Table of Contents
Zinc-base die-cast scrap represents the largest single source of secondary zinc. A large part of the die-cast scrap originates from grills, fuel pumps, carburetors, trim, and hardware from scrapped automobiles and other machinery, or from punchings or factory rejects of these components. A typical mixture of die-cast scrap contains from 3 to 5 percent aluminum and 1 to 3 percent copper. In recent years, however, there has been a tendency to replace the copper with considerably smaller amounts of magnesium.
Because of its aluminum content, sweated metal from this type of scrap is unsuitable for galvanizing, alloying in many brasses, or manufacture of zinc dust. Present practice in refining die-cast scrap involves distillation in a retort made of silicon carbide and graphite. Die-cast scrap is not usually distilled by itself but is charged into the retort along with a roughly equivalent quantity of galvanizer’s dross. An important factor in the processing cost is the limited life of the retorts. After approximately 40 batches have been distilled, the retort must be replaced because of damages resulting from repeated removal of solid distillation residues. Other disadvantages of distillation methods are the high temperatures required and the fuel necessary to furnish the heat of vaporization. A more desirable process would operate at a much lower temperature with only a small fraction of present thermal requirements.
Experimental work has been performed by the Bureau of Mines on development of processes for removal of aluminum from molten zinc-base die-cast scrap employing low-cost fluxes. The metallurgical principle involved is that a reactive metal impurity can be removed from a less reactive matrix metal by melting under a flux consisting of a salt or oxide of a metal less reactive than the impurity. A replacement reaction occurs in which the less reactive salt or oxide becomes reduced to the metallic form, and the more reactive metal impurity takes its place as an oxide or salt. For the reaction to proceed spontaneously, it is necessary that the thermodynamic relationships be such that the overall change in free energy be represented by a large negative number. If the change in free energy is only slightly negative the reaction may be made feasible by continuous removal of one of the products of reaction. While theoretically feasible from a thermodynamic point of view, many refining salts or oxides cannot be used because their physical characteristics, such as melting point or vapor pressure, make them unsuitable at the melting point of the metal to be refined. These characteristics can be modified, however, by the addition of other salts. Thus a high-melting-point refining oxide may be converted into a liquid flux by dissolving it in one or more low-melting-point salts. Similarly, a refining salt, whose boiling point may be below the melting point of the metal to be refined, might be modified by the addition of another salt, thereby lowering the vapor pressure of the refining salt. These two techniques were used in the present work.
The three separate flux systems that were investigated during the course of the investigation are listed below with the active components of each underlined:
System 1……FeCl3 + NaCl.
System 2…..Fe2O3 + CaCl2 + NaCl.
System 3…. Spent sal skimmings (essentially a mixture of ZnCl2, NH4Cl, and ZnO).
System 1— FeCl3 + NaCl
Theoretical Considerations
Ferric chloride was a logical choice as the first flux investigated for the removal of aluminum from molten zinc-base die-cast scrap because information available on the three-component system Fe-Al-Zn indicates that iron and aluminum are capable of forming intermetallic compounds of low solubility in zinc. If ferric chloride (FeCl3) could be made to react with the aluminum in a molten Zn-Al alloy to form volatile aluminum chloride (AlCl3) and free iron, then the latter would be available to combine with more aluminum to form inter-metallic crystals that could be filtered away from the residual molten zinc. Thermodynamic data appeared to be favorable for the first stage of the reaction given by the following equation;
2 FeCl3 + 2 Al → 2 Fe + 2 AlCl3.
The iron set free could react with more aluminum to form a solid intermetallic compound according to this equation:
2 Fe + 5 Al → Fe2Al5
Upon adding the two equations and canceling out the Fe terms appearing on opposite sides, the following hypothetical overall equation is obtained:
2 FeCl3 + 7 Al → Fe2Al5 + 2 AlCl3
If this process could be made to work, only 0.286 mole of FeCl3 would be required to remove 1 mole of aluminum. Thus, it can be seen, that both elements of the metal salt are active in removing aluminum. A patent has been granted on this process.
Experimental Procedure and Equipment
One practical consideration argues against the success of using FeCl3 as a flux; this salt boils at 315° C, and zinc die-cast alloy melts at approximately 400° C. At first glance, this would appear to necessitate conducting the reaction under a pressure of more than 1 atmosphere. However, two techniques were evolved that made it possible to maintain FeCl3 in close contact with the molten alloy at atmospheric pressure.
The first method comprises the addition of suitable salts to depress the vapor pressure of FeCl3. Sodium, potassium, and calcium chlorides, or mixtures of these salts, have been found satisfactory. A mixture of 3 parts FeCl3 to 1 part NaCl will form a highly fluid flux at the melting point of zinc die-cast alloy with a minimized risk of loss of FeCl3 by vaporization.
In a typical test procedure 1,000 grams of zinc-base die-cast alloy containing 4.2 percent aluminum are melted at 450° C in a fused silica crucible heated by a resistance-wound pot furnace. Temperature is regulated by a Chromel-Alumel thermocouple and automatic controller. A mixture of from 58 to 70 grams of FeCl3 and about one-third of this much NaCl is stirred into the melt using a motor driven glass agitator. The salts begin to melt almost immediately and form a liquid flux covering the surface of the melt. Fumes of AlCl3, are given off and because of the exothermic nature of the reaction, the temperature rises approximately 150° C. Rough thermochemical calculations indicate a theoretical rise of 300° C assuming no loss of heat from the system. After the initial increase, the temperature is allowed to drop back to 450° C. After approximately 90 minutes fluxing time, the end of the reaction is signaled by the cessation of fuming and the solidification of the remaining flux. The intermetallic compound, believed to be Fe2Al5 and being lighter than the molten zinc, tends to float as a mush on top of the melt. The metal is poured into a mold, allowed to cool and the remaining flux removed.
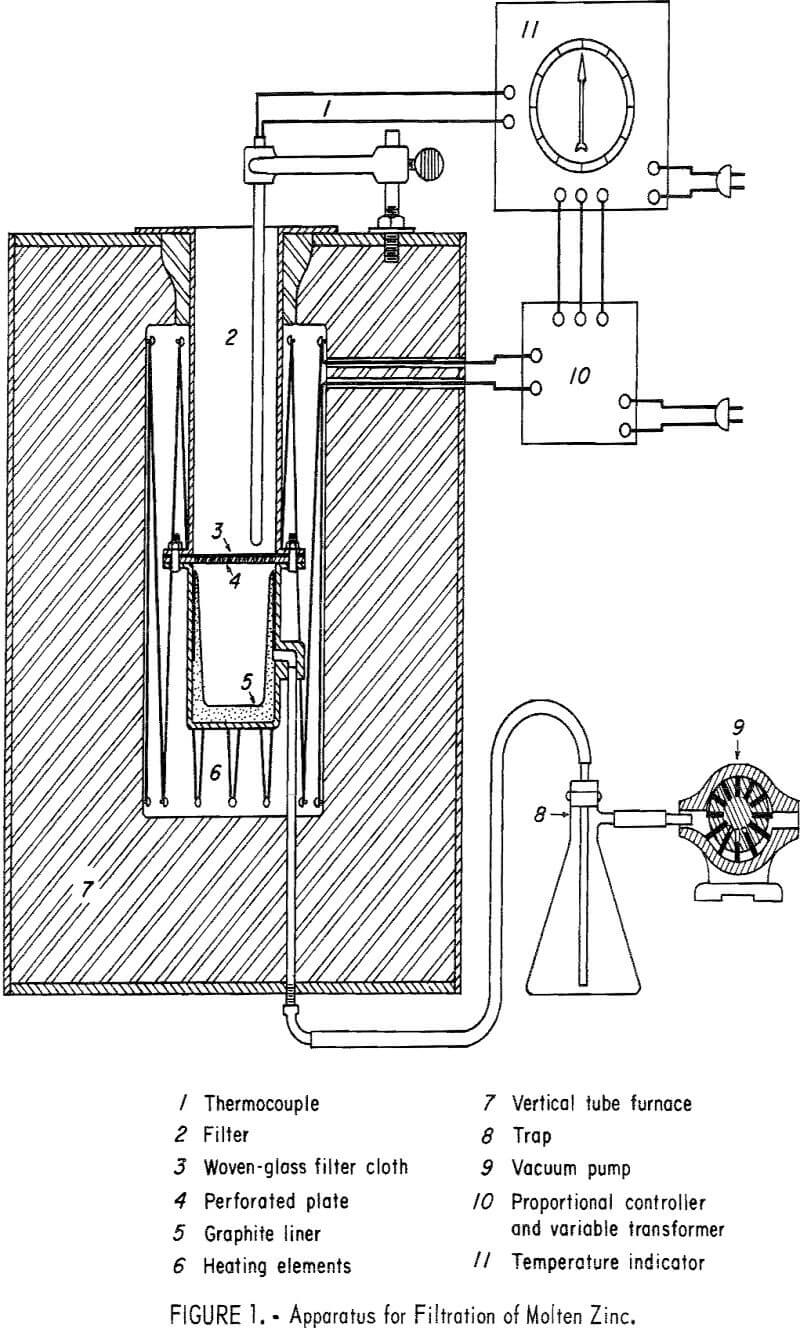
The metal is then transferred to a special apparatus where it is remelted and filtered. The filter (fig. 1) consists of an upper chamber, the bottom of which comprises a perforated plate covered by a glass fabric cloth. The lower chamber, a graphite-lined receiver capable of being evacuated, is attached to the upper chamber by bolted flanges. The entire apparatus is held within an electrically heated vertical tube furnace. In operation, the melt from the fluxing step is placed in the upper chamber and the furnace temperature set between 500° and 600° C. After 60 minutes holding time, a vacuum pump connected to the receiver is switched on, and the liquid phase is pulled through the glass cloth and into the receiver. The Fe-Al intermetallic compound along with some entrapped zinc remains on the cloth as a filter residue. The aluminum content of the alloy may be lowered to several tenths of 1 percent by this procedure.
Results
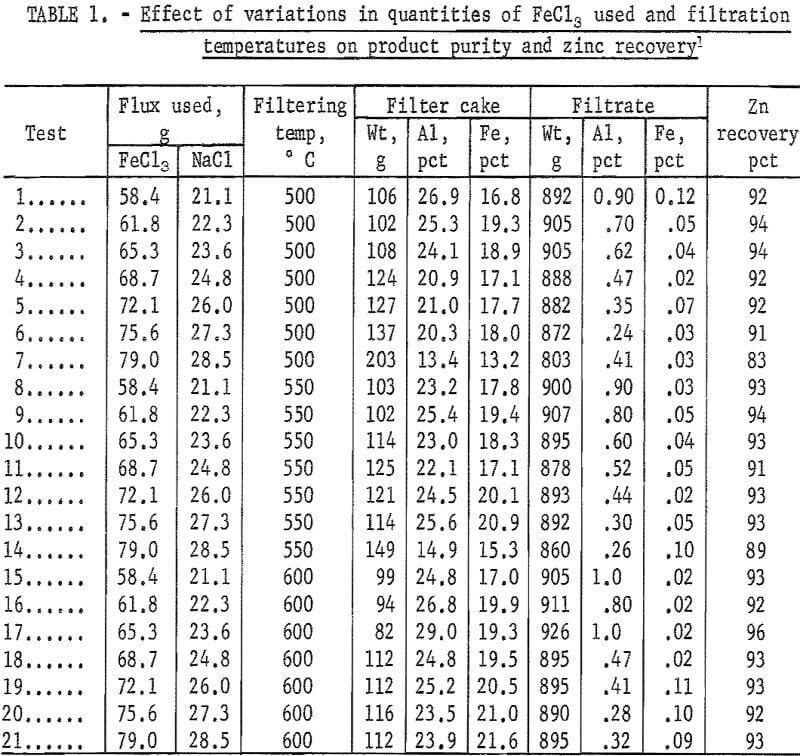
The results of 21 tests showing the effects of varying the quantities of FeCl3 used and the temperature of filtration are summarized in table 1. From the standpoint of best aluminum removal consistent with good zinc recovery, the optimum quantity of FeCl3 appears to be 75.6 parts per 1,000 parts of die-cast alloy of 4.2 percent Al content. This is shown in tests 6, 13, and 20. There is some indication that higher filtration temperatures than 550° C resulted in greater iron contents in the filtrates.
The small amount of aluminum remaining in the filtrate can be removed if desired by a further fluxing treatment with ZnCl2. The zinc remaining in the filter cake can be recovered by either conventional atmospheric pressure distillation or vacuum distillation.
Should this process be performed on a large scale, the exothermic nature of the fluxing operation would make it advisable to add the flux in increments to the molten alloy while simultaneously making further alloy additions in the form of cold ingots in order to maintain the temperature at a constant level.
In the filtration step of the process other filtering media than glass cloth could be used. Woven asbestos, metallic screen, porous carbon, crushed refractory material, or stone might be substituted. Means other than filtration could be employed to separate the Fe-Al intermetallic compound. Simple skimming at some loss in efficiency could be performed. Centrifugation could also be used as a separation technique.
Alternate Procedure
The second method of avoiding loss of FeCl3 through evaporation is to make up pellets of this salt by a briquetting operation and submerge them below the surface of the molten die-cast alloy. A flux cover comprising a eutectic mixture of NaCl and CaCl2 is maintained over the liquid metal. This accomplishes the dual purpose of absorbing any unreacted FeCl3 vapor bubbling to the surface as well as protecting the metal from oxidation. Having a lower specific gravity than the molten metal, the pellets are held submerged by means of a perforated inverted cup and attached rod, both made of graphite. Only one test was made using this technique; 1,000 grams of zinc-base die-cast alloy containing 4.13 percent Al were melted at 555° C and covered with a flux containing 31.7 grams of NaCl and 68.3 grams of CaCl2. The above ratio of salts, close to the eutectic, was molten at this temperature. Cylindrical-shaped FeCl3 pellets weighing 4 grams each were made up in a press. While slowly stirring the melt, the pellets were held one at a time below the surface by means of a perforated inverted cup. A total weight of 68.7 grams of pellets was evaporated into the melt over an interval of 145 minutes. The melt was then cooled and the flux removed. It was then reheated in the filter for 1 hour at 500° C and filtered as before. A total of 872 grams of filtrate containing 0.25 percent Al and 0.06 percent Fe was obtained. Filter residue amounted to 110.8 grams containing 22.7 percent Al and 19.6 percent Fe. Zinc recovery was 91 percent.
Distillation of FeCl3 into the molten metal was also considered in the present work. Attempts to externally vaporize FeCl3 in a closed retort and introduce the vapor below the surface of the melt through a tube were unsuccessful, however, possibly because of the decomposition of FeCl3 into Cl and FeCl2. FeCl2 has a much higher melting point than FeCl3 thus it tends to solidify and block the tube.
System 2—Fe2O3 + CaCl2 + NaCl
Efforts were made to develop a flux composition containing low-cost Fe2O3 which would be suitable for removing aluminum from scrap zinc-base die-cast alloy. Ideally the reaction would proceed according to the theoretical equation:
7 Al + Fe2O3 → Fe2Al5 + Al2O3
The Fe2Al5, existing as a high-melting-point compound, could then be filtered away from the remaining molten zinc as in the previous FeCl3 flux process. The problem was to find some solubilizing agent that was capable of converting Fe2O3 into a liquid flux at the melting temperature of zinc. A number of various salts singly and in combination were explored in an attempt to at least partially solubilize Fe2O3. Limited success was attained with a flux composed of Fe2O3 and a eutectic mixture of 2 parts CaCl2 to 1 part NaCl. Several tests were made in which this flux mixture was reacted with molten die-cast alloy containing 4.0 percent Al. After removal of the flux and the customary filtration procedure, it was found that slightly more than half the aluminum originally present in the alloy was removed, and an end product containing 1.9 percent Al was obtained. Lack of time prohibited further investigation of this flux system, but it is possible that further experimental efforts might improve on the degree of refining.
System 3-Spent Sal Skimmings
Theoretical Considerations
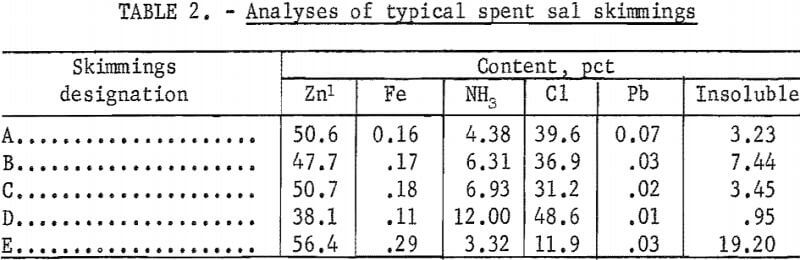
Depleted sal skimmings, a waste product of the galvanizing industry, were found the most effective of the three flux systems studied for removing aluminum from molten die-cast alloy. This material consists of the residual zinc ammonium chloride salt (ZnCl2·3NH4Cl) flux, after it has been used for some time and has lost its effectiveness in protecting the surface of the zinc bath employed in the hot dip galvanizing process. As the flux becomes depleted some NH3Cl is lost by sublimation and ZnO, generated by oxidation of the molten metal, starts to build up. The chemical composition of spent skimmings therefore consists primarily of ZnCl2, lesser amounts of ZnO and NH3Cl along with minor quantities of insoluble siliceous materials, and trace amounts of lead and iron. In addition from 2 to 5 percent free metallic zinc in the form of prills and shot is usually present. Analyses of typical spent sal skimmings are given in table 2.
If sal skimmings were used in an effort to remove aluminum from die-cast alloy, ZnCl2 would be expected to be the active component. The principle reaction mechanism is believed to be 2 Al + 3 ZnCl2 → 3 Zn + 2 AlCl3. However, there are probably other side reactions occurring simultaneously that are not as yet completely understood. When tests were made using varied ratios of spent sal skimmings to die-cast alloy in order to establish optimum conditions, it was found that a Cl-to-Al molar ratio of less than 3 satisfied the requirements of virtually complete removal of aluminum from the die-cast alloy. This would appear to indicate that a portion of the aluminum was being tied up in some form other than AlCl3. The compound AlOCl was identified in the ash remaining after the fluxing operation, and this could account in part for fewer than 3 moles of Cl in the flux being sufficient to remove 1 mole of Al present in the alloy.
The residual ash after fluxing is not completely free of chlorine because of the formation of AlOCl and also because the AlCl3 probably does not entirely evaporate from the salt phase. If some way could be found to reduce the chlorine content of the residual ash down to 2 percent or less, then this material, because of its high zinc oxide content, might be marketable as a feed for primary zinc smelting.
Experimental Procedure and Equipment
Preliminary fluxing tests were carried out using 500-gram charges of die-cast scrap. The operation was conducted in graphite crucibles contained within an electrically heated resistance furnace. Temperature was regulated by means of a Chromel-Alumel thermocouple and automatic controller. Because of the copious fumes of AlCl3 evolved during the reaction, the tests were conducted under an exhaust hood.
The experimental procedure consists of heating the metal charge to melting temperature, adding the required amount of sal skimmings, and continuously stirring with a motor-driven graphite agitator. Initially, the spent skimmings liquefy and entirely cover the surface of metal. Zinc prills and shot present in the flux melt and in doing so add to the overall metal recovery. As in FeCl2 flux, a temperature rise is noted at the beginning. As the reaction proceeds, ZnCl2 is consumed, and the flux loses its liquid character, becoming more viscous. Eventually, the flux tends to dry up as the ratio of ZnO to ZnCl2 increases. The liquid-liquid interface present at the beginning of the reaction is lost. At this time the end of the reaction can also be noted by a diminution of AlCl3 fuming. The crucible is then removed from the furnace and the metal poured into a mold. The residual ash is scraped out of the crucible, weighed, and analyzed along with the metal.
Results
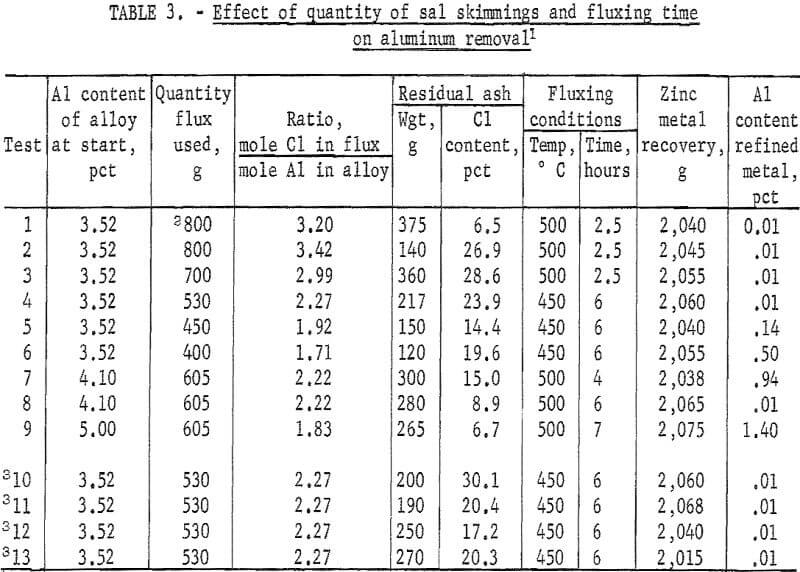
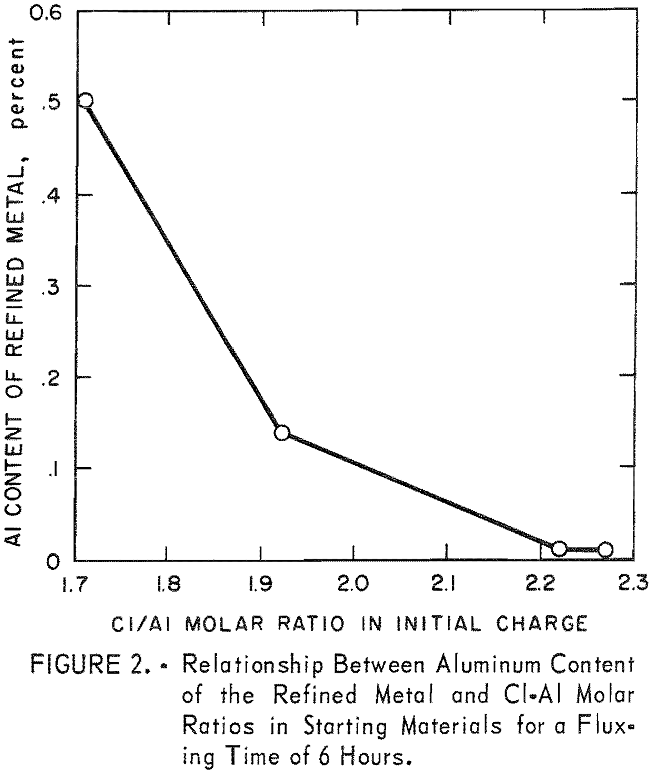
It was determined from these initial tests that aluminum content of the alloy could be reduced to 0.01 percent. In view of these promising results, a larger gas-fired furnace was constructed and tests performed using 2,000-gram metal charges. Results of these experiments are given in table 3. Of particular interest is the fact that the weight of metal recovered at the end of the test is greater than that at the beginning despite aluminum having been removed. This is due to the recovery of metallic zinc from both the free zinc and ZnCl2 contents of the sal skimmings. The relationship between aluminum content of the refined metal and Cl-to-Al molar ratios in the starting materials for a fluxing time of 6 hours is shown in figure 2.
Conclusions
Flux refining has the following advantages over current methods for removing aluminum from zinc-base die-cast scrap:
- The operation is relatively simple and does not require elaborate or expensive equipment.
- Temperatures involved are relatively low and must be maintained for only short periods.
- Part of the heat requirements is furnished by the exothermic nature of the fluxing operation.
- Less energy is needed to remove by flux refining the small amount of aluminum present in die-cast alloy than that required to distill off the entire zinc content.
Also, the choice of spent sal skimmings over the other flux compositions mentioned in this report might offer the following benefits:
- Sal skimmings are a low-cost waste product.
- No metal filtration step is necessary,,
- The aluminum content of the refined die~cast metal is reduced down to the trace range.
- Metallic zinc is recovered from both the free zinc and zinc chloride in the sal skimmings.
- Removal of the residual chlorine in the ash remaining after the fluxing operation could convert this material into a marketable product suitable for use as a feed for primary zinc smelting.
Future research possibilities growing out of experimental work done thus far on flux refining with spent sal skimmings include the following:
- Testing an electrostatic precipitator to recover aluminum chloride from the fumes.
- Determining the effects of small amounts of copper on the properties of the zinc product, when it is used for various commercial applications.
- Producing a zinc oxide concentrate by removal of excess chlorine from residual ash.
