Many assay methods for the determination of gold or silver, or both, in cyanide solutions have been published, which with care in manipulation, and modification in some cases, will give results that are satisfactory. It is possible to classify or group these methods as follows:
- Evaporating the solution in a porcelain or agate-ware dish containing litharge, or in a “boat” made of lead foil.
- Forming a lead sponge containing the precious metals by means of zinc shavings, zinc dust, or a piece of aluminum.
- Decomposing the cyanide solution with an acid and precipitating the precious metals by the use of one or a, combination of some of the following: Copper sulphate, sodium sulphite, hydrogen sulphide, cement copper.
- Precipitating the silver by zinc dust held in a Gooch crucible and determination with a standard solution of sulphocyanate.
- Electrolysis.
- Colorimetry.
Eliminating Group 4 because of its applicability to silver solutions only; Group 5, because of the time and apparatus required; and Group 6, because of the skill required, and the difficulty of maintaining the standards; which method of the remaining groups will give accurate results in the shortest time?
In the Hammond Laboratory of the Sheffield Scientific School, where many ores are tested for treatment by the cyanide process, the resulting solutions will cover a wide range, when their contents of base metal compounds are considered, and it is in the laboratory work just as much as in mill work that a reliable method that will not require too much time is needed. This is especially important in teaching.
What criticism I have to make has been brought about by doing what every operator does—trying the various methods to find the one that will give “good results.”
Group 1 requires too much time; a large hot-plate surface if many determinations are to be made; scraping of the dishes clean to remove all particles; breaking up the mass; fluxing and fusing in a crucible. Evaporating in a lead boat is uncertain, because some lead foil may be quite thin and perhaps pitted, so that the solution will leak through as the evaporation proceeds and the cyanide solution becomes concentrated.
Group 2 includes the method suggested by Alfred Chiddy and others that are modifications of it.
The idea of the formation of a lead sponge to contain the gold and silver as suggested by Chiddy is a clever one, and it appealed to every one having anything to do with cyanide solution. To be able to remove from the dish a small sponge of lead that could be cupelled was a great advancement in the work. It is difficult to get good results if it is followed as printed, so that its modification as suggested by Stines, Magenau, Holt and others is a natural outcome. Any of the methods of this group that will give a sponge of closely cohering lead, containing all the gold and silver, in a reasonable time, is a “good one;’’but when the sponge breaks into small pieces they must be collected in some manner and filtration is the next step.
When “the lead has been collected on a filter paper it then becomes necessary to scorify or dry the paper and reduce it in a crucible with the necessary fluxes.
Picric Acid Method for Determining Weak Acid Dissociable (WAD) Cyanide
It has seemed to me that it is right here that an opportunity exists for a new method, either for the formation of a good sponge or to save the broken sponge formed by any of the other methods, and at the same time eliminate scorification.
Group 3 includes all those methods that permit the use of a large quantity of solution and from which the precious metals may be precipitated as mentioned above. Whether it is necessary to use so large a quantity, aside from experimental work perhaps, I shall leave to the individual operator. My own objections to this group are: The quantity of solution involved; the time required; and the necessity of filtration and scorification.
In order to present this new method clearly I have numbered each successive step in making the determination and have included the photograph to show apparatus, etc.
I. Procedure for New Method:
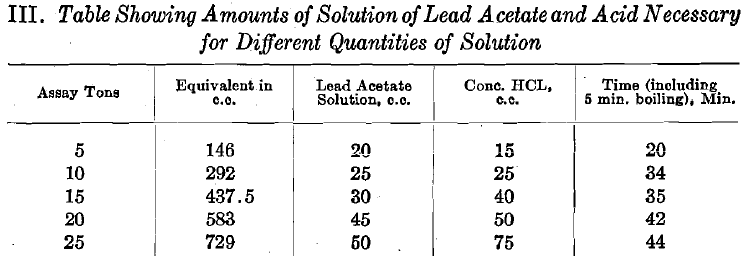
- Into a 250-c.c. beaker (No. 2 low form) pour 5 assay tons (146 c.c.) of cyanide solution.
- Add 20 c.c. of a 20 per cent, solution of lead acetate.
- Add 15 c.c. of concentrated hydrochloric acid.
- Place a ¼-in. rod of zinc in the beaker (No. 1 in the photograph).
- Place beaker on a hot plate—bumping does no harm if it will not break the beaker by raising it off the plate.
- As soon as the solution boils, leave it so for 5 min.; then remove from hot plate.
- Fill with cold water; then decant about half and again fill with cold water.
- Twist the zinc rod quickly between the finger and thumb and draw it out of the sponge.
- If any small particles of lead are left adhering to the rod at about where the top of the solution touched draw the sponge up the side of the beaker with a glass rod.
- Touch the zinc rod to the sponge to free the particles.
- Wash the sponge three or four times with cold water to remove zinc chloride.
- Press it against the side of the beaker with a glass rod to remove the water.
- Decant the water and wash again.
- Place the dewatered sponge on a piece of sheet lead 2 in. square, then fold it. It is now ready for cupellation.
II. Procedure When the Sponge Breaks:
When the sponge breaks into large pieces it is possible to unite them by pressing together with a glass rod. If there are many small particles it may not be possible to unite them by pressing together. Then this method is suggested:
15. Fit a 2-in. funnel into a filtering flask.
16. Cut a piece of sheet lead 3 in. square; fold as you would a filter paper.
17. Cut off the comers; then open it to fit the funnel.
18. Place about 5 g. of test lead in the lead cone and push it down with the finger, then smooth out the folds and creases so that it will fit well.
19. Lift it from the funnel and prick seven or eight holes in the apex or point by means of a pin, then put it back into the funnel.
20. Complete 9 and 10; then start the filter pump.
21. Tip the beaker and draw most of the lead sponge into the lead cone by means of a glass rod (without a rubber tip).
22. Pour the remainder of the solution into the cone, rinse beaker and wash contents of cone three or four times.

Fig. 1.- Apparatus used in the New Method for the Determination of Gold and Silver in Cyanide Solutions.
23. Tamp the lead down with the flat knob of a glass rod. (See 4 in Fig. 1.)
24. Stop suction, remove cone from funnel and fold it in carefully. (See 5 in Fig. 1.) It is now ready for cupellation.
25. Draw a hot cupel to the front of the muffle and place the cone in it. (See 6 in Fig. 1.) When the water has been driven out, push the cupel into a hotter part of the muffle and finish the cupellation in the regular manner.
IV. Notes and Comment:
The generation of hydrogen along the zinc rod is sufficiently active to prevent the adherence of the lead. A strip of aluminum does not work so well.
The method has been used in the assay of a number of solutions containing a variety of base-metal compounds and in each case the sponge remained whole. With solutions from cobalt ores that contain much silver the sponge is apt to break.
The amount of time as given in the table will vary with the heat of the hot plate—those given are averages.
In order to keep the weight of the lead to be cupelled down to a minimum, thin sheet lead should be used.
A cone as described in 16 and 17 will correspond to a filter paper 7½ cm. in diameter and, made of heavy sheet lead, will weigh about 7 g.
The pin holes should always be as near the point as possible.
A screen analysis of the test lead used in developing this method is as follows: + 30, 7.0 per cent.; + 60, 28.6; + 100, 23.2; — 100,41.2per cent.
It is quite possible to have a cone manufactured as are bottle caps. This would lessen the weight about 50 per cent.
V. Scorifying the Precipitate:
The following method may be used for scorifying the precipitate obtained by any of the methods of Group 3:
26. Collect the precipitate in a 9-cm. filter paper; wash it down into the point.
27. Make a cone from a disk of sheet lead 3½ or 4 in. in diameter.
28. Punch 10 or 12 holes at the point. .
29. Fold the filter paper into a small wad and place it in the point of the cone.
30. Pour on top of the paper 10 g. of test lead.
31. Fold the lead cone so as to include its contents and place it in a glazed scorifier at the mouth of the muffle.
32. When the paper has become dry and begins to char, the gases will burn as they come from the pin holes. As soon as the flame ceases place the scorifier in a hotter part of the muffle. The lead cone will hold the paper firmly while it is burning; so there is no danger of its unfolding and scattering the precipitate.
Discussion:
E. J. HALL, New York, N. Y. (communication to the Secretary).— The Chiddy method for determining gold and silver in cyanide solutions has been subjected to so many proposed modifications, of which L. W. Bahney’s is one, it is natural to suppose the method is defective.
There is no denying that this method is capable of giving inaccurate results, but so is any other method of quantitative determination. However, proof has not come to my notice that the troubles are inherent and not in the detail application, and I am inclined to think from our experience that in many cases it is the latter.
Errors in this method may be occasioned by:
(1) The retention of zinc in the sponge, which tends to desilverize the molten lead in cupellation, similar to the action in the Parkes process, removing gold and silver in a crust of zinc oxide. Failure to remove zinc may result from lack of concentration of hydrochloric acid, due to neutralization by salts present, volume of liquid too great for the specified amount of acid, or the use of weak acid. (HCl rapidly loses its strength unless kept in perfectly tight containers. The HCl gas escapes even from the ordinary glass-stoppered acid bottles after the plaster seal has been broken.) Undissolved zinc is likely to be held in the upper part of the lead sponge, which floats above the liquid, and unless the solution is boiled it will escape action. Mr. Bahney’s modification makes this difficulty impossible. It is not, however, a necessary evil when the assay is properly conducted.
(2) In the assay of foul solutions the sponge is apt to disintegrate, as pointed out. Occasionally this tendency may be overcome by slightly oxidizing the solution after adding zinc and boiling to reduce impurities. The lead acetate and remaining acid is then added. When the sponge breaks up it is usually sufficient to decant most of the liquid and then wash the lead into a boat, drain off remaining water, and cupel. If the lead is fine enough to remain in suspension, prohibiting decantation, it may be rapidly filtered on SS No. 597 filter paper. Fluxing is not necessary, as the paper may be pressed between blotters, sponge separated, paper folded once, held by one end with a pair of tongs and the bottom ignited with a bunsen burner, allowing ashes to fall into a lead boat containing the sponge; then wrapped and cupelled.
(3) The furnace temperature necessary to start cupellation is considerably higher than that required for a good finish. The major portion of cupellation loss occurs when the last gram or two of lead is being oxidized. This loss increases rapidly with increase in temperature, particularly with small beads such as are usually obtained from cyanide solutions.
There is about 1.7 g. of lead in 10 c.c. of a saturated lead acetate solution, the quantity usually employed. The maximum weight of sponge will not exceed this weight and is often less than 1 g. when the solution has been heated for a long time or HCl concentration is high. The sheet lead required for wrapping is 2 to 3 g., so the whole packet will not weigh over 3 to 5 g.
For the reasons given above it is impossible to obtain good results in cupelling lead buttons of this size unless the cupels are moved to the mouth of muffle or coolers introduced in the furnace immediately after the buttons have opened. This is particularly true when large cupels are used, as they give up their heat slowly. If gold is the principal metal sought it should always be protected by silver.
That gold and silver in all cyanide solutions can be best determined by any one method is not a reasonable assumption. For rich solutions where 1 to 2 assay tons will suffice and prompt returns are not required the evaporation method in a lead boat is most attractive, as the working time and attention are least. A lead boat 1.75 by 3.75 in. made from a sheet 3 by 5 in. will permit evaporation of 2 assay tons of solution in 60 to 75 min. A covering of test lead on the bottom of boat will facilitate evaporation and reduce the tendency to spit. If the solution is foul or carries suspended matter, scorification is desirable.
Mr. Bahney objects to this method because the lead foil may leak. Assayers’ sheet lead holds water better than the foil and is readily obtainable.
Solutions that are reasonably free from impurities and suspended matter give good results by the Chiddy method and its modifications. Suspended matter not soluble in HCl will collect in the sponge, as well as other impurities, and scorification should follow. Under these conditions one of the so-called precipitation methods is preferable.
It is unfortunate that Mr. Bahney did not include some comparative figures, particularly as the assumption that some gold and silver might be retained on the zinc stick seems reasonable.
Method for the Determination of Gold and Silver in Cyanide Solutions, BY L. W. BAHNEY, 1915
