Table of Contents
Leaching of porphyry copper mine strip wastes and low-tenor ores provides a substantial part of U.S. copper needs. Dump leaching requires years to extract part of the copper, and leach dumps often become impermeable owing to precipitation of iron salts which greatly reduces the copper leaching rate. In an effort to improve dump leaching technology, the Bureau of Mines did research as part of a program to assure adequate domestic supplies of minerals. This improved technology is needed to provide increased dump permeability for optimum copper leaching and recovery, thereby extending and conserving copper reserves.
Dump leaching of low-tenor ores is a simple procedure whereby leach solution is distributed over the top of the dump to dissolve the copper minerals as the solution percolates through the bed. The enriched leach liquor is collected at the base of the dump, treated to recover the copper, and recycled to the dump. Copper recovery methods currently used include either solvent extraction followed by electrowinning or cementation of the copper using scrap iron. Copper recovery by cementation has been practiced for hundreds of years, but the solvent extraction-electrowinning technique is a relatively recent development.
In the more familiar cementation technique, metallic iron reacts with dissolved copper to form metallic copper and dissolved ferrous iron. Ferrous iron remains in solution at a leach solution acidity of pH 3; however, oxidation of ferrous iron by the air precipitates ferric compounds at a pH of about 2 or more. The precipitation of ferric compounds from recycled leach solution is probably the most troublesome problem encountered in leaching operations because precipitates plug both distribution pipelines and the dumps themselves. Strict control of the iron content and maintaining solution acidity near pH 2 are necessary to minimize the problem.
The more recent use of solvent extraction followed by electrowinning enables copper recovery without the addition of troublesome iron to the leach system. The copper leaching, solvent extraction, and electrowinning process was first used commercially by Rancher’s Exploration and Development Corp. at its Bluebird Mine in Arizona. One reason for adopting this method was to improve copper leaching by decreasing the precipitation of iron salts in the leach dumps. Although economic comparisons between the old and new copper recovery techniques have been reported, the results of studies to compare these two techniques with respect to their effects on copper leaching rate have not been described. The Bureau of Mines, aware of the problems encountered in maintaining leach dump permeability, has tested and reported the beneficial effects of removing ore fines prior to leaching. Also reported was the enhancement in copper extraction rate by oxygen injection into a nearly dormant leaching system. To supply the need for further information concerning copper dump leaching, this report describes the results of comparative testing of the two copper recovery techniques, leaching- cementation and leaching-solvent extraction-electrowinning, using 7-ton ore samples under laboratory conditions.
Materials and Methods
Low-grade, open pit mining waste was used in the leach testing. One sample of material contained li percent minus 0.5-inch fines, whereas the other sample contained 32 percent minus 0.5-inch fines. One objective of the investigation was to determine the effect of the amount of minus 0.5-inch fines on bed permeability using alternate copper recovery techniques.
Mineralogy of the Samples
The host rock was an altered quartz monzonite consisting primarily of randomly oriented feldspars, quartz, and chlorite grains. Smaller amounts of sericite and possible clay minerals also were present. Pyrite was the most abundant sulfide mineral. The copper minerals consisted primarily of
chalcocite, digenite, chalcopyrite, covellite, and enargite. Chalcocite was the most abundant copper mineral in the sample and accounted for about 66 percent of the copper. The distribution of copper among the various minerals is shown in table 1.
Sample Preparation and Characterization
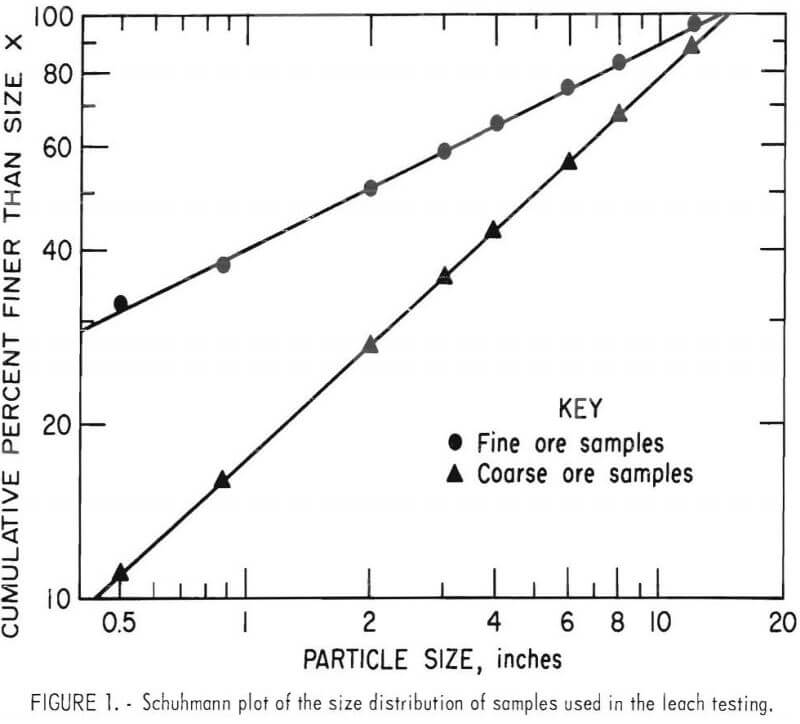
Size distributions for both samples are presented by the Schuhmann plots shown in figure 1. The Schuhmann relationship when written in linear form is
log Y = A log X – log K…………………………………………………………..(1)
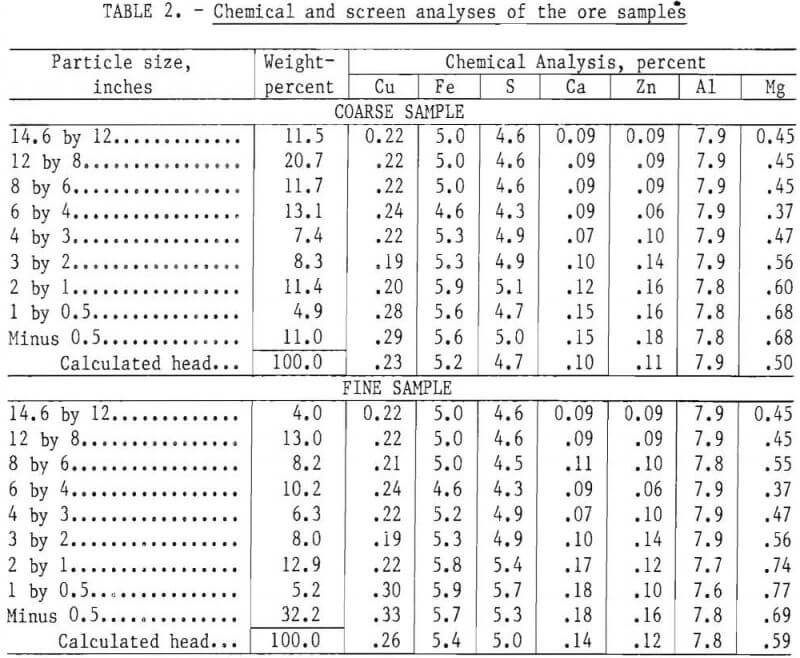
where Y is the cumulative weight fraction passing a screen with aperture X, and A and K are constants. The ore was screened into several sized fractions, and these fractions were carefully recombined into two coarse samples and two fine samples with distributions fitting the Schuhmann equation. The sizing was done to facilitate developing a mathematical leaching model, and the results of that study will be reported separately. Maximum ore particle size in the samples was 14.6 inches. The coarse samples contained 11 percent minus 0.5-inch fines, and the fine samples contained 32 percent minus 0.5-inch fines. Chemical analyses of the ore fractions are listed in table 2.
Test Procedures and Equipment
Leaching tests were done in glass-fiber-reinforced polyester columns 4.5 feet in diameter and 10 feet high with a capacity for nearly 8 tons of ore. Figure 2 shows two leach columns, solution pumps, and surge tanks.

The samples were leached by downward percolation of pH 2 solution at initial flow rates of 72 to 76 gallons per square foot per day. This flow, much higher than flows conventionally used in dump leaching, was used to make readily apparent any changes in bed permeability. Solutions were applied to the ore through a rotating arm that contained 1/8-inch-diameter orifices. Leach solution draining from the columns was circulated back through the ore until the copper concentration reached 1 to 2 grams per liter, simulating conventional operations, and then the flow was stopped to recover the copper either by cementation or by solvent extraction. Typical concentrations of other ions in solution, in grams per liter, were 1-5 zinc, 1-5 aluminum, 0.5-1.5 magnesium, and 0.5 calcium. Leaching was resumed using the solutions depleted of copper. Sulfuric acid was added as necessary to maintain a pH of 2 to minimize the precipitation of ferric compounds.
Leaching Mechanisms
Copper minerals in the ore including chalcocite, digenite, chalcopyrite, covellite, and enargite dissolve in acidic ferric sulfate solution with the formation of cupric sulfate and ferrous sulfate. Ferrous iron must be oxidized to the ferric state to enable recycling the leach solution because ferrous salts do not dissolve the copper minerals. The principal copper mineral in the ore, chalcocite, was dissolved as shown by reactions 2 and 3:
Cu2S + Fe2(SO4)3 → CuSO4 + 2FeSO4…………………………………………(2)
CuS + Fe2(SO4)3 → CuSO4 + 2FeSO4 + S……………………………………..(3)
A more detailed description of leaching mechanisms has been presented by Sullivan.
Copper Recovery by Cementation
Copper was removed from leach liquor containing from 1 to 2 grams of copper per liter by pumping the solution through a vertical glass pipe, measuring 6 inches in diameter and 90 inches high, filled with detinned scrap iron. Adjusting the influent pH to 1.5 using sulfuric acid prevented the precipitation of ferric salts in the cementation tower. Finely divided metallic copper in the effluent was settled, and the clear solution, containing about 0.1 gram of copper per liter, was used for another leaching cycle.
The following chemical reactions may occur when copper is precipitated by metallic iron:
CuSO4 + Fe → FeSO4 + Cu……………………………..(4)
Fe2(SO4)3 + Fe → 3FeSO4………………………………(5)
2FeSO4 + H2SO4 + ½O2 → Fe2(SO4)3 + H2O……………………….(6)
H2SO4 + Fe → FeSO4 + H2…………………………………(7)
Reaction 4 is the familiar cementation reaction that theoretically requires 0.88 pound of iron to precipitate 1.0 pound of metallic copper. In practice, however, reactions 5 and 7 contribute to inefficiency, and iron consumption ranges from 1.2 to 2.5 pounds of iron per pound of copper. Both of these reactions not only waste iron but add iron to the system, and this iron eventually oxidizes (reaction 6), forming troublesome precipitates on top of and within the leach dump.
Copper Recovery by Solvent Extraction-Electrowinning
As an alternative to cementation, copper was recovered from leach solution by a combination of solvent extraction and electrowinning operations. Solvent extraction separated copper from iron contained in the leach solution and provided a concentrated copper electrolyte suitable for electrowinning. The reversible exchange of copper between organic and aqueous phases is shown by the generalized equation:
Cu2+(aqueous) + 2RH(organic) ↔ R2Cu(organic) + 2H+ (aqueous)……………………….(8)
The organic extractant, LIX-64N, employed as a 10-volume-percent solution in kerosine, extracted copper but not iron from the leach solution in exchange for hydrogen ions. Copper was stripped from the organic phase with acidic spent electrolyte from electrowinning to enrich the copper electrolyte and to regenerate the organic phase for reuse.
The continuous countercurrent solvent extraction system used in this work comprised four extraction and four stripping mixer-settlers. Aqueous and organic flows in the extraction section typically were 0.24 and 0.18 gallon per minute, respectively. Acidic raffinate containing less than 0.1 gram of copper per liter was returned to the leaching circuit. Aqueous and organic flows in the stripping section typically were 0.04 and 0.18 gallon per minute, respectively. The barren organic phase was recycled to the extractors, and the strip liquor, containing about 25 grams of copper per liter, was depleted to about 18 grams of copper per liter in the electrowinning cell.
The electrowinning cell contained nine lead anodes and eight copper cathodes measuring 6¾ inches by 8 inches. A current density of 16 amperes per square foot was employed for electrowinning.
Experimental Results
Four long-term leaching tests were made to determine the effect of the two copper recovery methods on copper leaching rates. A pair of comparative tests was made on the relatively coarse ore sample containing 11 percent minus 0.5-inch fines. Another comparison was made using the relatively fine sample containing 32 percent minus 0.5-inch fines. Experimental results and observations including copper extraction rate, acid consumption, leach solution iron content, column permeability, and bacterial activity are described.
Leaching the Coarse Ore
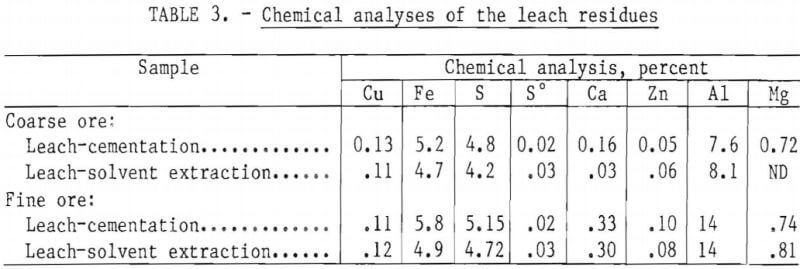
Two 7-ton batches of ore containing 11 percent minus 0.5-inch fines were leached simultaneously for a total of 416 days. Leaching was interrupted periodically to remove copper from the leach liquor when a copper concentration of 1 to 2 grams per liter was reached. Copper removal was done 32 different times in each leach test. The degree of copper extraction was monitored using leach liquor assays, solution inventory, and an initial ore-sample analysis. Copper extractions were later corrected based on the initial copper content determined by analyses of the leached residue and copper products. Table 3 shows the chemical analyses of the leached residue products for all four leaching tests. Solution iron concentrations and acid consumptions were recorded.
Copper Extraction
Copper leaching progress is shown by the two curves in figure 3. After 416 days, 48 percent of the copper was extracted by cementation, whereas 51 percent of the copper was extracted using solvent extraction and electrowinning. Because of the small difference in copper extractions from the two ore samples, the copper recovery system apparently had little, if any, effect on copper extraction.
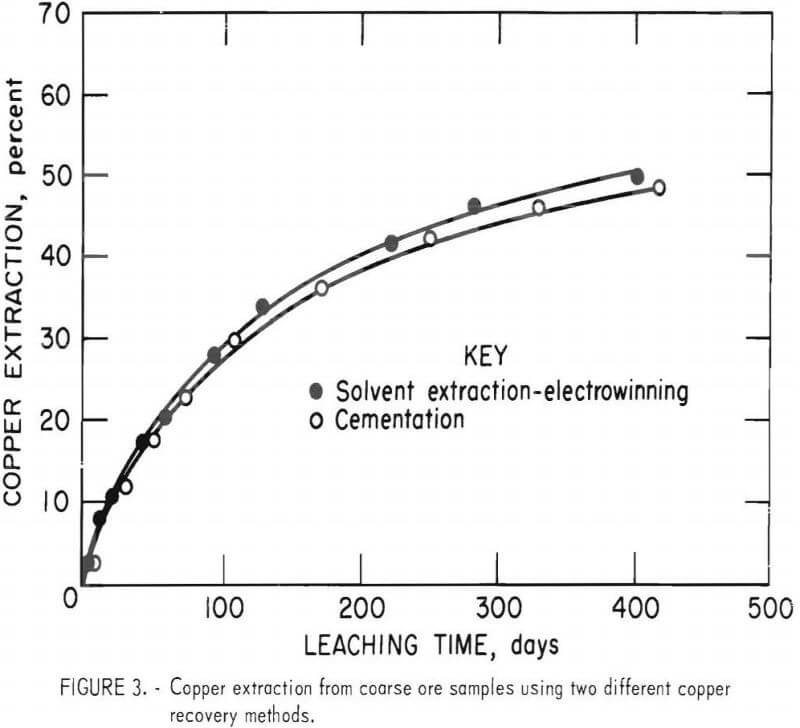
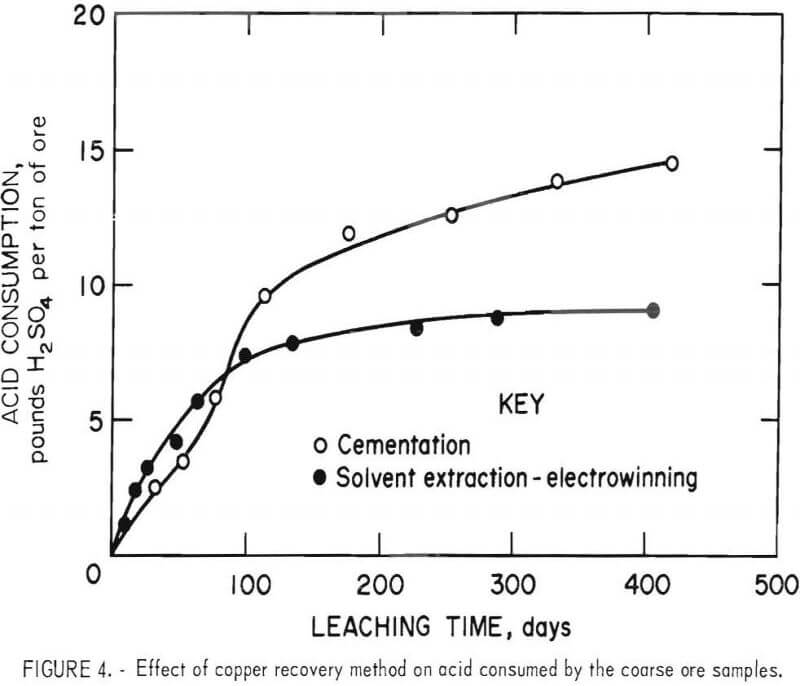
Acid Consumption
Sulfuric acid additions required to maintain pH 2 leaching conditions in the two ore columns are shown in figure 4. For the 416-day leaching period, acid consumptions using the cementation and solvent extraction alternatives were 15 and 9.2 pounds of H2SO4 per ton of ore, respectively. The higher acid consumption by the leach-cementation test appeared to result from two reactions, 6 and 7, whereby acid was used by the oxidation of ferrous iron and by reaction with metallic iron. Expressed in another way, the weight ratios of acid consumed to copper leached were 5.4 and 3.5 for the cementation and solvent extraction alternatives, respectively. Thus, sulfuric acid was conserved when solvent extraction-electrowinning was employed for copper recovery.
Iron Content of the Leach Solutions
Iron was not present in the leach solutions initially, but it soon appeared as a result of the dissolution of iron minerals and the cementation of copper with iron. The iron concentration increased in both leach solutions, although the rate of increase was more rapid in the leach-cementation test. After 131 days of leaching, iron concentrations in solutions from the leach-cementation and leach-solvent extraction tests were 31 and 14 grams per liter, respectively. At this time a measured amount of leach liquor was bled from each circuit and replaced with water to decrease leach liquor concentrations to 10 grams of iron per liter. Iron was bled periodically to simulate large-scale leaching practice, in which dissolved iron is controlled after cementation by oxidation and settling in ponds.
Nearly all of the iron in the leach liquors was in the ferric state except for a period of about 2 days following copper removal by cementation. Ferrous iron was oxidized by dissolved oxygen (reaction 6), and the rate of oxidation apparently was accelerated by the bacterial activity of iron-oxidizing bacteria. Ferrous iron concentration in the leach solutions generally was 0.01 gram per liter.
Iron compounds precipitated on the ore particles during leaching, but the quantity of the accumulation could not be precisely measured. During the leach-cementation test, the iron added to the system through 33 cementation operations was 12.3 pounds of iron per ton of ore. Iron periodically bled from the system was 6 pounds per ton of ore. Iron periodically bled from the system was 6 pounds per ton of ore, and iron remaining in solution at the completion of the test was 0.7 pound per ton of ore. Based on these amounts, 5.6 pounds of iron per ton of ore accumulated in the ore bed. The additional iron that dissolved from the ore and then reprecipitated was not determined.
Ore Permeability
A decrease in ore permeability sufficient to cause ponding of leach solution on top of the ore was not observed during the leaching of either ore column, although the amount of minus ½-inch fines increased from 11 percent to 17 percent in each test. Although more iron precipitated in the ore when cementation, rather than solvent extraction-electrowinning, was employed, plugging within the column was not noticeable at the solution circulation rate of 72 gallons per square foot per day.
Bacterial Activity
Bacterial activity by micro-organisms probably aided the oxidation of both iron and sulfur. The oxidation of iron, aided by bacterial action, was helpful because iron oxidation at pH 2 is very slow under sterile conditions. The presence of iron- and sulfur-oxidizing bacteria in the ore was verified by culturing leach solution samples.
Leaching the Fine Ore
Two 7-ton batches of ore containing 32 percent minus 0.5-inch fines were leached for 568 days to determine the copper leaching rate as a function of copper recovery method and ore size distribution. The test procedure used was similar to the method used for leaching the coarse ore samples except that copper was removed from the leach liquor 25 times and the leaching continued for 568 days rather than 416 days. The tests were terminated when it became apparent that leaching had ceased.
Copper Extraction
Copper leaching progress is shown by the two curves in figure 5. Copper extraction after 568 days of leaching was 48 percent when copper was recovered by cementation, whereas 60 percent was extracted when copper was recovered by solvent extraction and electrowinning. Similar copper extractions were achieved in both tests during the first 200 days of leaching; however, a noticeable lag in copper extraction became apparent after 200 days in the leach test using cementation for copper recovery.
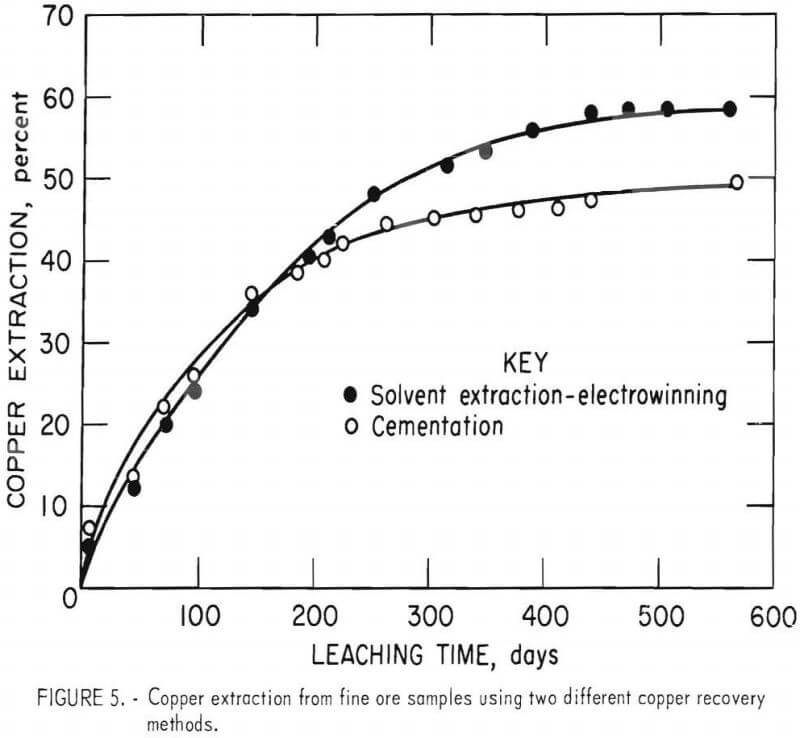
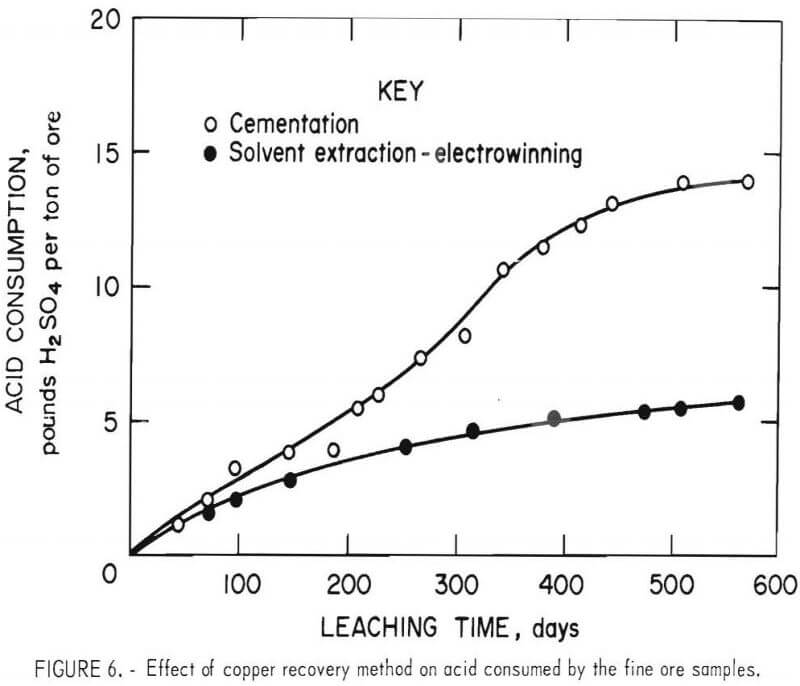
Acid Consumption
Sulfuric acid additions required to maintain pH 2 leaching conditions in the two ore columns are shown in figure 6. During the 568 days of leaching, acid consumption using the cementation and solvent extraction alternatives were 14 and 5.7 pounds of H2SO4 per ton of ore, respectively. The weight ratios of acid consumed to copper leached were 5.07 and 1.46 for the cementation and solvent extraction alternatives, respectively. Again, the higher acid usage by the leach-cementation test appeared to result from reactions occurring during and following cementation.
When the solvent extraction-electrowinning method was used, a comparison of acid consumption for the coarse and fine ore samples was made. Less sulfuric acid was added in leaching the fine ore, and this was believed to result from the leaching of more iron sulfide minerals that generated sulfuric acid as shown by reaction 9:
FeS2 + 7Fe2(SO4)3 + 8H2O → 8H2SO4 + 15FeSO4…………………………………….(9)
Iron Content of the Leach Solutions
The iron content of the leach liquors, none initially, increased during the 568-day leaching period to 20 and 11 grams of iron per liter for the leach-cementation and leach-solvent extraction tests, respectively. Unlike the tests using coarse ore, iron was not bled from the system to maintain a level of 10 grams of iron per liter. Ferrous iron in the leach liquor treated by cementation increased after 151 days from a negligible amount to 70 to 100 percent of the total iron concentration. A low concentration of ferric iron reflected by the high ferrous iron content caused a slower copper extraction rate.
Iron accumulation in the ore bed as a result of iron added to the system by cementation was 0.6 pound per ton of ore during the first 151 days of leaching. Only 1.6 pounds of iron per ton of ore were known to have accumulated in the ore by the end of the test. This small accumulation plus the unknown accumulation resulting from the leaching of iron contributed to the total amount of iron that precipitated in the ore.
Ore Permeability
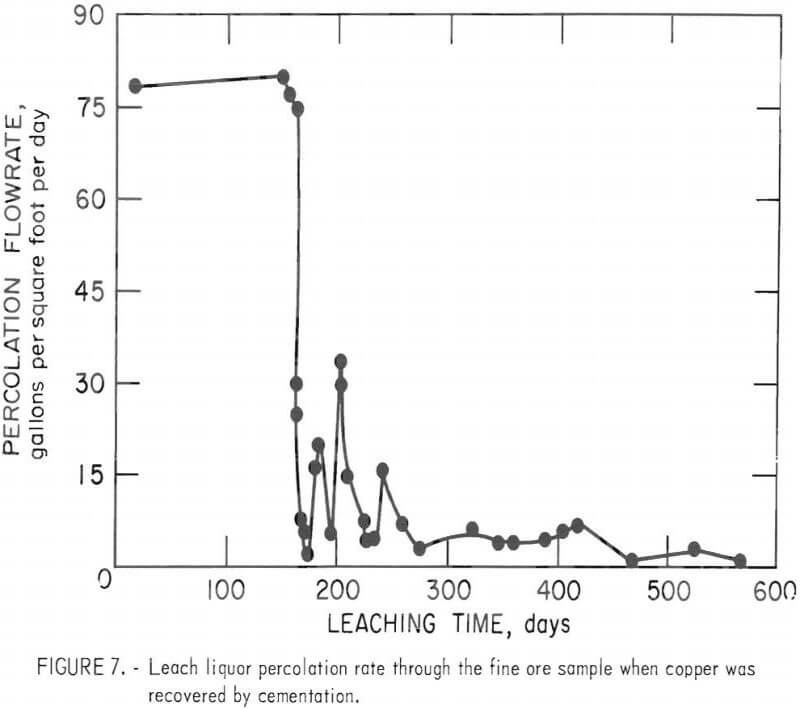
The initial solution circulation rate during these tests was 76 gallons per square foot per day. Only slight ponding of leach solution on top of the ore occurred when copper was removed by solvent extraction, and the top surface appeared free of precipitated iron salts. A marked decrease in ore permeability became evident when copper was removed by cementation and the percolation rate dropped to only 1.9 gallons per square foot per day after 162 days of leaching. The precipitation of iron salts and the high amount of fines in the ore impeded the percolation rate. Ponding was severe, and the deposition of iron compounds on the surface was quite apparent. No increase in the amount of minus ½-inch fines was evident by the residue screen analyses; therefore, the decrease in permeability was attributed to the deposition of iron salts on the ore surface. The flow rate was manually controlled to maintain a 2- to 3-inch-deep pond on top of the ore bed. The maximum percolation rate was cyclic, as shown in figure 7. The cyclic behavior was believed due to solution breaking through a thin layer of iron precipitate, followed by plugging caused by continued precipitation.
Bacterial Activity
The negligible leach liquor ferrous iron concentration, when solvent extraction was used, indicated effective bacterial activity within the ore. Gas samples taken deep within the ore column contained 20 percent oxygen, a condition necessary for good bacterial activity.
The high ferrous iron content during the latter part of the leach- cementation test reflected a decline in bacterial activity. A gas sample taken from within the ore column on the 150th day of leaching, when bacterial activity appeared low, contained only 4.2 percent oxygen. Thus, poor
bacterial activity was attributed to oxygen starvation caused by poor permeability in the ore bed. The copper leaching rate decreased because of the low concentration of ferric iron in the leach liquor.
Conclusions
Comparative leaching tests were made to determine copper extractions from low-grade chalcocitic ore using either cementation or solvent extraction-electrowinning methods to recover copper from the acidic leach liquors. The copper recovery system had little, if any, effect on copper extraction using relatively coarse ore containing 11 percent minus 0.5-inch fines. After 416 days of leaching, copper extractions were 48 and 51 percent, respectively, when cementation and solvent extraction were used. Solvent extraction-electowinning, however, enabled better copper extraction from ore containing 32 percent minus 0.5-inch fines. Copper extractions in comparative tests after 568 days of leaching were 48 and 60 percent, respectively, when cementation and solvent extraction were used. Cementation of the copper, unlike solvent extraction, contributed additional iron to the system; the cementation iron, in addition to iron leached from the ore, precipitated in the least permeable ore bed and markedly reduced the flow of leach solution. Plugging of the ore bed inhibited the oxidation of ferrous iron to ferric iron, the oxidant needed for dissolution of copper from the sulfide minerals.
Poor conversion of ferrous iron to ferric iron within the bed was attributed to the arresting of aerobic bacterial action by oxygen starvation in the poorly permeable ore bed.
Consumption of sulfuric acid, necessary to maintain a leach liquor pH of 2, was minimized by using solvent extraction and electrowinning for copper recovery. Cementation contributed additional iron to the system, and oxidation of this iron required additional acid for pH control.
