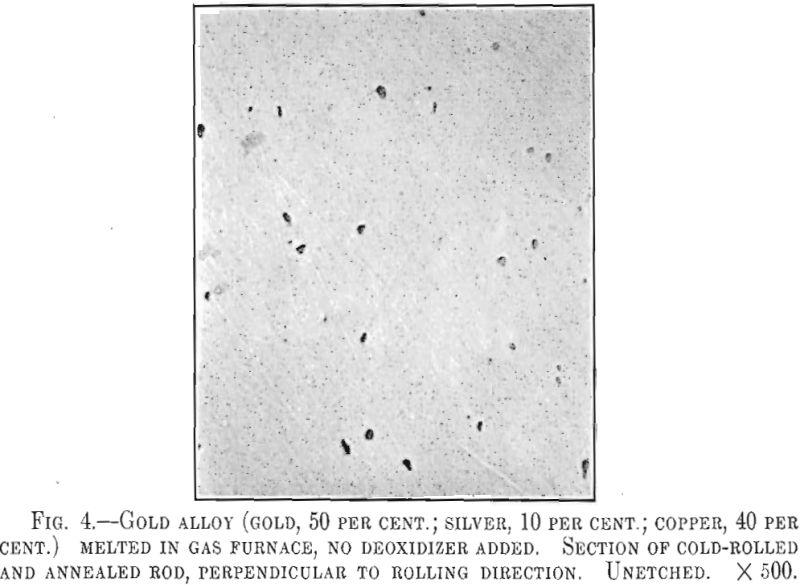
rounded particles, evidently Cu2O. The presence of this oxide is undesirable as the polished alloy will show a bluish sheen. The action of phosphorus is the same as in sterling silver, the deoxidized alloy being more suitable for highly polished work.
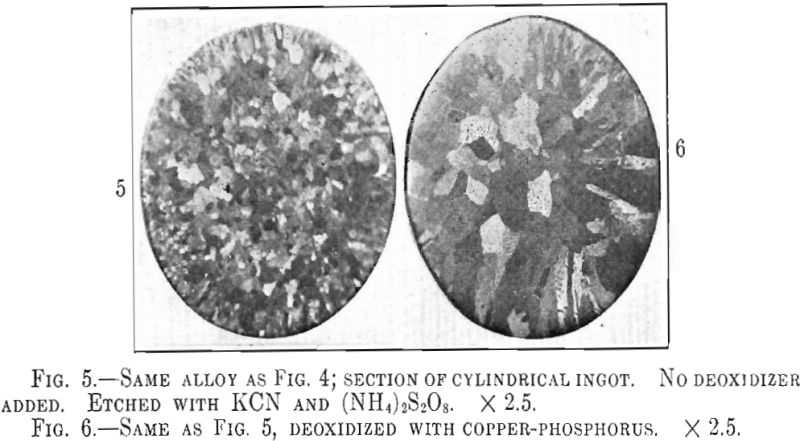
Webster, Christie and Pratt comment on the difference in grain size of cast tough pitch copper as compared to cast phosphorized copper. Precisely the same effect was found in the 12-kt. red gold alloy castings. In the untreated ingot the grain growth is hindered by the precipitation of Cu2O particles resulting in more or less equiaxed crystals as shown in Fig. 5, while in the phosphorized alloy the crystals grow unrestricted producing the large dendrites of Fig. 6. Both ingots were cast at the same temperature so as to avoid grain size differences due to temperature effects. The large crystals of the phosphorized alloy do not appear to cause any difficulty in mechanical working but care must be taken in annealing as the crystals of a deoxidized alloy seem to grow very rapidly above a certain annealing temperature. Large crystals in annealed metal are objectionable as they are the cause of the rough “orange peel” effect noticeable in overheated metal.
Calcium Boride in Gold Alloys
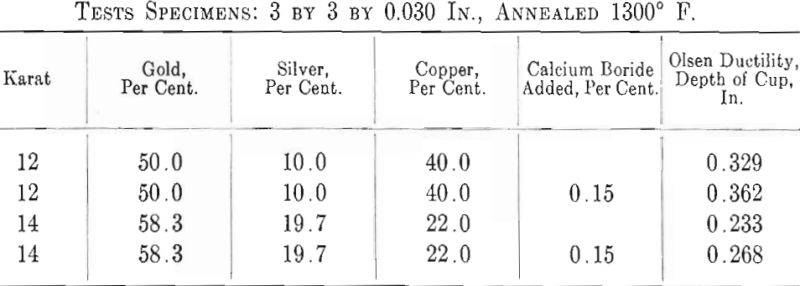
Mention has been made by Wise of the possible use of this material as a deoxidizer in gold alloys. The author conducted several tests with such a deoxidizer containing 85 to 90 per cent, calcium boride, CaB6, and a few per cent, of carbon, iron and silicon. It is claimed that this deoxidizer is insoluble in copper and copper alloys and therefore any excess can be fluxed off and none will be precipitated in the alloy during solidification. This, of course, is a distinct advantage as too many deoxidizers precipitate harmfully in the solid alloy. Experiments made by the author have proved that calcium boride is very efficient and produces clear melts and good sound castings. Ductility tests on two gold-silver- copper alloys gave substantial increases in favor of the treated alloys, as indicated by the results below.
Casting Temperature
To determine what effect casting temperature might have on the rolling properties and ductility of gold alloys, tests were conducted on two representative alloys, one a 14-kt. white gold, the other a 10-kt.
green gold of the high-zinc, low-silver type. The metals used for alloying were fine U. S. Mint gold, fine silver, electrolytic copper, Bunker Hill grade zinc and Mond nickel. Four melts were made of each alloy, heated to a temperature high enough to insure rapid and thorough alloy-
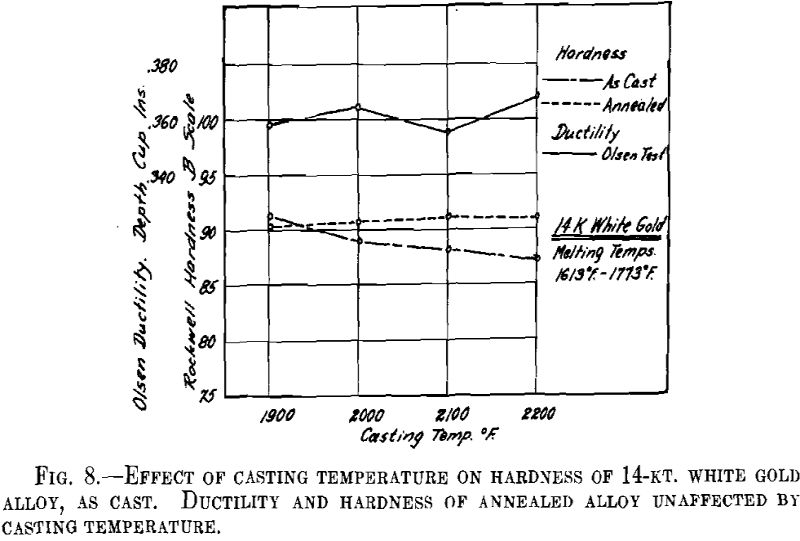
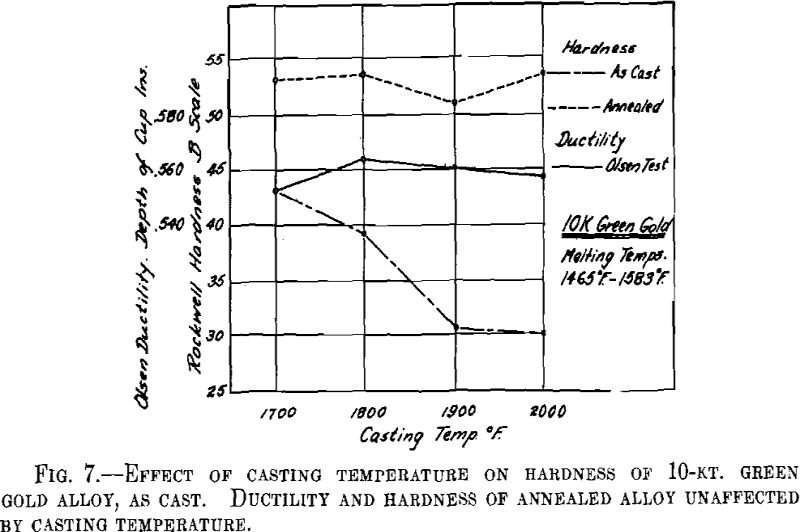
ing, then cooled to the desired temperature and cast to ingots 2 by 2 by 0.400 in. A liberal amount of boric acid was placed in the graphite cruciblc to reduce zinc losses. At the high temperatures zinc burned rather vigorously on removing the flux and pouring the melt. Good ingots were obtained for all casting temperatures except that at the minimum temperature for both white and green gold ingots the surfaces showed “ripples” indicative of rapid solidification. The green gold ingots were cold-rolled to 0.040 in. in three stages with intermediate annealings at 1200° F., while the white gold ingots were similarly rolled to 0.030 in. with annealings at 1300° F. The values obtained for hardness of the cast alloy and for hardness and ductility of the annealed sheet are presented below and plotted in Figs. 7 and 8.
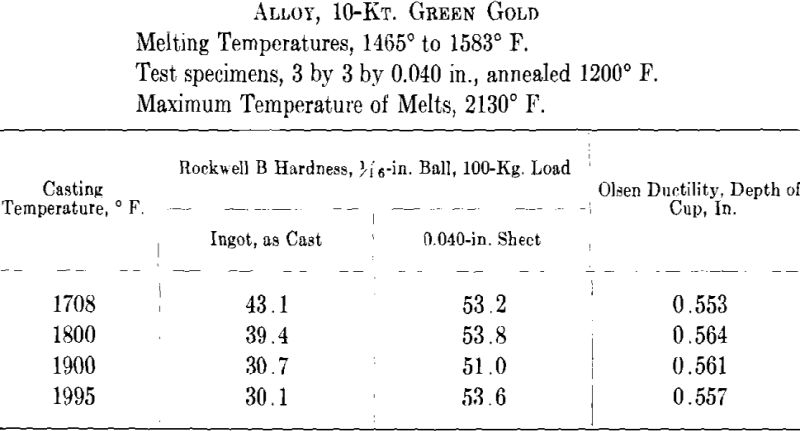
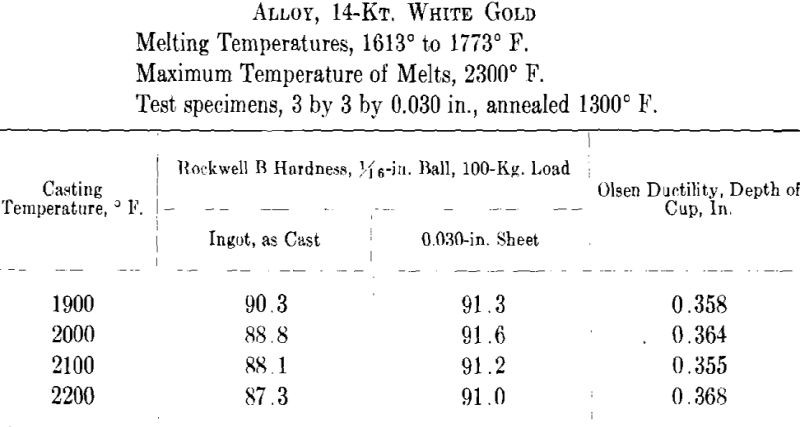
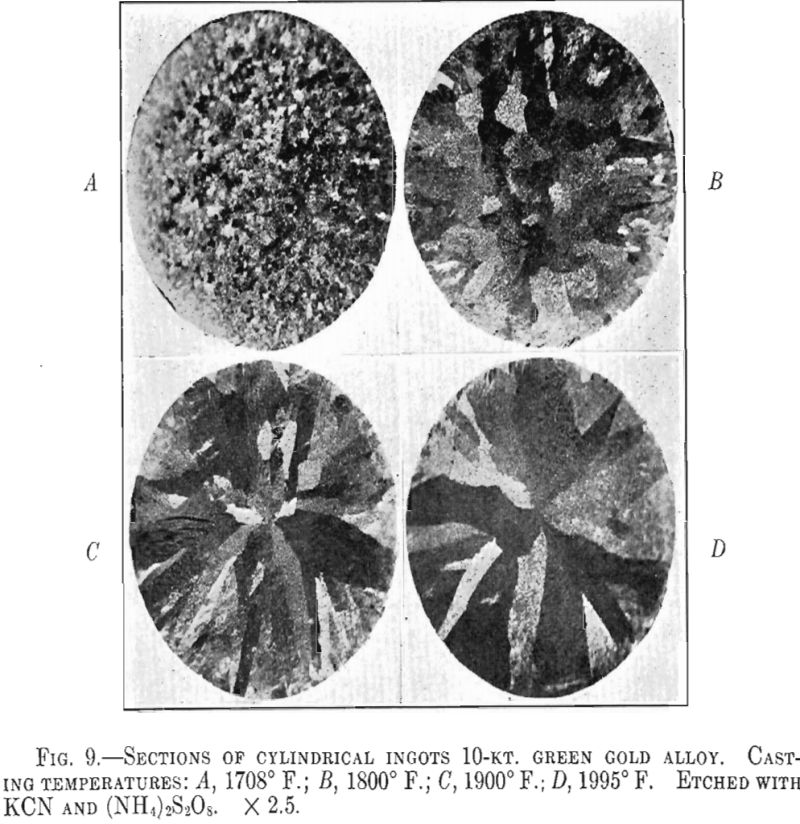
It appears from the above figures that the hardness of the alloy, as cast, decreases with increasing casting temperature. This is accompanied by a corresponding increase in grain size as is shown by the photomicrographs of cast cylindrical sections of the 10-kt. alloy, Fig. 9, A to D. The large grain size, except for developing coarsely rough edges on the initial rolling, did not produce any marked difficulties in cold-working. After the first annealing, grain size differences seem practically obliterated. Hardness readings taken of the annealed ductility test samples do not show any marked variations for either alloy. Similarly, the Olsen ductility values do not seem to show any definite effect attributable to casting temperature, the differences noted being within experimental error.
It appears therefore that for these particular alloys and probably for all other common gold alloys casting temperature has practically no effect on the rolling properties and the ductility of rolled and annealed sheet.
Of course, if an alloy is cast at too low a temperature the metal will solidify before it has a chance to fill out the mold, thereby producing a
“cold-shut.” The alloys should be poured at temperatures from 150° to 25.0° F. above the liquidus temperature, depending on the size of the melt, casting speed, temperature of the mold, size and proportions of the ingot, etc. On the other hand, excessively high temperatures are undesirable inasmuch as the metal losses due to vaporization of zinc and cadmium will be correspondingly large.
Effect of Impurities
Nowack investigated the effect of small quantities of iron, tin, bismuth, antimony, aluminum, tellurium and lead on the mechanical properties of pure gold and 90 per cent, gold-10 per cent, copper alloy. The results he obtained may be summarized briefly as follows:
Iron.—This metal forms a solid solution with gold up to about 15 per cent, iron by weight. Alloys with as much as 10 per cent, iron could be worked without fracturing. An alloy with 1 per cent, iron was as workable as pure gold.
Tin.—A solid solution occurs to about 5 per cent, tin and with larger amounts of tin compounds are formed, such as AuSn, AuSn2 and AuSn4. Nowack found that an alloy with 1 per cent. Sn showed a heterogeneous structure even after annealing. The working qualities of this alloy were fair, while an alloy with 0.1 per cent, tin rolled as easily as fine gold. Ten per cent, tin produced an alloy which was very brittle.
Bismuth.—The metal forms a solid solution to about 4 per cent, while alloys with more bismuth consist of solid solution and a eutectic of this constituent and free bismuth.14 An alloy with 0.1 per cent, bismuth was found to break easily on rolling. One with 0.01 per cent, bismuth could be rolled somewhat further but was still appreciably more brittle than fine gold. Similar results were obtained for the 90 per cent, gold-10 per cent, copper alloy containing bismuth.
Antimony.—A solid solution forms up to about 0.5 per cent, and then a compound AuSb2. An alloy with 0.1 per cent, antimony was found to roll fairly well but with 1.0 per cent, the presence of the compound made the alloy quite unworkable.
Aluminum.—A solid solution appears to exist from 0 to 2.5 per cent, aluminum and then numerous compounds with larger amounts of aluminum. Nowack found that an alloy with 3 per cent, aluminum was practically useless. One with 0.1 per cent, worked readily while with 1 per cent, aluminum fairly good properties resulted
Tellurium.—Nowack found that tellurium affected greatly the working qualities of fine gold. Tellurium is insoluble in gold in the solid state and forms the brittle compound AuTe2. An alloy containing as little as 0.1 per cent, tellurium is unworkable. Only an alloy with 0.01 per cent, or less can be rolled.
Lead.—Like tellurium, lead has an extremely bad effect on gold. The solubility of lead in gold is about 2 per cent.18 Compounds Au2Pb and AuPb2 are formed with greater amounts of lead. An alloy with as little as 0.06 per cent, lead is unworkable. With a lead content of 0.005 per cent, the alloy can be rolled like fine gold. Nowack tried the effect of annealing on the 0.06 per cent. alloy and found that even after a long period at 650° C. traces of AuPb were still visible and were the cause of the alloy cracking when only slightly strained. Lead has a similar detrimental effect on a 90 per cent, gold-10 per cent, copper alloy, 0.06 per cent, rendering the alloy unworkable.
In an attempt to produce free-cutting low-karat gold alloys for screw machine work the author experimented with alloys containing from 0.5 to 1.5 per cent. lead. While the effect of the lead on the rolling and drawing qualities was not found to be as detrimental as Nowack determined for fine gold, the ductility was nevertheless sufficiently impaired to discourage the use of lead in these alloys. The results obtained with 0.5 per cent. lead are indicated in the following table:
Sulfur in White Gold
In connection with a study of the effect of impurities in gold alloys the author considered it of interest to test the influence of sulfur on the nickel-zinc white gold alloys. These alloys which have been so popular for jewelry in recent years contain from 12 to 18 per cent, nickel depending on the karat value of the alloy. Compared to the colored golds these alloys are quite hard, averaging B 84 to B 93 Rockwell hardness in the “as cast” condition. Because of the presence of large amounts of zinc, 4 to 10 per cent., the white golds are not as subject to gas porosity as are alloys containing no zinc. They do occasionally give trouble in cold- rolling by cracking and splitting. It has been shown by Merica and Waltenberg that of all the elements naturally occurring in cast nickel only sulfur is responsible for the nonmalleability of untreated nickel. These authors state that 0.01 per cent, sulfur in remelted electrolytic nickel is sufficient to render it nonmailable.
As a test of the effect of sulfur in commercial white gold the author made four 14-kt. white golds containing 0.01, 0.02, 0.05 and 0.1 per cent, sulfur. In addition, an alloy free of sulfur was cast as a check. The alloys were made of electrolytic copper, redistilled zinc, nickel pellets and fine gold. The sulfur was added to the melts in the form of a nickel- sulfur alloy containing 3.28 per cent, sulfur which was obtained from the International Nickel Co. The ingots were cast about 2 by 2 by 0.430 in. thick and were numbered 1 to 5 from 0 to 0.1 per cent, sulfur, respectively.
Ingot 5 (0.1 per cent, sulfur) split and cracked so badly on the first few passes in the rolls that it had to be scrapped. Ingot 4 (0.05 per cent, sulfur) split from end to end in the plane of rolling after 28 per cent, reduction of area but it was possible to further cold-work after annealing so as to obtain ductility test sheets. Ingot three (0.02 per cent, sulfur) showed some tendency to split but the effect was not as pronounced as in No. 4. Only the ingot with 0.01 per cent, sulfur and the one with no sulfur could be rolled without any difficulty. The samples rolled down for ductility tests were 3 in. square by 0.030 in. thick and were annealed at 1300° F. and air-cooled. These were tested in the Olsen ductility testing machine with the following results:
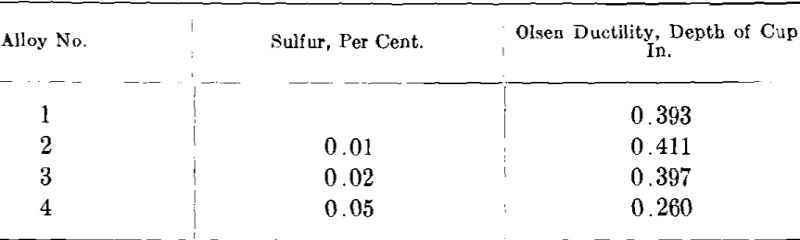
From these figures it would appear that as much as 0.02 per cent, sulfur has little influence on the ductility of annealed samples but that in quantity of 0.05 per cent, the ductility is very materially affected.
Examination of the cast sample of alloy 4 showed the sulfur to be distributed as sulfide in a discontinuous network. At high magnification the sulfide is seen to consist of small irregular particles arranged in a structure resembling eutectic. The rolled and annealed alloy shows the sulfide extended in the direction of rolling and apparently present both in the grains and at their boundaries. When annealed at high temperature, such as 1600° F., extensive coalescence of the sulfide particles occurs to form larger rounded particles. The fact that the sulfide does not appear in thin intercrystalline films as in nickel probably accounts in part for the fact that considerably larger amounts of sulfur can be present in white gold than in nickel without seriously affecting the ductility. The effect of sulfur is however sufficiently detrimental to warrant the use of a sulfur-free nickel in the manufacture of white gold alloys. For this purpose Mond nickel of a purity of 99.5 per cent, nickel and higher, containing only small traces of sulfur is the best form of the metal to use.
Summary
- Defects in gold and silver alloy ingots and their causes have been described.
- It has been shown that a correctly proportioned mold is necessary in order to obtain ingots which shall give as little scrap metal as possible.
- Gases in gold and silver alloys produce blisters when the alloy is rolled down and annealed. These gases can be rendered harmless by the use of deoxidizers. The characteristics of various deoxidizers are discussed.
- In so far as ductility measured by the Olsen test is concerned casting temperature has apparently little effect on the ductility of rolled and annealed sheet.
- A summary has been given of the effects of various impurities on fine gold. The harmful effect of lead in low-karat, red golds and of sulfur in 14-kt. white gold is shown.