I was manager of a mining enterprise where the ores were composed principally of iron pyrites (much decomposed), in a quartz matrix, with native gold in very irregular grains. Some portions, however, carried their metallic value in a matrix of calcite and siderite. The mill in which the ores were treated consisted of two 800-lb. stamps, two amalgamating-plates, four pans, two automatic washers, two Frue vanners, and the necessary accessories for assaying, retorting and refining gold. The plant, originally erected with American capital, and directed by competent Americans, passed into the hands of Mexican owners. On taking charge of the business I found on hand a large amount of ore assaying 35 grammes of gold per metric ton (about 1 oz. Troy per ton of 2000 lbs.); but the company was losing money, and was about to abandon the business.
Obviously, the difficulty lay in the treatment of the ore. The extraction of gold scarcely reached one-tenth of the assay-value ; the loss of mercury was considerable; and high freight-charges excluded the alternative of exporting the ores.
This situation naturally suggested that amalgamation was not applicable to these ores, and that a more appropriate method must be adopted.
The cyanide- and chlorination-methods had been tried already, without practically satisfactory results. For the first few months I employed a combined system, extracting a part of the gold by direct amalgamation, and cyaniding the concentrates. This saved 32 per cent, of the assay-value, but the cost of milling still exceeded the proceeds. Meanwhile I observed that on certain occasions during the amalgamation there was a very perceptible escape of hydrogen sulphide. This I could not satisfactorily explain; but, on the other hand, it accounted for the great loss of mercury which had made amalgamation so expensive and ineffective.

To remedy this (and also diminish the great quantity of cyanide of potassium that had to be used, especially when the gangue of the mineral was calcite), I began with a reverberatory roasting of the crude ore delivered to the mill, and a subsequent washing with water, until the latter came off perfectly clear. The operations of amalgamation, concentration and cyaniding were then performed as before, and the result was a gold-extraction of 63 per cent, of the assay-value, with a loss of 11 per cent, of the mercury used.
This loss surprised me; nevertheless, under these conditions the milling of the ores began to be remunerative, and I could afford to study the subject with more leisure and less anxiety. On further investigation, the gold which had escaped amalgamation was found to be in a peculiar state of aggregation, reminding one strongly of the “ platinum sponge ” in its tendency to condense some gases. I think that the gold could be found there in another form also, analogous to so-called “ black platinum.”
Once this fact was discovered, the explanation of the previous phenomena was not difficult. The very finely-divided sponge and black gold, coming into contact with the mercury, provoked an energetic electro-chemical action; and this decomposed a relatively large quantity of water, the oxygen of which was absorbed by the sponge, while the hydrogen, combining with the sulphur of the pyrite, produced hydrogen sulphide. Of the latter, a part escaped as free gas, and a part attacked the mercury, producing mercury sulphide, which explains the great loss of that metal.
Of course the actual reactions are much more complicated than this rough statement; but the principal result, the formation of mercury sulphide, has been conclusively proved by analysis. The loss of the gold is also explained, whenever the sponge or the black gold is present under such conditions as to operate like the electro-positive element of an electric couple ; that is to say, when it will receive, condense and hold oxygen, and be returned by the electro-negative element of the couple in question. This I will prove later on.
The investigation was continued, to find a method of treatment which would both reduce the loss of mercury and increase the extraction of gold. Since the gold occurred in the gangue in grains of varying size, sometimes, but not always, impalpable, it seemed impossible to dispense with amalgamation entirely. On the other hand, a subsequent cyaniding was impaired by the foregoing pulverizing with stamps, which gave a large amount of slimes, through which it was difficult to pass the cyanide solutions. Moreover, these solutions were immediately transformed into carbonates and ammonia salts, and the consumption of cyanide was excessive. Treatment with chlorine was also difficult, and by neither of the two methods was I able, in my laboratory experiments, to obtain more than 40 per cent, of the assay-value.
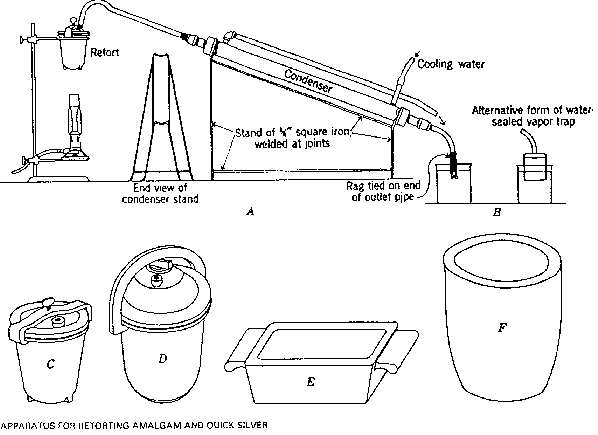
I resolved, therefore, to continue experimentally the amalgamation, supplemented at first with cyaniding, and prefaced with roasting, as above described. Having abundance of hydraulic power, I began the use, with certain modifications, of the Siemens and Halske method of precipitating the gold. This increased by 15 per cent, of the assay-value the extraction of gold, and also reduced the expense of treatment. Precipitation with zinc was therefore abandoned. The increased extraction was undoubtedly occasioned by the employment of the electric current, since the treatment had not been otherwise modified, and the average composition of the ores had not changed. A comparative experiment, in which a given lot of concentrates was cyanided, and one part was treated with zinc-precipitation, and the other with the electric current instead, entirely confirmed this hypothesis. In the first case 60 per cent., and in the second 66.30 per cent, of the assay-value was obtained.
The examination of various works on electro-metallurgy and electricity furnished me with little or nothing in the way of further guidance, except certain hints as to the movements of bodies and substances in solution, produced by the electric current, and the irregular decomposition of the electrodes, which seemed to indicate the key to the problem. Without recapitulating here the statements of Parret, Becquerel, De la Hive, Wiedemann, Jurgensen, Quincke, Herschel, and Nobilli, I will simply say that their investigations, together with my own experience, led me to attempt the treatment of the ores in question by amalgamation only, with the aid of the electric current.
Before devising a process of my own, however, I tried those of Body, B. C. Moloy, and others, without satisfactory results. Finally, after some experiment and change in my first apparatus, I perfected a method by which the loss of mercury was reduced to 0.08 per cent., and the extraction of gold brought up to 95 per cent, of the assay-value, while the cost of treatment was lowered until it only amounted to $0.42 per ton for crushing, and $0.19 for amalgamation and the electric current.
At first, this method consisted in reverberatory roasting of the ore and washing abundantly with water, then passing it through the mortars, where it was pulverized and began to amalgamate. The mortars were provided with interior amalgamating-plates, in communication with the poles of a dynamo that produced a current of 150 amperes, 14 volts. The two stamp-batteries discharged into a common channel, in which; side by side, were placed the large amalgamating-plates, one communicating with the positive pole, the other with the negative. This arrangement gave encouraging results; but in view of the energetic decomposition of water which attended it, the electro-motive power was diminished by subdividing the amalgamation-plates and uniting them, not in series, but in tension. This produced the much-desired result. The liberation of gases diminished considerably, and the loss of mercury became insignificant.
Similar dispositions were made for the pans and the washers. Over the wooden shoes were placed amalgamating-plates 2 decim. square, and on the side-walls of the buckets similar plates 0.5 m. square, united, in tension, with the poles of the dynamos, in such a manner that the electro-motive power would not exceed 1.5 volts. Later, the aggregate surface of the plates was enlarged to some 15 m. square, which gave the best results. A further increase of surface would have been useless.
On an average, 9 tons of ore were treated every 24 hours; the extraction of gold was 94 per cent., and the loss of mercury was insignificant.
Still later experiments led to the abandonment of the preliminary roasting of the ore; and this resulted in the maximum gold-extraction of 95 per cent, of the assay-value.
The Patio Process
Subsequently, I had occasion to occupy myself with the treatment of ores by the patio process. The ores consisted of a quartz mass, carrying a mixture in variable proportions of miargyrite, polybasite, silver-glance, pyrites, oxides of iron and manganese, and finally some native silver and calcite; zinc-blende was occasionally present.
The plant comprised 50 stamps of 850 lbs.; 30 arrastres; 4 mechanical washers; a patio (yard) with a capacity of 1360 tons; and corresponding apparatus, operated by abundant hydraulic power.
All the theories known to me regarding the reactions of the patio process are inconsistent with the phenomena observed in practice. As a consequence, the process, empirically, and more or less ignorantly, performed, has often been unfairly discarded by reason of failures due in reality, not to its principles, but to its improper application.
Frederick Sonneschmid, who was sent to Mexico by Charles III. of Spain, with the idea of introducing there the German methods of treating silver-ores, did not hesitate to report that the patio process was superior to the barrel-amalgamation developed by Born and his successors.
The theory of Sonneschmid, based upon twelve years of practice in Mexico, may be stated as follows :
Sonneschmid assumed that the magistral, in which he regarded the copper sulphate as the chief operative reagent, liberated from the salt hydrochloric acid, which transformed to silver chloride the metallic or sulphuretted silver contained in the ores; and that this silver chloride, in the presence of the excess of salt or hydrochloric acid, was reduced to the metallic state, and amalgamated with part of the mercury, giving up its hydrochloric acid to another part, to form mercury chloride. In addition to the mechanical loss of mercury in the process, there was thus a double chemical loss, due to the formation of mercury chloride, partly by the hydrochloric acid from the silver, and partly by the free hydrochloric acid in the mass.
This theory, as further developed, but not essentially changed, by Karsten, Rammelsberg and Regnault, has been generally adopted. Up to a certain point, it explains the most important phenomena of the process.
The consumption of mercury in this process is generally measured in its proportion to the amount of silver obtained. A loss of 12 oz. of mercury per mark (= 8 oz.) of silver extracted is generally considered good practice; and it is commonly reckoned that of the 12 oz., 8 constitute the chemical loss, and 4 the mechanical. But when docile ores are intelligently and carefully treated, the loss of mercury may be less than 8.25 oz. per 8 oz. of silver—a fact which disproves the theory that the silver chloride is reduced wholly by the mercury; since this loss is much smaller than the chemical equivalent involved in such a reaction.
Experiments made at the Hacienda, de Regia, by my father, Eng’r Miguel Bustamente, showed that, when the quantity of salt was augmented, and the treatment was slightly “ cold,” the total loss of mercury never exceeded 4 oz. per mark of silver extracted.
By another series of experiments, made to ascertain the influence of the impurities of the sulphates of copper employed, he demonstrated that the English sulphate of copper, the purest used in Mexico, did not give as good results as the acid sulphate of copper produced by the Mints in treating gold; and, finally, that the most effective and economical of all is the impure sulphate of copper, with a large quantity of iron, known as “ magistral,” and obtained by the roasting of chalcopyrite.
These results, repeatedly confirmed by myself and others, likewise contradict the generally admitted principles and theories cited above.
The fact is, that some of the reactions pointed out by theoretical chemists take place; but there are a multitude of other reactions which may and do also occur; and the accessory ingredients of the ore have no less (and probably even more) influence in the patio than in other reduction-methods, because the latter may neutralize, by means of appropriate mixture of charges, some of the elements disturbing the desired reaction; whereas in the patio no one has taken pains to make such mixtures, but all are content simply to divide the ores into “ docile ” and “ rebellious.”
This is not surprising, since the greater number of plants are in the hands of ordinary amalgamating-workmen, ignorant of chemistry and mineralogy, and attached to the routine practice of their fathers. Regular docimastic assays are rarely maintained, and still more rarely used with advantage as checks or guides in daily operations. The assays of the residue are carelessly made, and the treatment is generally guesswork. Nevertheless, the general results in treating “ docile ” ores are good. If the loss of mercury could always be kept down to 4 oz. per mark of silver obtained, and the extraction of silver and gold up to 95 per cent, of the assay-value, and if this could be done with a larger proportion of the “ rebellious ” ores, the patio would be the ideal method for this country.
Some ten years ago, as a student of metallurgy, I presented, in my examination-thesis, a theory of the patio process which I wish now to re-state, without pretending that it solves the whole problem, but believing that it takes account of certain reactions, constantly occurring in the process, which have been overlooked hitherto, though they have a marked influence on the results of the treatment.
The first chemical operation upon “ docile ” ores is the salting (ensalmorar), which consists in the addition of chloride of sodium (from 1.5 to 4 per cent, of the weight of the ore). The salt should be as pure as possible, as its quality has a marked influence on the consumption of sulphate of copper afterwards, and on the pureness of the silver, as well as on the time spent in making up the torta.
In the majority of cases I have found the use of an excess of nearly pure salt to result in greater silver-extraction, saving of time in the treatment, and notable diminution of the loss of mercury.
After the mixing (repaso), which may be done by peons, horses, rollers, pans, cradles, Archimedean screw, Chilean alacran, arrastres, etc., comes the “ incorporation ” of the sulphate of copper, or the magistral, and then of the mercury. The quantity of copper sulphate added (varying from 1.5 to 6 per cent, of the weight of the ore) depends upon many circumstances, the principal being the dullness of the workmen and the good or bad quality of the impure sulphate employed. The quantity of mercury added is calculated to be 4 or 5 times the weight of silver expected to be obtained at the end of the operation.
The exact estimate of the quantity of sulphate of copper to be employed is of great importance. If too little is added, the treatment is checked; the sulphate is converted into suboxide of copper; and the mercury, floured and oxidized, cannot be easily recovered by washing the torta without some injurious change in the compounds of silver.
If, on the other hand, the sulphate be in excess, the chloride reactions are very energetic, the mercury being rapidly converted into chloride (with liberation of 62.8 cal; of heat) ; whereas, the formation of silver chloride (liberating only 29.2 cal.) cannot take place. By subsequent reactions and outside influences, among which are the admitted effects of light and organic matter, a portion of the mercury is converted into an oxide, which is, like calomel, almost insoluble in the more or less concentrated solution of salt to which the principal reactions of the patio process are ascribed. A considerable loss of mercury is thus caused; and the compounds of silver are so transformed or rendered inert as to hinder their reduction, and produce the indications known to the workmen as those of “ hot ” treatment.
The addition, as a remedy, of lime, ashes, precipitated copper, etc., cools the torta, and destroys the calomel which may have been formed; but it neither reduces the oxidized mercury nor modifies the passivity of the argentiferous compounds.
All the current theories of the patio attribute to cupric or to cuprous chloride the chloridization of the silver in the ore —the copper becoming a sulphide or sulph-antimonide, etc. But many trustworthy laboratory experiments have disproved this proposition. The test is not difficult. Place pure pulverized argentite in a beaker; add cupric chloride in more or less concentrated solution; and the result is nil, as could have been foretold from the principles of thermo-chemistry; since the heat of formation of the chloride of silver is only 29.2 cal., while that of cupric chloride is much greater, namely, 71.2 cal. Adding chloride of sodium makes no difference, even after three months. But on the further addition of iron, or metallic zinc in shavings, an almost instantaneous reaction follows; and the more intense the light during the experiment the more energetic will be this reaction. The black silver sulphide is changed to white. This reaction, no doubt, led Kroncke to employ the cuprous chloride in the method which bears his name. When an excess of iron or zinc is added, the energetic reaction rapidly deposits metallic silver—which is not surprising.
This experiment, studied in the light of Berthelot’s thermo-chemical law, confirms the conclusion that the reaction is not a simple chloridization of the silver by the cuprous chloride (the formation heat of the latter being but 29.2, while that of the former is 62.2 cal.), but is in large part due to the metallic iron (or zinc). This conclusion can be further supported by similar experiment, in which cuprous silver is used instead of cupric chloride. The resulting reaction is very slow, and quite insignificant.
On the other hand, the hypothesis of the effective agency of the iron encounters at once the objection that, apart from American pan-amalgamation, the various silver-amalgamation processes do not involve a large consumption of iron; and, moreover, that they produce silver of much higher purity than that obtained in pans, which rarely assays as much as 0.750 fine. It is true that the crushing of ore with modern apparatus exposes it to a certain quantity of iron; that the animals which tramp the tortas are shod with iron; but these factors cannot be significant; since, both formerly and to this day, crushing in stone arrastres and the trampling of the torta by men are performed in remote mining districts of Mexico, with technical results not greatly, if at all, inferior to those of more modern practice.
These objections led me to a series of laboratory experiments which, I think, demonstrate:
- the formation of ferric chloride (Fe2Cl6), the formation-heat of which, in solution, is 255.4 cal.;
- its subsequent reduction to a lower chloride, with liberation of chlorine, which, acting in the nascent state upon the compounds of silver, transforms them into chlorides;
- the reaction of these chlorides upon the hydrated oxides in the mixture of ore and reagents, and on the metallic iron, resulting in metallic silver with a new formation of chloride of iron, releasing oxygen, and probably affecting a partial regeneration of sulphate of copper;
- a new formation of chlorides of copper and a continuation of these reactions until the termination of the treatment. This is a resume of my theory of the patio process.
What is the role of the copper in these reactions ? Its presence is certainly indispensable. It has always been supposed to play the double role of the chlorination of the compounds of silver and its own sulphatization. As to the latter reaction: the formation-heat of the sulphate of silver is 3 cal.; that of the sulphate of copper 20.8 cal.; and that of the sulphate of iron, in the most unfavorable case, 41.6 cal. Undoubtedly, therefore, if iron oxide be present, this last reaction will be the one to take place. The state of division of this sulphate of iron; the liberation of oxygen in the formation of perchlorides from the oxides of iron contained in all ores; the humidity ; the action of light and of atmospheric agents;—all contribute to the formation of the, sulphate of iron, liberating 94.4, and not to that of copper, liberating only 57 cal. This is only an application of the well-known principle of “maximum work.”
Continuing: the formation-heat of the chloride of sodium (NaCl) is 58.5; that of sodium sulphide, dissolved, 186.8; that of iron sulphide 94.4; and that of copper sulphide 57 cal. The latter, therefore, will undoubtedly be most easily attacked by the chloride of sodium, since it requires the smallest number of calories to make it resign to the sodium its sulphuric acid, with formation, undoubtedly, of proto-chlorate of copper, which liberates 71.2 cal.
This simple comparison of the formation-heats shows at once the usefulness of the sulphate of copper in the patio process, and also explains the small success of those experimenters who have, in practice, substituted sulphate of iron. The presence of copper is, moreover, of the utmost importance for the preservation of mercury in the metallic state, after the oxides of iron have been transformed into proto-chlorides; the formation-heat of the corrosive sublimate being only 59.6 cal.
It remains to be explained why the “magistral” (i.e., the sulphates of copper and iron obtained by the reverberatory roasting of chalcopyrite) yields, in this process, better results than the English sulphate of copper, chemically the purest in the market. This explanation is very simple, and completes my theory of the patio process.
All those who have practiced photography have witnessed the effect of light in reducing the silver-salts and transforming the proto- into the per-salts of iron; also the strong solvent action of iron perchloride upon the salts of silver—especially silver chloride, whether it has or has not been affected by light. The solvent power of iron perchloride upon silver chloride is greatly superior to that of the chloride of sodium, though the latter may be more generally known; and it naturally facilitates and accelerates the reactions in the patio. In particular, the chlorination and consequent loss of mercury is diminished, for two reasons:
- because the quantity of chloride of copper formed is made relatively small; and
- because the proto- and perchloride of iron immediately formed, instead, from the sulphate of iron of the magistral, directly aid in attacking the argentiferous compounds.
The reduction to silver of the dissolved silver chloride may be effected either:
- through the precipitation of silver as an unstable oxide by the oxides of iron naturally existing or artificially formed in the ore, or
- by the conversion of silver proto-chloride into perchloride, leaving free silver, which amalgamates with the mercury, eluding in this way further chlorination and solution.
Consequently, mercury should not be chemically lost in this treatment. In fact, the necessary chemical loss has often been shown in practice to be imaginary. The mechanical loss is the only inevitable one.
The two principal signs observed in the usual tests which have hitherto served, and will doubtless continue to serve as a practical guide in the operation of the patio process, confirm part of the theory here presented.
- The test of a “ cold ” torta, made immediately after the incorporation by trampling, shows mercury, sometimes in part more or less confluent, but usually in small drops, or in the exceedingly fine state of division (floured) which we call liz. Rubbing this together, and then attempting to strain it by squeezing, we obtain scarcely any signs of amalgam. The mercury is very white, resembling its natural color, or tending more or less to a yellowish color on the surface, owing (as the experts say) to the formation of sub-oxide of copper. The film of this oxide, covering the surface of the mercury, is undoubtedly due to the decomposition of the chloride of copper by the oxides of iron in the ore; and the quantity of chlorine thus liberated from the copper salt is not sufficient to form the needed amount of perchloride of iron, which, acting in the nascent state, and favored by the heat liberated in its own formation, is the true agent in the chloridization of the silver-compounds. Hence the “ coldness ” of the torta, with the unfavorable conditions which that implies. This phenomenon led me to suspect for the first time the important part played in the patio process by the iron oxides and salts of the ore.
- On the other hand, the torta is “ hot” when an excess of sulphate of copper has been added. In this case, perchloricle of iron is very rapidly formed, and tends to be reduced with similar rapidity to the proto-chloride, converting the mercury to calomel (Hg2Cl2), until the reaction provoked by the immoderate use of sulphate of copper has terminated. In this case, practically all the reagents employed are consumed in the chlorination of the mercury, without useful result. The greater part, if not the whole, of the iron oxides in the ores is changed to proto-chloride; and if, after the over-heated torta has cooled, pure sulphate of copper be employed to continue the treatment, much difficulty will be experienced in recovering the conditions lost.
Inventors, reasoning upon the reactions of the Freiberg barrel-amalgamation, have proposed the use of metallic iron in the various phases of the patio process, as a means of minimizing the loss of mercury. The main result of such a measure has been the requirement of a larger quantity of sulphate of copper, together with delay in the progress of the treatment. The reason is easily seen : the metallic iron precipitates metallic copper, and this reaction cools the torta. The consumption of mercury increases instead of diminishing.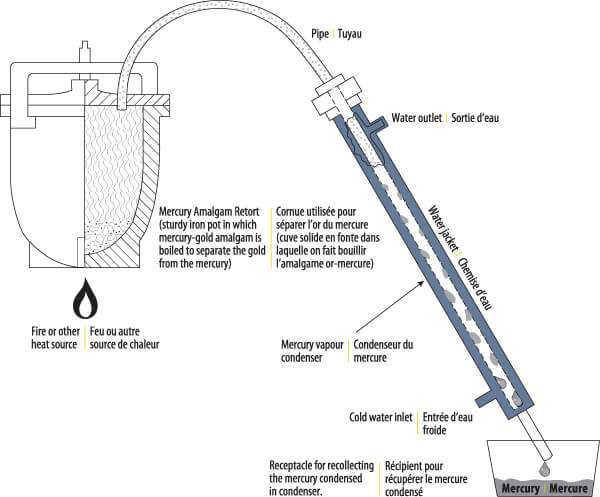
In view of these facts and considerations, it is, in my judgment, the best practice to conduct the treatment of the torta moderately and with vigilance, and, upon the least sign of excessive heat, to apply the remedy at once, in the form of a quantity (calculated as exactly as possible) of lime, precipitated copper, or ashes, to forestall the effects of a “ hot ” torta upon the mercury.
As already observed, the proper amount of sulphate of copper required in this process for any particular ore should be as carefully determined, according to the law of chemical equivalents, as the amount and nature of flux required in a smelting process. And it follows that for this process, as for smelting, different ores might be so mixed, after proper analysis of each, as to diminish the necessary amount, or increase the effectiveness of the metallurgical reagents added. Empirical mixtures of “ docile ” and “ rebellious ” ores are known to have given excellent results in many Mexican localities; and there is a wide and promising field for the thorough study and systematic application of this practice, which would raise it from the plane of local tradition or happy accident to that of definite purpose and fore-knowledge.
In this, as in other respects, the Mexican amalgamation-process has never had opportunity to exhibit its full technical and economical capacity. I firmly believe that it can successfully compete with smelting, especially in a country like ours, in which, by reason of topographical conditions and the cost of fuel, freights will always be high.
With regard to methods for diminishing the loss of mercury and amalgam, I would here recall the experiments in connection with the amalgamation of gold, described in the first part of this paper. I have similarly employed the electric current in connection with the patio process also—not to affect the treatment itself, but to join the metallic particles. The result was, as I had expected, the same as that which had been accomplished with gold. The quantity of silver and mercury recovered was considerably increased; and I succeeded in saving 97 per cent, of the humid-assay value of silver with a loss of only 5.1 per cent of the mercury employed.
The apparatus consisted of a series of amalgamated copper plates connected to the poles of the dynamo, and grouped in tension, so as to obtain, per sq. meter of surface, from one to two volts and 40 amperes of current. These plates were so suspended by means of the canals and inside of the drain of the patio in such a manner as to interrupt, to a certain extent, the free passage of the slimes and water, but without seriously hindering or complicating the washing of the torta. I am fully conscious that, after more than seven years spent in establishing facts, overcoming difficulties, and perfecting details, my work in the economic utilization of the facts and theories set forth above is, like my attempt to state them here, still far from complete and satisfactory. Nevertheless, this paper, begun two years ago, is now published, in the hope that the suggestions and experiments of others may aid in the improvement and the due recognition of our Mexican patio process, so little understood, so often undervalued, and so worthy of a better fame and fate.
