The occurrence and distribution of phosphorus is one of the most important questions with which the steel-maker has to do. Large sums are invested in processes for the removal of this element from ores before they come to the blast-furnace, and much greater sums in freeing the pig-iron from it. Mechanical engineers are becoming more and more critical of the amount of total phosphorus left in structural steel, the tendency being to require that it be reduced to the lowest possible figure. The old maximum of 0.1 per cent, is now considered much too high for first-class structural steel, and demands for 0.03 to 0.01 per cent, are by no means unheard of.
The first to speak, in a scientific way, of the harmful effect of phosphorus on iron and steel appears to have been Meyer, who, in 1781, made mention of what we now term “cold-shortness,” ascribing it to the presence of this metalloid, but not suggesting any remedy. It is, however, doubtful if a clear idea of this subject was possessed by the scientific world until after the invention of the pneumatic or Bessemer process, in 1855-56. One of the chief obstacles in the way of this renowned metallurgist,—one, indeed, the removal of which was the sine qua non of success,—was the phosphorus in the iron blown. The effect of the phosphorus does not seem to have been apprehended until many and costly experiments had been made, and then the required minimum percentage of it in the iron was secured only by the use of low-phosphorus ores. It was not until 1879-80 that practical success was reached, by the Thomas process, in the actual removal of the phosphorus from the iron.
We now know something of the effect of the total amount of phosphorus on iron and steel, but very little as to the effect of the condition of the phosphorus. The state of combination in which this element exists may well influence its action quite as much as the total quantity present. In this respect it may, and probably does, bear a close analogy to carbon; not, indeed, in the particular of existing in the metalloidal state, but in that of a two- or even a three-fold condition, as phosphide, as phosphate, and perhaps as a mixture of the two. But so far from knowing the condition of the phosphorus in the metal, we do not yet know, as we should, its condition in the burden or in the ore. It is well known that the condition of the iron in the ore, whether as ferric, ferrous, or ferroso- ferric oxide, materially affects blast-furnace practice, and extends even to the quality of the iron made. Why may not the same principle obtain in respect of the phosphorus? It is highly probable, indeed one may say it is certain, that in the vast majority of ores phosphorus exists as a phosphate, i.e., in its highest state of oxidation; and we are content to report it as phosphoric acid without regard to the base or bases with which it may be combined. We assume that the ultimate effect of the phosphorus, as well as what may be termed its intermediate effects, will be the same, no matter whether we have to do with phosphate of iron, phosphate of aluminum, phosphate of lime, or mixtures of these, as, for instance, in the reverted phosphates, so important to the agriculturist.
It may not be of so much importance to know the state of combination of the phosphorus in ores well within the Bessemer limit, i.e., with say 50 per cent, of iron and 0.01 to 0.03 per cent, of phosphorus ; but the case appears to me quite otherwise when we come to ores carrying 2.31 and even 5.41 per cent, of phosphorus, as do some of the red fossil-ores (Clinton) of Murphy’s Valley, Jefferson county, Ala. If ores with 60 to 65 per cent, of iron and less than 0.04 per cent, of phosphorus are deservedly remarkable and eagerly sought for, ores lying at the other extreme are no less remarkable. I have examined ores from the locality just mentioned which gave 2.31 and 5.41 per cent of phosphorus, the samples having been taken from the same seam at points 2 miles apart. The seam is somewhat thin (2½ to 3 feet), but it extends over a considerable territory. The iron-content is about 40 per cent. It is the typical ore for the basic Bessemer or Thomas process, in that it could be used as a concentrator for ores less rich in phosphorus but richer in iron. I am not yet able to express an opinion as to the condition in which the phosphorus exists in these ores, except that it is as a phosphate.
Thinking that perhaps a study of the naturally occurring compounds containing phosphorus in the shape of phosphoric acid might

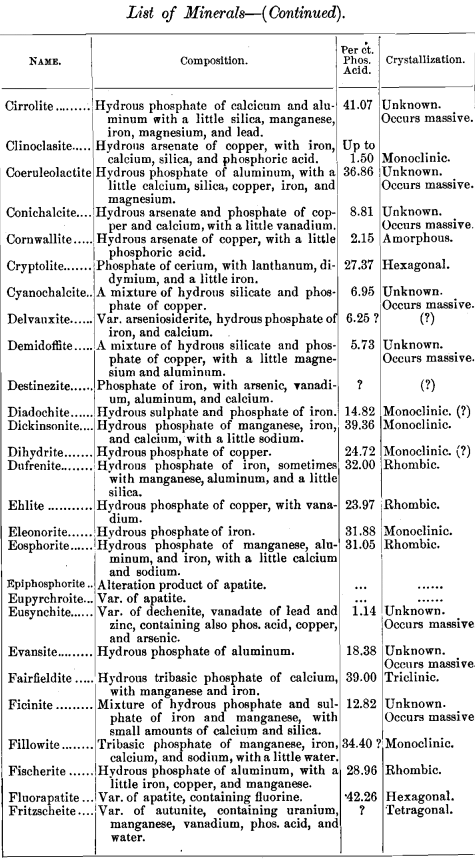
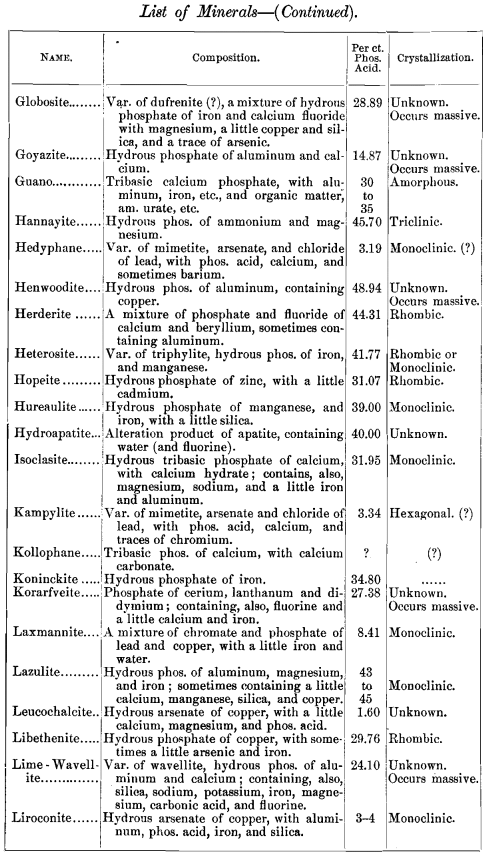
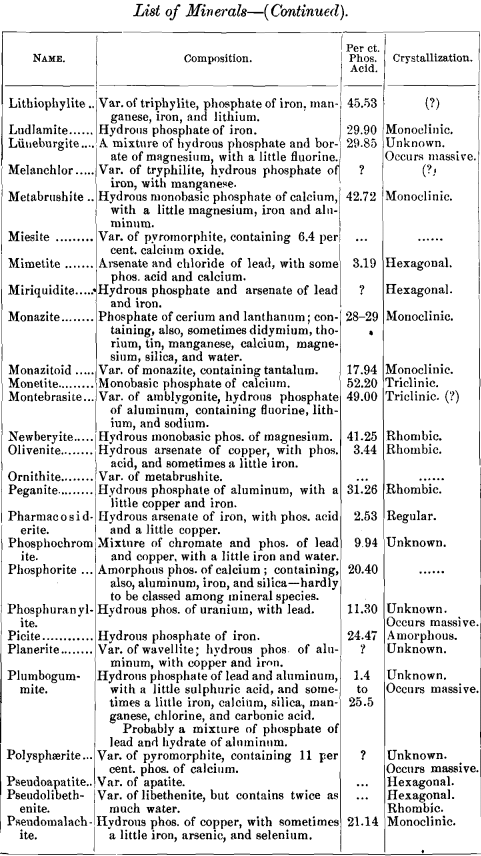
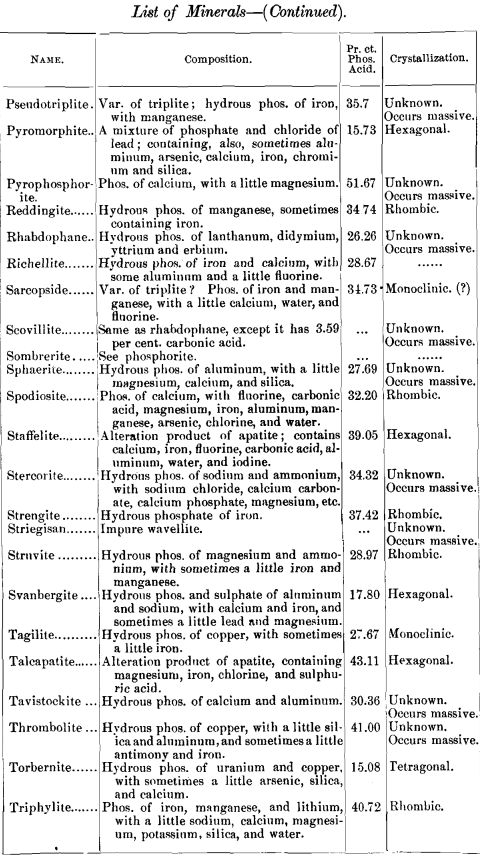
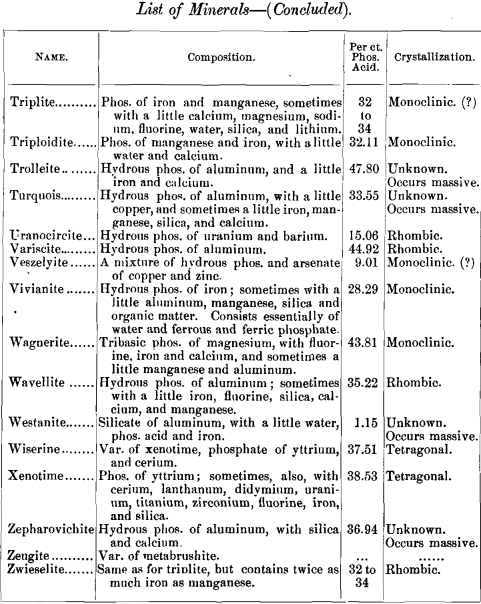
lead to some interesting conclusions, I have prepared a list of minerals carrying one per cent, and over of phosphoric acid. The list includes also the composition of each mineral and its crystal habitus. There are 141, most of them showing no crystal system, but occurring massive. Of those which are crystallized, most belong to the monoclinic system ; next in order being the rhombic, with almost as many as the monoclinic; then the hexagonal, with about half as many as the monoclinic; and at considerable intervals the triclinic and tetragonal, with one (pharmacosiderite) in the regular system.
Any additions or corrections that can be made to the list will be cordially welcomed. It was my intention to discuss some of these minerals more at length, but a protracted illness has prevented all kinds of work, and I submit the list as it is.
The various rock-phosphates, such as South Carolina or Charleston rock, Navassa, Florida, etc., cannot be classed among mineral species. They may be regarded, for the most part, as more or less pure tribasic calcium phosphate, the impurities consisting of iron, aluminum, carbonic acid, silica, etc.
