Table of Contents
This cyanide precipitate is unique in that it is apt to vary widely in composition in the course of very short periods of time, and a method of refining and melting that would prove highly satisfactory at one cleanup would yield almost a hopeless “mess” at a second. Table I gives a general conception of the wide range in grade and composition of those raw precipitates. It is evident that no one standard flux could be expected to produce a high-grade bullion with such a widely variable precipitate, and that any method of treatment with acid requires most careful consideration.
Refining by Acids
Naturally, our first efforts to improve our precipitates were directed along the lines of acid treatment. Table II embodies the results of careful sampling and analysis of one cleanup at the time when wet refining reached its maximum development at tins mill. We have now discarded all methods of wet refining, successfully replacing them by a more scientific method of flux calculation described herein, but it is believed that a general description of this wet refining is warranted as it throws some interesting sidelights on acid refining in general.

Column A gives the analysis of the raw precipitate; column B, that of the precipitate after heating with steam, with frequent addition of hydrochloric acid until a point was reached where the batch had a strong tendency to remain acid. The bath was then diluted with hot water, and repeatedly washed by decantation, until the liquor by test indicated that all soluble lime salts were removed. After the last decantation, commercial sulphuric acid was added, the bath heated by steam, and agitated with air until all action ceased. The bath was then diluted with hot water, and repeatedly washed by decantation, until by test all soluble zinc salts had been removed.
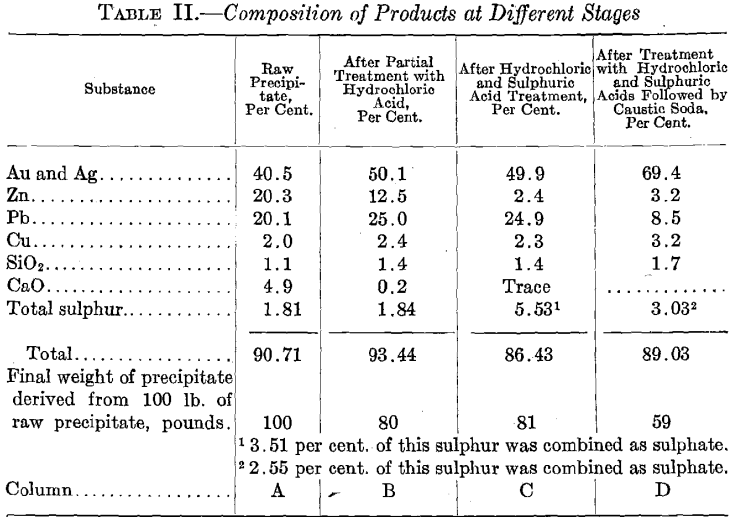
Column C gives the analysis of the precipitate after the sulphuric acid treatment. This analysis is unique. We note first a slight decrease in the grade of the precipitate in spite of the fact that a large portion of the zinc has been removed. The increase in total sulphur content is indicative, as it shows how well finely divided lead reacts with sulphuric acid to form lead sulphate. In this case a large proportion of lead sulphate was formed, thereby actually lowering the grade of the precipitate in spite of the removal of zinc. This explains why it often happens in treating with sulphuric acid raw precipitate containing much lime, as well as lead, that a precipitate is obtained actually lower in grade than the original raw precipitate.
Column D shows analysis of the precipitate after being treated by hydrochloric and sulphuric acids and lastly with hot caustic soda. To the residue, after decanting the last sulphuric acid wash, sufficient caustic soda was added to make a strong solution. The batch was then vigorously heated and agitated with steam for 2 hr., diluted with hot water, and repeatedly washed by decantation, until, by test, all soluble lead salts had been removed.
The effect of the caustic soda bath is well shown by the final analysis. The grade was well brought up and a marked decrease in the quantity of lead and sulphur shows the solubility of lead sulphate in hot caustic soda.
Refining by Fusion
The present method of flux refining has been arrived at through a vast amount of experiment. In general, it embodies the principle of the addition of an oxidizer, and the proper proportionment of fluxes to carry off the oxidized impurities as metallic bases in a fluid slag.
A rather wide experience in the melting of precipitates has shown that the various impurities of precipitates are oxidized and driven into the slag in a definite order. Zinc tends to oxidize first, and the resulting zinc oxide will combine with the acid radical of the flux and therefore enter the slag. As in blast-furnace practice, the zinc tends to render slags infusible; and for this reason borax glass is always used in a greater amount than silica to furnish the acid radical for the slag. Borax glass greatly increases the fusibility of zinky slags.
Sulphur tends to oxidize second, and if an alkaline oxide is present in the melt, alkaline sulphate is formed, which is rather easily fusible. This sulphate is not soluble in the slag, and, being lighter, rises to the surface of the melt, forming the well-known sulphate “cover.” The sulphate “cover” is nearly always quite pure, so that after any melt the melter may easily separate it from the slag, and, by weighing and computing its sulphur content, obtain a close check on the analysis of the precipitate. This cover is either Na2SO4 or K2SO4 depending on the alkaline oxide present. (The sulphate cover is an intensely interesting material to the chemist. Many unsuspected elements are known to concentrate therein. In the Liberty Bell material, Mo, As, Sb, Te, Se, and V have been detected.) Where precipitate is melted without oxidizers, sulphur generally makes itself known by the formation of matte—a very undesirable byproduct.
After all sulphur is oxidized, lead will oxidize, and the resulting lead oxide enters the slag proper. It is well known that lead oxide combines with silica in nearly all proportions under the influence of heat, and that the resulting lead silicates are easily fusible between rather wide limits. For this reason more silica may be used in the melt when much lead is present, and for the same reason the amount of borax glass may be diminished.
After lead, copper will oxidize to Cu2O and combine with the acid radical of the slag.. The color of such slags is red. Where an excessive amount of oxidizer is present, copper will oxidize to CuO, and slag containing this oxide is green.
This selective oxidation is most clearly evident in the order of oxidation between copper and lead. It is practically impossible to drive copper into the slag as long as metallic lead is present in the bullion. Here quantitative separations between lead and copper are easily obtained.
Graphite crucibles have a marked reducing action on lead slags. No matter how rapid the melting, or how fast the resulting slag is removed from the crucible, if much lead is present, some will be reduced to metal. In such cases it is practically impossible, in direct melting, to slag copper, even though oxidizers are added far beyond the calculated requirement.
Where melts are conducted in clay or clay-lined graphite crucibles, this reducing action is of course avoided, and as a result copper will be found to enter the slag as easily as lead, though not until all lead is oxidized. Hence, in melting precipitate in graphite crucibles no attempt is made to slag copper. If a desirable grade of bullion can not be made unless copper is slagged, the melter will then, of course, use clay or clay-lined crucibles.
As an illustration of this, I give the data regarding a melt of precipitate containing a rather high amount of copper. This precipitate had received hydrochloric acid treatment only, and gave upon analysis the following composition:

All of this precipitate having been fluxed alike with an oxidizing flux, equal portions were melted down in graphite and clay-lined crucibles. The resulting bullion from the clay-lined crucible shows 982 total fineness, while the bullion from the graphite crucible showed 812. I should add that, up to date, we have not found an entirely satisfactory clay or clay-lined crucible. At best they last one heat.
Fluxes—The common oxidizing fluxes found on the market are manganese dioxide, potassium and sodium nitrate. Manganese dioxide can be obtained quite pure but at best it is costly because of its comparatively low quantity of available oxygen. It also requires extra weight of acid flux to retain the MnO in the slag. However, it gives satisfactory results. Potassium nitrate is well known and needs but passing notice. Sodium nitrate is highly recommended and deserves special mention. It is very high in available oxygen and the crude product from Chile can be obtained practically pure so far as fluxing is concerned. It is laid down at Telluride at less than one-fifth the cost of potassium nitrate, and is one of the very cheapest fluxes on the market. It is also metallurgically more to be desired in the fluxing of sulphur, the resulting sodium sulphate being decidedly more fusible than the corresponding potassium salt and as a result the separations are better. The reaction between sodium nitrate and sulphur is 2NaNO3 + S = Na2SO4 + 2 NO. Sodium carbonate or soda-ash is more desirable than the bicarbonate, since it goes considerably farther weight for weight as a flux, and lowers the freight bill. We use this flux only to supply alkali for the sulphur in the melt, when manganese dioxide is the oxidizer. Silica is an excellent acid flux, though its use must be guarded. Slags high in silica are not well adapted to the melting of precipitate, because of the higher temperature they require, especially in the presence of zinc. By reason of its cheapness, it is desirable to use as much silica as possible to replace borax in the melt, but our experience leads us never to go below a ratio of borax glass to silica in the slag of 2 to 1, where much zinc is present, or below a ratio of 1 to 1, even, where much lead is to be slagged.
In Table III, I have embodied results consistent with our practice and have arranged the same for convenience and rapidity of flux calculation.
Explanation of Table III.—In the substance column, I have placed the common impurities of the precipitate as well as the more frequently used fluxes. Column 1 contains the molecular weights of the substances in round numbers. Columns 2, 3 and 4, I have labelled “Per Cent. Oxygen.” These columns indicate the weight of oxygen that the substance will combine with or evolve in per cent, of the original weight of substance. The terms “acid,” and “base” oxygen I use for brevity. This is an effort to distinguish between oxygen that will enter the acid or basic part of the slag. The metallurgist‘s conception of a slag is that it is a fusible mixture of definite chemical combination between various acid and basic oxides. By “available oxygen” is meant the quantity of oxygen which the substance will evolve and which will be available in oxidizing metals or other impurities in the precipitate.
Column 5 gives the weight of manganese dioxide required to oxidize a unit weight of the substance on the same line in Column 1. These figures are derived from the chemical equation:
2MnO2 = 2MnO + O2
With pure manganese dioxide these figures represent approximately the amount of oxidation obtained in practice.

Columns 6 and 7 are similar to Column 5; they represent the weights of KNO3 and NaNO3 required to oxidize a unit weight of substance. The figures are derived from the equations:
2KNO3 = K2O + 2NO + 3O
and
2NaNO3 = Na2O + 2NO + 3O
Authorities seem to agree that these equations represent the breaking up of the niters in presence of an oxidizable substance with heat; and I find that this agrees well with practice, where the melts are conducted in graphite crucibles. It has been frequently noted, however, that, when the melts are conducted in clay or clay-lined crucibles, the oxidizing power of the niters is markedly higher than is indicated by these equations; the reason being that graphite has a reducing action on slags. When clay or clay-lined crucibles are used, about 80 per cent, of the niter called for by the above equations will give the same oxidation in practice.
Columns 8 and 9 give the weight in pounds of potassium carbonate or sodium carbonate required to form potassium sulphate or sodium sulphate with 1 lb. of sulphur which has been oxidized to SO3. Thus K2CO3 + SO3 = K2SO4 + CO2. We use these carbonates only when manganese dioxide is the sole oxidizer.
Column 10 indicates the quantity of available “acid” oxygen in mixtures of borax and silica. This table could be carried out infinitely but I have only listed mixtures that experience and practice indicate to be feasible.
In “Flux Calculation, Case I,” given in the appendix, I have gone through the regular flux calculation with some detail. For the type precipitate I have taken the lowest grade of raw precipitate that we have yet melted.
The analysis of this precipitate was:

It will be noticed that I use a type slag of 2 of acid oxygen to 1 of basic oxygen. This is the result of experience. Such slags have just a noticeable tendency to string and are the best, most easily fusible, and least corrosive to the crucible. They are invariably of low grade, and have been made repeatedly as low as $20 per ton in precious metal. This type of slag has been found by far the most desirable, and we now make it as a regular practice, irrespective of the composition of the precipitate or fluxes used.
Since 100 lb. of precipitate was assumed at the start, 92 per cent, of this flux was added to the estimated dry weight of precipitate. It is interesting to note that this melt gave a resulting bullion fineness of 972 total fine. As we assumed that all the copper would go into the bullion, and that the balance of the impurities and base metals were removed in the slag we did expect a bullion of 25 ÷ (25 + 1.4) x 1,000 = 950 total fine, approximately. This melt, of course, was conducted in a graphite crucible.
“Flux Calculation, Case No. 2” was the calculation for the precipitate treated with caustic soda, listed in Column D, Table II. The bullion made was 942 total fine, which is in remarkable accord with the calculated fineness, assuming that all copper went into the bullion, as 69.4 ÷ (69.4 + 3.2) x 1,000 = 955 total fine.
The calculation shows the use of soda ash and manganese dioxide.
It is also interesting that a distinction was made between sulphide and sulphate sulphur.
Sintering
After removing the precipitate from the press the computed flux is added and the whole well mixed while wet. The mixture is then loaded into cast-iron pans, and placed in a large cast-iron muffle furnace; and the temperature of the furnace is gradually raised to a red heat. In this operation moisture is removed, and the precipitate gradually agglomerates and settles to about one-third of its original volume. During this process practically all the chemical reactions are completed and the mass sinters to a point where subsequent “dusting” is avoided.
When sintered the charge is taken from the furnace and cooled, and is easily removed from the pans. This gives an excellent product for crucible melting. In one case, where a precipitate containing 90 per cent, of gold and silver was obtained by acid-treatment, the melting of over 11,000 oz. of fine bullion was done in 4 hr. in one No. 275 oil-burning tilting-furnace. This included the time taken in starting up the furnace in the cold. With precipitate of average grade, i.e., 65 per cent. Au and Ag, a melting rate of 1,300 oz. of bullion per hour is common.
The advantages gained by sintering are many. The completion of practically all chemical reactions in the muffle furnace prevents any excessive boiling in the crucible, and the crucible may be loaded to the top during the period of melting. The slow application of heat in the muffle furnace gives ample chance for complete chemical reaction. Hence, we arrive, in the use of oxidizers, at practical results which correspond with theory. The great decrease in the volume of the precipitate during sintering enables us to get much more weight in the crucible at a charge, and this again decidedly increases the melting rate. Metal losses during the sintering are exceedingly small. At one time the vents of our muffles were connected to a dust chamber, but nothing was ever recovered; and frequent tests of the gases leaving the muffles, by passing them through water or cotton soaked in a solution of sodium sulphide, failed to show more than traces of valuable metal.
These flux calculations have now been used for a period of 18 months, during which time about 60 melts have been conducted on all grades of raw and acid-treated precipitate; and in no case has there been a failure to realize a close approximation to the grade of bullion calculated.
Slags
The slags vary so widely in composition that at a glance no relationship seems to exist. They all, however, conform to one single relationship: namely, the ratio of “acid” to “base” oxygen is approximately 2:1.
After reviewing the analyses of some 20 slags, I find that the range of the composition of slags may be considered to fall within the following limits. The slags were made from all grades of raw and acid-treated precipitate.

The composition of the sulphate cover remains quite constant.
When soda niter or sodium carbonate are used the sulphur in the sulphate cover is from 20.5 to 21.5 per cent. This is close to the theoretical requirement of sulphur in Na2SO4. When potassium niter is used the sulphur content lies between 17 and 18 per cent., likewise close to the theoretical requirement for sulphur in K2SO4.
As an illustration of the distribution of the bases and compounds in all the products of the melt, I will take Case 1. Here 134 lb. of slag and 5½ lb. of cover were made by actual weights for every 100 lb. of dry precipitate melted.
A partial analysis of this slag gave:

The sulphur in the cover was not determined. If we therefore compute the actual weights of materials in precipitate and flux and in the slag, we arrive at the following figures:

If we assume that the sulphate cover contains an average of 21 per cent, sulphur, the 5.5 lb. of cover would indicate 5.5 x 0.21 = 1.16 lb. sulphur or 1.16 per cent, of dry precipitate. This is a very close check to the 1.2 per cent, obtained by actual analysis; and we thus arrive at results that are entirely satisfactory within the limits of experiments error.

Totals in the original flux calculation as 28.89 : 14.50 or a ratio of approximately 2 to 1.
The cyanide slags are stored, and once a year are melted up in a small blast furnace with brick charges, made from the ash obtained by burning old mill launders and tanks. This furnace which has a capacity of 5 tons of slag and brick a day also handles copper scrap directly, and will deliver about 15 tons of pig copper per day. This local smelting lasts only about 10 days a year. Any matte made is controlled by running the furnace more oxidizingly and thus driving the metals into the bullion and slag. As a result of this the only byproducts shipped are lead bullion and pig copper.
In spite of the seemingly high costs of local smelting, the ultimate gain is greater, by reason of the invariably greater recovery, as compared with that realized by shipping the material away.
