Table of Contents
- Experimental Procedure and Discussion of Results
- Original and Synthetic Nicaro Materials
- Nickel Sulfate-Cobalt Sulfate Electrolytes
- Nickel Sulfate-Cobalt Sulfate-Ammonium Persulfate-Ammonium Fluoride Electrolytes
- Nickel Carbonate-Cobalt Carbonate Solubility Tests
- Nickel Sulfate-Ammonium Fluoride Electrolytes
- Ammonium Fluoride Electrolytes
- Nickel Chloride-Cobalt Chloride Electrolytes
- Electrolysis
- Electrodialysis
- Synthetic Nicaro Liquor Electrolytes
- Determination of Decomposition Potentials for Nickel and Cobalt Electrolytes
- Cyanide and Phosphate Electrolytes
- Nickel Sulfate-Cobalt Sulfate-Potassium Sulfate-Potassium Dichromate Electrolytes
- Ammonium Sulfate Electrolytes
- Nickel Sulfate – Boric Acid Electrolytes
- Selective Precipitation of Cobalt From Nicaro Liquors
- Selective Precipitation of Nickel From Nicaro Liquors
- Separation by Aeration
Preliminary work on electrolytes of neutral sulfate, neutral chloride, ammoniacal sulfate, and ammoniacal chloride solutions offered little promise for separating cobalt from nickel by the anodic deposition of cobalt peroxide.
Electrolytes composed of synthetic Nicaro liquor, dichromates, cyanides, and phosphates offered little or no promise.
An ammonium sulfate electrolyte showed separation of cobalt from nickel only at low cathode current density and high temperature.
Although leaching with ammonium fluoride extracts nickel preferentially to cobalt from carbonate mixtures, no further purification was achieved by electrodeposition.
Versene complexed nickel preferentially to cobalt at a pH of 9 in Nicaro liquor. Straightforward distillation then resulted in a precipitate of 99 percent cobalt and a filtrate of 97 percent nickel. However, a very large amount of Versene was needed to complex the nickel.
The most successful separation of nickel and cobalt was achieved with the nickel sulfate – boric acid electrolyte. Nickel:cobalt ratios greater than 500:1 were obtained when the cathode and anode current densities were equal and approximately 1 ampere per square decimeter. Nickel containing an average of 0.4 percent cobalt was obtained for 549 ampere-hours of operation, after which the 1-percent-cobalt limit was exceeded. This indicates that further study is needed to determine the specific operating conditions under which Specification-grade nickel can be obtained by the continuous process described.
The best separation of nickel and cobalt was achieved by employing the differential solubility of the two elements in a nickel sulfate-boric acid electrolyte when it was used to leach Nicaro basic carbonate. Nickel containing an average of 0.4 percent cobalt was deposited with a power consumption of 1.3 kw.-hr. per lb. of nickel.
Introduction
During World War II the United States built a nickel plant at Nicaro, Cuba, to augment the supply of this strategic metal. In an effort to increase further the efficiency of the nickel production at the Nicaro plant, the General Services Administration in 1955 initiated research projects with the Federal Bureau of Mines, Battelle Memorial Institute, and the Nickel Processing Corp. This investigation covers only one phase of the research program; it was conducted by the Bureau of Mines at its Northwest Electrodevelopment Experiment Station, Albany, Oreg. The specific purpose of the project was to develop an electrolytic process for recovering Specification-grade nickel from either Nicaro pregnant liquor or basic carbonate precipitate currently in production. These Nicaro products contained nickel and cobalt in the ratio of 64:1. For the purpose of this investigation, “Specification-grade nickel” refers to nickel containing a maximum of 1 percent cobalt. This investigation was conducted along two main lines: (1) Separation of cobalt from nickel solutions, using chemical or electrolytic methods; and (2) selective leaching of nickel from basic carbonate precipitate containing nickel and cobalt, followed by recovery of nickel by electrodeposition.
Over 250 literature references were reviewed pertaining to the separation of nickel and cobalt, with primary emphasis on electrolytic methods; the bibliography at the end of this report is not complete but contains all of the significant references considered in this experimental work. Although many claims have been made for successful separation techniques, there appears to be considerable disagreement as to the feasibility of many of the methods proposed. Most electrolytic separation methods depend on anodic deposition of a hydrated cobaltic oxide. The problem of separating nickel and cobalt electrolytically is by no means new, and efforts to devise a practical method date back several decades.
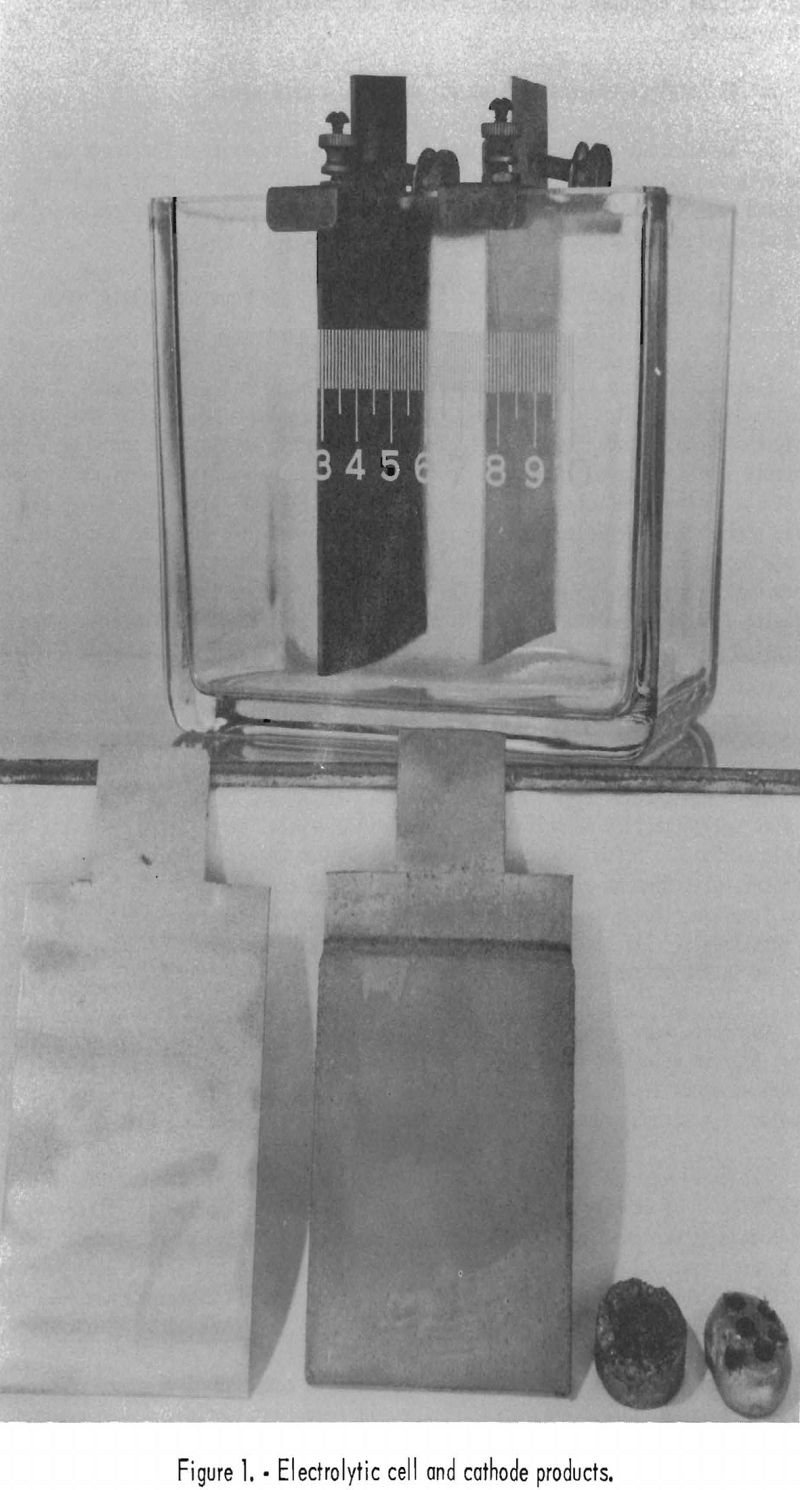
Figure 1 shows a typical electrolytic cell with electrodes (top); a sandblasted aluminum cathode, a nickel-plated cathode, a pressed button of nickel strippings, and a nickel button arc-melted and drilled for sampling (bottom, left to right).
Experimental Procedure and Discussion of Results
Original and Synthetic Nicaro Materials
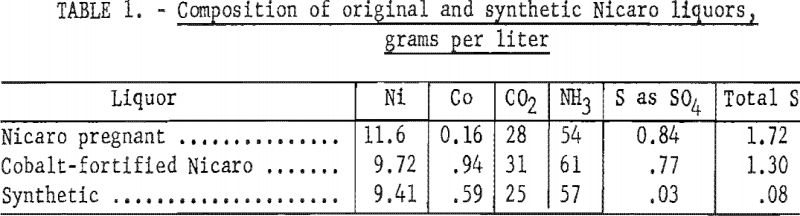
Nicaro pregnant liquor and basic carbonate precipitate were not available at the inception of this project, and materials resembling these products were synthesized to use for exploratory experiments. As a nickel and cobalt separation process in the Nicaro circuit might be included under conditions that would yield a higher recovery of cobalt, a cobalt-fortified Nicaro pregnant liquor and bulk precipitate were prepared. The composition of the Nicaro and synthetic materials to which reference is made in this text is shown in tables 1 and 2.
Nickel Sulfate-Cobalt Sulfate Electrolytes
Deposition of cobalt as a peroxide at the anode from a neutral solution of nickel sulfate and cobalt sulfate has been claimed in a patent by Coehn and Salomon. Several tests were made in an effort to separate cobalt from nickel by such a method.
The following procedure was used in all electrolytic tests in this section:
- The electrolyte was regenerated by circulating it through a synthetic Nicaro mixture, composed of nickel carbonate and cobalt carbonate in the ratio of 94:6 by weight.
- Electrolyte temperatures of 25° and 50° C. were used.
- An aluminum cathode was spaced 5 cm. from a lead anode. The anode was used with and without bags of Dynel filter cloth to determine if cobalt could be oxidized more easily at the anode. The lead anode was cleaned cathodically and a film of lead dioxide formed anodically in sulfuric acid.
- Cathode current densities of 1 and 2 amperes per square decimeter were used.
Electrolysis of a solution containing 94 grams of nickel sulfate and 6 grams of cobalt sulfate per liter failed to produce an anode deposit. Qualitative spectrographs analysis of the cathode deposit indicated over 5 percent cobalt. In the next 6 electrolytic tests, 25 grams of ammonium sulfate per liter was added to increase the conductivity of the electrolyte. The results were the same as those obtained in the first test. The electrolyte was made strongly ammoniacal for the next experiment. It was impossible to regenerate this electrolyte with carbonates, but no serious effects were noted from depletion of the electrolyte. Because there were no anode deposits and the cathode deposits contained 5 to 10 percent cobalt, no cobalt was separated from nickel by this procedure.
Nickel Sulfate-Cobalt Sulfate-Ammonium Persulfate-Ammonium Fluoride Electrolytes
Coehn and Salomon claimed that anodic deposition of cobalt as a peroxide is more selective if a persulfate is present in a neutral electrolyte of nickel and cobalt sulfates. Smith reported that cobalt was deposited at the anode as a hydrated oxide from an electrolyte containing nickel and cobalt as the double ammonium fluoride. These two ideas were used separately and then combined in this series of experiments. All electrolytic tests in this section employed the procedure described in the preceding section.
Two tests were conducted with an electrolyte containing 94 grams of nickel sulfate, 6 grams of cobalt sulfate, and 25 grams of ammonium persulfate per liter. Although an anode deposit was evident in both cases, there was too little material to sample. The cathode deposits contained over 10 percent cobalt.
Two tests were made using an electrolyte prepared with 94 grams of nickel sulfate, 6 grams of cobalt sulfate, and 72 grams of ammonium fluoride per liter. No anode deposit was produced, and the cathode deposits contained over 5 percent cobalt. In a third test 3.2 grams of cobalt nitrate per liter was added to the electrolyte. No cathode deposit was produced, and the small anode deposit contained not only over 10 percent cobalt but also 5 to 10 percent nickel.
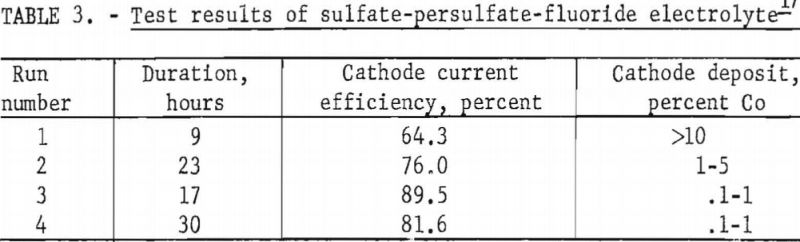
Four tests were carried out from an electrolyte composed of 94 grams of nickel sulfate, 6 grams of cobalt sulfate, 72 grams of ammonium fluoride, and 20 grams of ammonium persulfate per liter. No anode deposit was obtained in these tests. The summarized results in table 3 indicate that, although the cobalt in the starting electrolyte was deposited readily at the cathode, very little if any additional cobalt was dissolved from the carbonate mixture used in regenerating the electrolyte.
Although no anode deposits were obtained in these experiments, it was noted that nickel carbonate appeared to be dissolved preferentially by the spent electrolyte from the synthetic Nicaro mixed carbonate used for regeneration. This observation initiated a series of tests to determine the solubility of nickel carbonate and cobalt carbonate in several electrolytes at various levels of pH.
Nickel Carbonate-Cobalt Carbonate Solubility Tests
One of the simplest methods for separating metal ions is by the selective precipitation of oxides or hydroxides under conditions of controlled pH. Several tests were made to determine whether the reverse of the procedure would show promise for separating nickel and cobalt that is, to determine whether nickel carbonate or cobalt carbonate could be dissolved preferentially in solutions by controlling the pH. The solutions investigated were dilute sulfuric acid, nickel sulfate, and nickel sulfate-ammonium fluoride.
Ten-gram samples of nickel carbonate or cobalt carbonate were weighed into 250-ml. volumetric flasks and made up to volume with dilute sulfuric acid of different pH values. After standing for 24 hours at room temperature, with occasional shaking, the solutions were filtered and the filtrates chemically analyzed. Table 4 shows the cobalt and nickel contents of the solutions. One value for cobalt in the table appears to be out of line. If this value is disregarded, there is a 4-to-7 preference for dissolving nickel between a pH of 2.83 and 5.35.
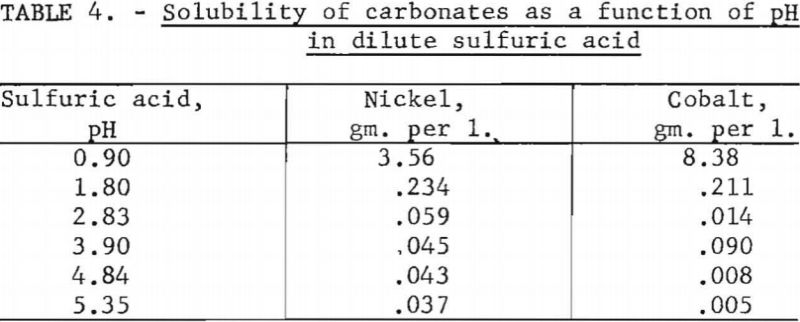
Tests were conducted to determine the solubility of cobalt carbonate as a function of pH in a solution containing 94 grams of nickel sulfate per liter. Ten-gram samples of cobalt carbonate were weighed into 250-ml. volumetric flasks and made up to volume with nickel sulfate solutions. The identical procedure described in the first experiment was employed. Table 5 shows the resulting values, which were corrected for the cobalt content of the nickel sulfate solutions. In contrast to the values reported in the first experiment with solutions of dilute sulfuric acid, the solubility of cobalt carbonate apparently was increased by the presence of nickel sulfate. Cobalt carbonate had the lowest solubility at a pH of 4.75.
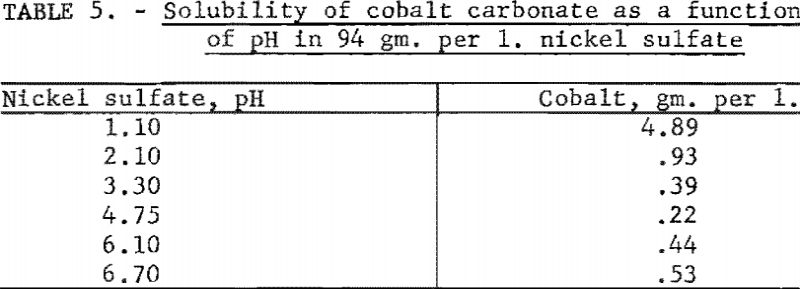
Several experiments were made to determine the solubility of cobalt carbonate as a function of pH in a solution composed of 94 grams of nickel sulfate and 72 grams of ammonium fluoride per liter. The same procedure as described in the first experiment was again followed. After correction for the cobalt content of the nickel sulfate solution, the solubilities of cobalt found for the various pH values are shown in table 6. These results indicated that cobalt carbonate was even more soluble in this solution than in the nickel sulfate solution.
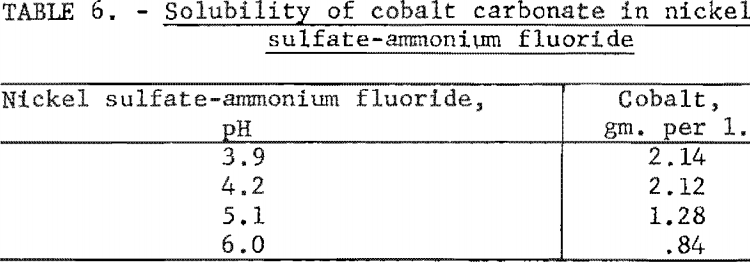
Nickel Sulfate-Ammonium Fluoride Electrolytes
Two series of electrolytic tests were conducted to determine if nickel sulfate-ammonium fluoride electrolytes would show any preference for dissolving nickel from a synthetic Nicaro mixture of nickel carbonate and cobalt carbonate. A third series of tests was made with original Nicaro basic carbonate precipitate to verify the results of tests made with the synthetic regenerant.
All electrolytic tests were made with an aluminum cathode and a lead anode. The specific gravity of the electrolyte was determined before and after each test to indicate electrolyte depletion. At the end of every test the cathode deposit was stripped, washed, dried, pressed, and arc melted, and a sample was obtained by drilling.
In the first series 4 tests were made with an electrolyte containing 94 grams of nickel sulfate and 54 grams of ammonium fluoride per liter. No cobalt was present in the electrolyte at the start of the series, but the electrolyte was circulated continuously through a mixture of nickel carbonate and cobalt carbonate containing 50 percent of each by weight. The duration of each run was approximately 16 hours at a cathode current density of 1 ampere per square decimeter.
At the end of the last run chemical analysis of the carbonate mixture showed approximately 80 percent cobalt carbonate and 20 percent nickel carbonate. Qualitative spectrographs analysis of the cathode deposits indicated 1 to 1.5 percent cobalt in each of the four deposits. Although a small amount of cobalt carbonate was dissolved by this electrolyte, the results were favorable.
A second series of electrolytic tests was conducted to gain additional information regarding the selectivity of a nickel sulfate-ammonium fluoride electrolyte for leaching nickel from a synthetic mixture of nickel and cobalt carbonates. Nine runs were made with a starting electrolyte containing no cobalt, 80 grams of nickel sulfate, and 38 grams of ammonium fluoride per liter. A cathode current density of 1 ampere per square decimeter was used. Each test was continued for 24 hours; then the electrolyte was regenerated by mixing thoroughly with a mixture of nickel and cobalt carbonates, the starting composition of which was 50 percent of each by weight. The suspension was allowed to settle and was filtered; the filtrate was used as the electrolyte for the next run.
At the end of the series the carbonate mixture contained approximately 65 per-cent cobalt carbonate and 35 percent nickel carbonate, substantiating the results of the previous experiments by showing the electrolyte to have a preference for dissolving nickel carbonate. Cobalt analyses of cathode deposits, shown in table 7, indicate that a 50-50 nickel carbonate-cobalt carbonate mixture is too rich in cobalt for use as a regenerant in producing nickel with less than 1 percent cobalt.
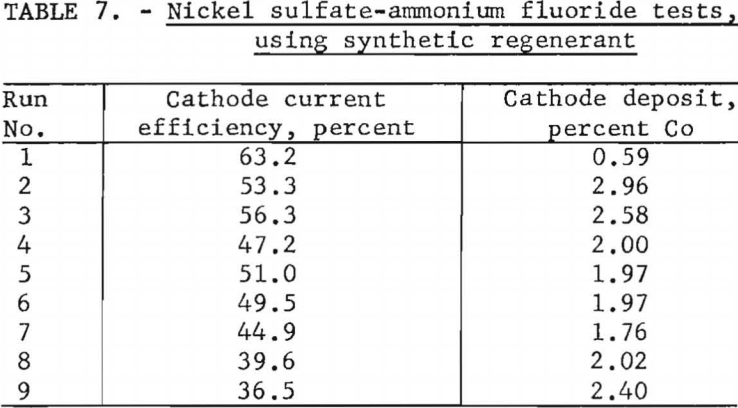
Starting with an electrolyte containing no cobalt, 100 grams of nickel sulfate, and 25 grams of ammonium fluoride per liter, 4 tests were made in the third series. The electrolyte overflowing from the cell was percolated through original Nicaro basic carbonate for regeneration and returned to the cell. The duration of each run was approximately 6 hours at a cathode current density of 0.5 ampere per square decimeter.
The data in table 8 indicate that cobalt occurring in the cathode deposit definitely increases with time. In other words, the electrolyte exhibited a lesser selectivity for dissolving nickel carbonate from Nicaro basic carbonate than from the synthetic mixture. The chief impurities in the cathode deposits as determined by qualitative spectrographic analysis were aluminum and calcium (0.01 to 0.1 percent) and silver, copper, magnesium, manganese and silicon (0.001 to 0.01 percent). Considerable nickel ammonium sulfate crystallized from the electrolyte, possibly indicating a loss of fluoride ions from the electrolyte by anodic deposition.
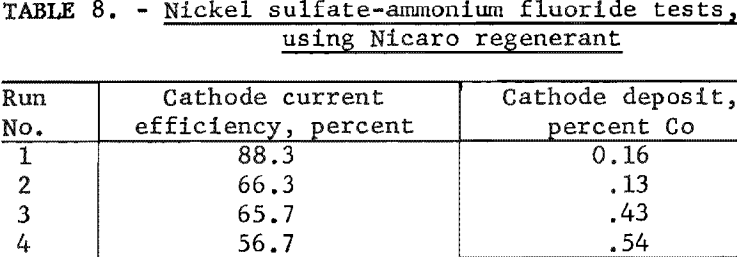
Ammonium Fluoride Electrolytes
The following experiments were carried out to determine if ammonium fluoride electrolytes would show preference for dissolving nickel carbonate from a synthetic Nicaro mixture of nickel and cobalt carbonates, since a preferential solubility of nickel carbonate has been shown to exist in some previous experiments. After the ammonium fluoride leaching experiments with synthetic materials, as well as with cobalt-enriched Nicaro precipitate, several electrolytic tests were conducted to determine if specification nickel could be deposited from ammonium fluoride leach liquors. A single leaching experiment was conducted with an ammonium bifluoride solution. Several exploratory tests were made also on leaching Ramona Loma Mulo serpentine with ammonium fluoride electrolytes.
In the first experiment a 20-gram sample of a 50-50 mixture of synthetic nickel carbonate and cobalt carbonate was leached at room temperature with 4 separate 1-liter batches of solutions, each containing 38 grams of ammonium fluoride per liter. The percentage of extraction of nickel and cobalt by each of the leaching steps is shown in table 9. These data indicate that 92.5 percent of the nickel and 7.9 percent of the cobalt were extracted from the carbonate mixture in 4 leaching steps.
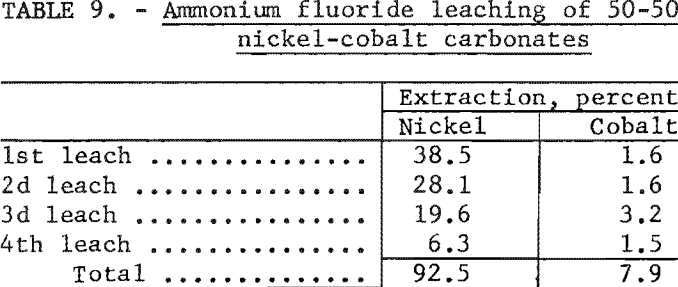
To determine the effect of higher ammonium fluoride concentration and higher temperature, the next test employed a solution containing 100 grams of ammonium fluoride per liter. By digesting a 20-gram sample of 50-50 nickel carbonate-cobalt carbonate at 90° C. for 24 hours with 1 liter of solution, 92.9 percent of the nickel and 19.2 percent of the cobalt were recovered in a single extraction.
In view of favorable preliminary results, a series of experiments was conducted, using a mixture of synthetic nickel and cobalt carbonates similar in composition to Nicaro basic carbonate. Ammonium fluoride concentration and leaching temperature were examined as variables. In all tests the sample consisted of 9 grams of C.P. grade nickel carbonate and 1 gram of C.P. grade cobalt carbonate. Each leaching step was made with 1 liter of solution and was maintained at temperature, with occasional stirring, for at least 6 hours. Table 10 shows the extent of extraction of nickel and cobalt at each leach. The best separation of nickel and cobalt was obtained by leaching at 70° C. with an electrolyte containing 25 grams of ammonium fluoride per liter. In 2 leaching steps a total of 89.7 percent of the nickel and 2.8 percent of the cobalt was extracted.
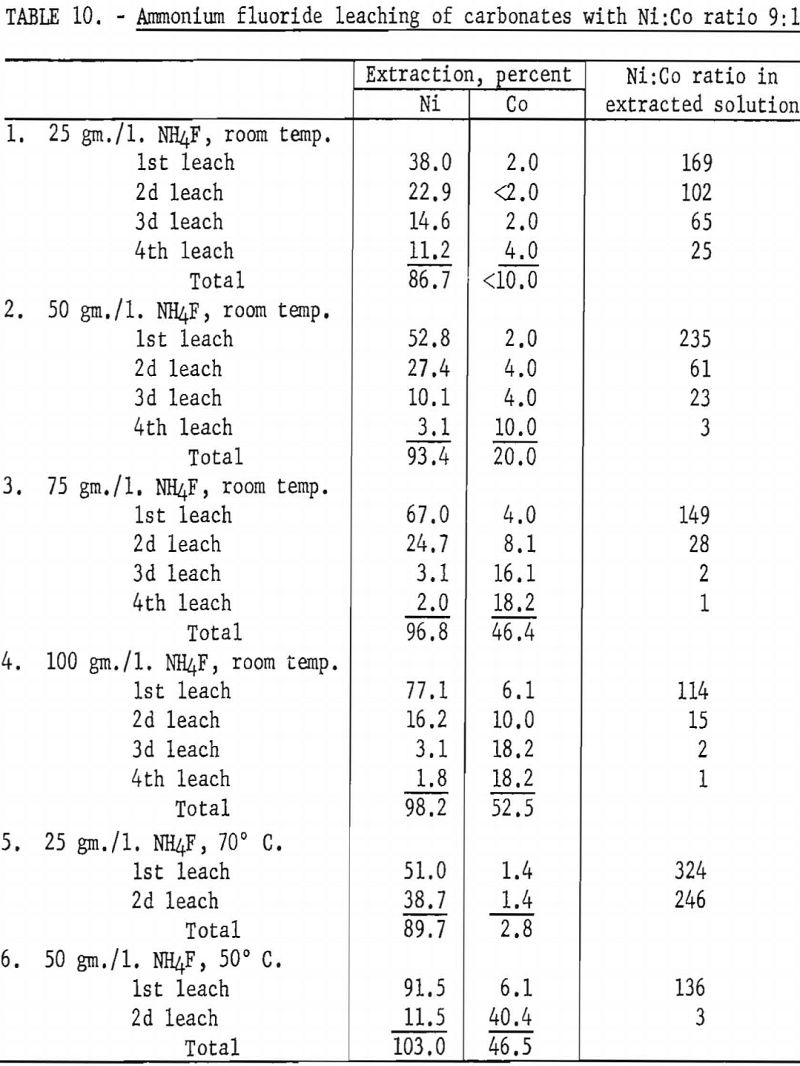
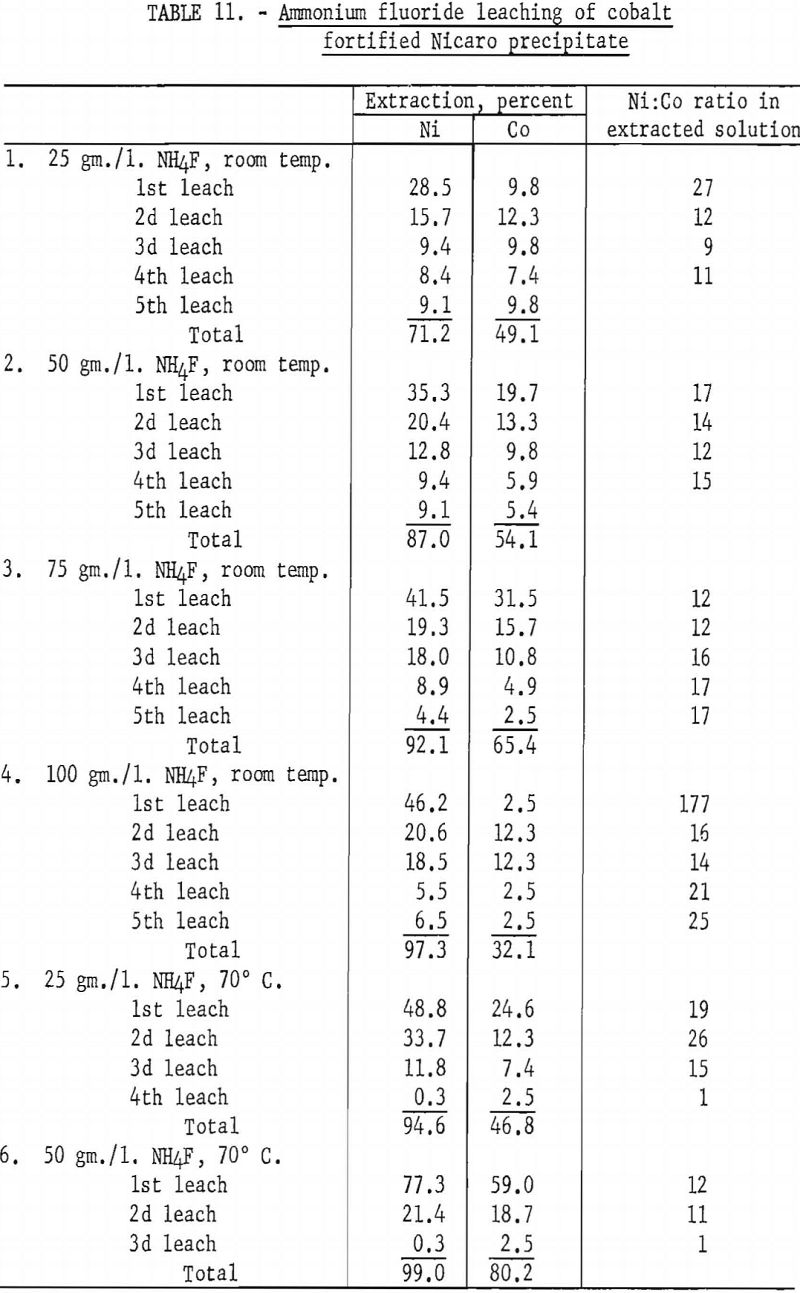
A series of tests was conducted next, using original Nicaro basic carbonate precipitate instead of synthetic carbonates to determine whether the solubility characteristics of this material were similar to those of the synthetic material. A 20-gram sample of cobalt-fortified Nicaro precipitate was leached with 5 separate 1-liter solutions containing 25 grams of ammonium fluoride per liter. Each leach-batch was maintained at room temperature for a minimum of 6 hours, with occasional stirring. The identical procedure was followed for ammonium fluoride concentrations of 50, 75, and 100 grams per liter. In addition, tests were made with 25- and 50- grams-per-liter concentrations at approximately 70° C.
Data obtained in these tests (shown in table 11) indicated that a high ammonium fluoride concentration and a low leaching temperature gave the best nickel extraction, as well as the best nickel and cobalt separation, which was not the case with synthetic materials. In using the Nicaro precipitate, the nickel extractions in the first leaching steps were not as high, and the Ni:Co ratio in the extracted solutions was considerably lower than when synthetic materials were used. These results show that the difference in composition between synthetic and Nicaro carbonates is sufficient to cause a marked variation in their behavior on leaching.
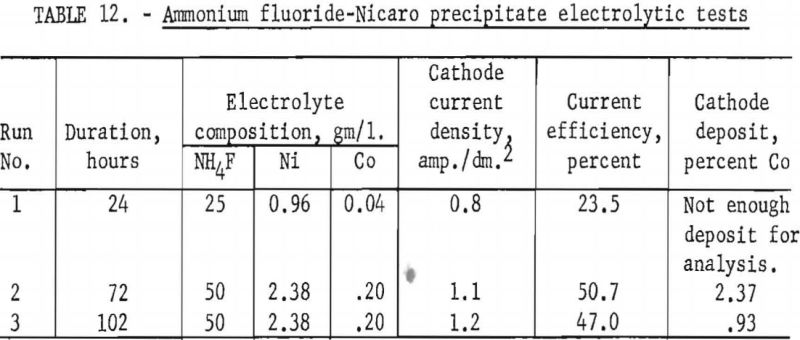
To determine whether Specification-grade nickel could be deposited from ammonium fluoride-Nicaro basic carbonate electrolytes, three tests were made with several of the leach liquors obtained in the tests described above. Aluminum cathodes spaced 4 cm. from graphite anodes were used. The electrolytes were regenerated by periodic additions of cobalt-enriched Nicaro precipitate to the electrolytic cell.
Pertinent data for these tests are shown in table 12. The deposits in each test were somewhat granular. A material balance indicated that 92 percent of the cobalt was deposited along with the nickel at the cathode, while 8 percent remained in the residue, which was insoluble in the electrolyte. Final analyses of the electrolyte indicated that the cobalt in the electrolyte was reduced from 0.20 to 0.009 gram per liter.
One experiment was conducted with a solution containing 100 grams of ammonium bifluoride per liter. A 20-gram sample of mixed synthetic carbonates, with a Ni:Co ratio of 9:1, was leached at 90° C. for 24 hours. In a single leach 91.7 percent of the nickel and 76.6 percent of the cobalt were extracted.
Two exploratory tests were conducted on leaching Ramona Loma Mulo serpentine with ammonium fluoride electrolytes. Work on differential thermal analysis of Ramona Loma Mulo serpentine, reported by the Bureau of Mines station at Rolla, Mo., indicated that 668° C. should be the optimum roasting temperature for the ore. At this temperature the serpentine lattice is reportedly destroyed, with a release of combined water.
In the first test a 10-gram sample was ground to pass 100-mesh and heated to 670° C. under vacuum in a tube furnace. After cooling to room temperature, the sample was leached at 70° C. for 6 hours with 1 liter of solution containing 50 grams of ammonium fluoride. No cobalt and only 4.7 percent of the nickel were extracted. The second 10-gram sample was reduced with hydrogen at 670° C. for 4 hours, then cooled under helium and leached at 80° C. for 6 hours with 1 liter of solution containing 25 grams of ammonium fluoride. Seventeen percent of the nickel was extracted.
Nickel Chloride-Cobalt Chloride Electrolytes
Electrolytic tests of an exploratory nature with nickel chloride-cobalt chloride electrolytes are described in the first part of this section. The second part concerns electrodialysis experiments using an electrolyte of nickel chloride and cobalt chloride in a high concentration of hydrochloric acid.
Electrolysis
All electrolytic tests employed continuous circulation and regeneration of the electrolyte with a synthetic Nicaro mixture of nickel carbonate and cobalt carbonate in the ratio of 94:6 by weight. Aluminum cathodes and lead anodes were used at a cathode current density of 1 ampere per square decimeter.
Two experiments were carried out with an electrolyte composed of 94 grams of nickel chloride and 6 grams of cobalt chloride per liter. A qualitative spectrographic analysis of the nonmetallic cathode deposit indicated the presence of more than 10 percent nickel and more than 5 percent cobalt. In the next two electrolytic tests the chloride electrolyte was made ammoniacal with ammonium hydroxide until the electrolyte became dark blue. An anode bag of Dynel filter cloth was used in one test. Metallic cathode deposits were obtained, but there was no separation of nickel and cobalt.
Electrodialysis
There is considerable evidence that cobalt chloride exists in a high concentration of hydrochloric acid as a CoCl4= anion complex while nickel remains as a cation. Two electrodialysis experiments were conducted to determine if nickel and cobalt could be separated by passing an electrolyte of nickel and cobalt chlorides in a high concentration of hydrochloric acid through a two-compartment cell in which the compartments were separated by a diaphragm.
The acidity of the hydrochloric solution necessary to form the blue cobalt anion complex was determined by a series of color tests. Two-gram samples of C. P. cobalt carbonate were dissolved in 200-ml. portions of hydrochloric acid ranging from 3 to 12 normal. The colors ranged from pink in the 3N to deep blue in the 12N hydrochloric acid. The blue color first appeared in the 7N hydrochloric acid, probably according to the following equation:

The electrolyte was prepared by dissolving 3,150 grams of nickel carbonate and 350 grams of cobalt carbonate in 7 and 2 liters of 7N hydrochloric acid, respectively. The 2 solutions were combined, and 12N hydrochloric acid was added to give a total volume of 11 liters. A cathode current density of 2 amperes per square decimeter was used with a stainless steel cathode and a graphite anode.
The first electrodialysis experiment employed a cation semipermeable membrane separating the anode and cathode compartments. It was postulated that if the feed electrolyte was introduced into the anode compartment, the negative cobalt complex would remain and concentrate in the anolyte, while the nickelous ions could be removed from the catholyte by deposition. However, chemical analysis showed that the anolyte had the same nickel:cobalt ratio (9:1) as the initial feed electrolyte, while the catholyte ratio decreased to 3:1, as expected. That the anolyte did not increase in cobalt concentration was contrary to expectations. The cathode deposit contained 10.2 percent cobalt.
The second electrodialysis experiment was made employing the identical procedure and electrolyte as described in the first experiment, except that three layers of glass cloth were substituted for the cation semipermeable membrane because the electrolyte flow through the latter was very slow. There was no separation of nickel and cobalt, probably due to diffusion of the anolyte and catholyte. The deposit contained 10.7 percent cobalt.
Synthetic Nicaro Liquor Electrolytes
Preferential deposition of cobalt at the cathode from an ammoniacal nickel-cobalt complex electrolyte has been reported in the Journal of the Chemical Society of Japan. Several tests were carried out with electrolytes similar to Nicaro product liquor to determine if cobalt and nickel could be separated in this type of electrolyte.
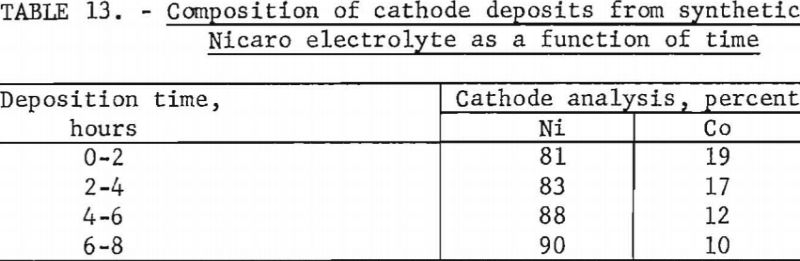
The electrolyte had a composition of 11 percent ammonia (half as hydroxide and half as carbonate) and approximately 1 percent nickel, with a nickel:cobalt ratio of 16:1. A current density of 1 ampere per square decimeter was used with hard lead cathodes and carbon anodes. All other conditions were the same as those reported in the Japanese work. After three preliminary experiments a fourth electrolytic test was made to determine the composition of the cathode deposits as a function of time. In this fourth test, cathodes were changed every 2 hours over a total time of 8 hours.
Chemical analyses of the cathode deposits are shown in table 13. Although more cobalt was deposited at the beginning of the electrolysis, little selectivity was indicated.
In another attempt to separate nickel and cobalt from an electrolyte similar in composition to Nicaro product liquor, 19 grams of ammonium fluoride was added per liter to determine if nonmetallic cobalt could be deposited at the anode. Lead electrodes at a current density of 1 ampere per square decimeter were used. No deposit was apparent at either electrode.
Determination of Decomposition Potentials for Nickel and Cobalt Electrolytes
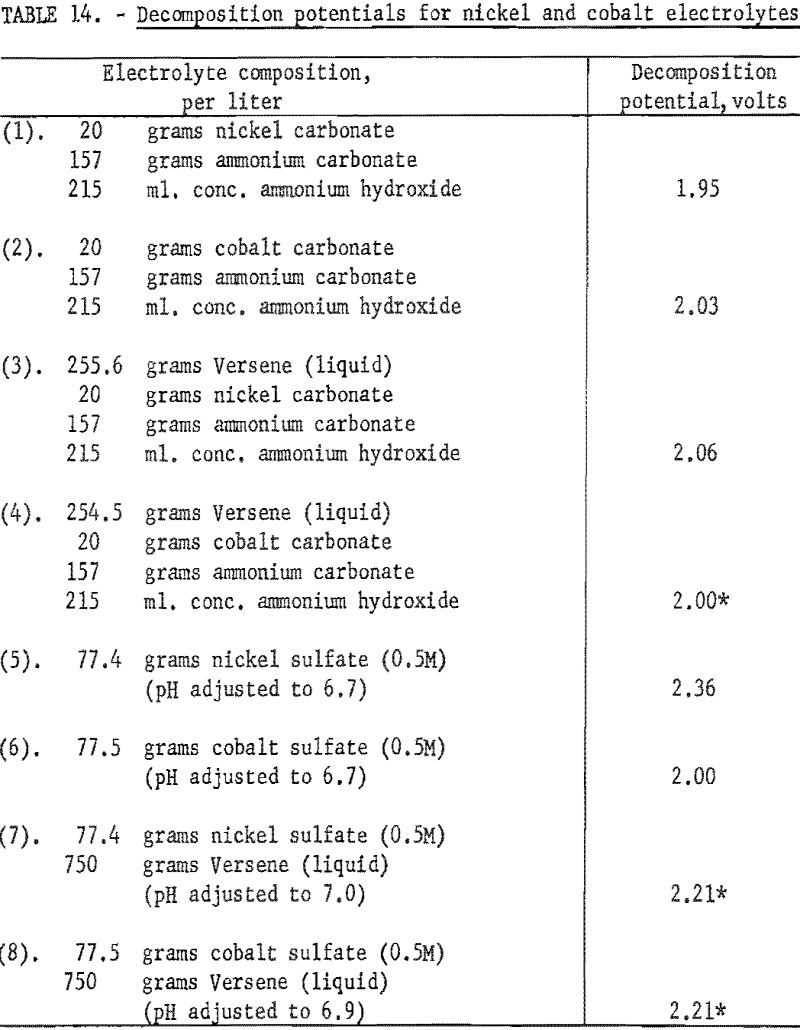
Decomposition potentials were determined for several electrolytes to determine if there might be enough difference between similar solutions of nickel and cobalt to offer any possibilities for electrolytic separation of the two elements. Versene was added to some electrolytes to complex the metallic ions and in this way alter the decomposition potentials.
In obtaining all of the following values, graphite anodes and hard lead cathodes were used. The electrodes were spaced 5 cm. apart, and all the electrolytes were at room temperature. The voltage was increased in 0.05-volt increments and the corresponding current recorded. The composition of all of the basic electrolytes was essentially the same as of Nicaro leach liquors, containing either nickel or cobalt but not both. The sulfate solutions were all 0.5 molar. In all instances where Versene or tetrasodium salt of ethylenediamine tetra acetic acid was added, the exact amount was added that would be required to complex the metallic ions completely. Duplicate determinations were made for each electrolyte.
The decomposition potentials were determined by plotting the data and extrapolating the steep portions of the curves to zero current. Results are shown in table 14. Some of the electrolytes in which there were possibilities for several complex ions to coexist appear to have 2 or 3 straight-line portions in the curve, giving different values upon extrapolation. These were noted in the table by a star. The decomposition potentials, as determined by these tests, indicate that electrolytic separation of nickel and cobalt would be hardly feasible.
Cyanide and Phosphate Electrolytes
One reported method of nickel and cobalt separation depends on the fact that cobalt forms a cyanide complex that is stable in acid solutions, whereas the corresponding nickel complex is unstable under the same condition. Since the cobalt complex would be negatively charged, it was postulated that nickel and cobalt may be separated by electrophoresis. Several attempts to prepare an electrolyte containing nickel cations and cobalt cyanide anions invariably resulted in precipitation of nickel cyanide. Contrary to expectations, the same results were obtained when the two ions were prepared in separate solutions and mixed in a solution of 1:1 sulfuric acid.
A nickel-cobalt cyano complex electrolyte was prepared, however, by mixing 200 grams of potassium cyanide, 94 grams of nickel sulfate, and 6 grains of cobalt sulfate per liter. It was difficult to maintain all of the constituents in solution, and no cathode deposit was obtained after 8 hours of electrolysis.
A second electrolyte was prepared from 10 grams of sodium cyanide, 94 grams of nickel sulfate, and 6 grams of cobalt sulfate.
A third electrolyte contained 10 grams of sodium cyanide, 94 grams of nickel chloride, and 6 grams of cobalt chloride. Qualitative spectrographic analysis of the cathode deposits from the second and third electrolytes indicated that over 10 percent cobalt was present in each.
A phosphate electrolyte was prepared from 100 ml. of orthophosphoric acid added to 50 grams of ammonium fluoride; the mixture was neutralized with nickel carbonate and cobalt carbonate. A high acidity was required to prevent precipitation of phosphates, and since there was heavy hydrogen evolution at the cathode, the test was discontinued.
Nickel Sulfate-Cobalt Sulfate-Potassium Sulfate-Potassium Dichromate Electrolytes
An electrolyte suggested by Perkin employed small amounts of potassium dichromate and potassium sulfate added to a neutral solution of nickel sulfate and cobalt sulfate. A complete separation of nickel and cobalt was claimed, with the nickel depositing as metal at the cathode and the cobalt as peroxide at the anode.
Two tests were made with an electrolyte prepared from 8.2 grams of nickel sulfate, 0.5 gram of cobalt sulfate, 7.0 grams of potassium sulfate, and 0.3 gram of potassium dichromate per liter. In the third test the electrolyte composition was changed to 94 grams of nickel sulfate, 6 grams of cobalt sulfate, 30 grams of potassium sulfate, and 15 grams of potassium dichromate per liter. No anode deposits were obtained and the cathode deposits appeared nonmetallic.
Ammonium Sulfate Electrolytes
A total of 21 tests was conducted in 3 series of experiments to determine if nickel and cobalt could be separated and Specification-grade nickel deposited from an ammonium sulfate-Nicaro basic carbonate electrolyte. The electrolyte was composed of approximately 25 grams of nickel as Nicaro bulk precipitate, dissolved in a solution containing 400 grams of ammonium sulfate per liter. In the first series the effect of nickel concentration on current efficiency was investigated. In the second and third series the effect of temperature and cathode current density on cathode-deposit composition was investigated. All runs were conducted in a cell with a sand-blasted aluminum cathode spaced 5 cm. from a lead anode.
The first series was conducted at 50° C. and consisted of 8 runs in which the electrolyte was regenerated at the end of each run by mixing thoroughly with Nicaro bulk precipitate and filtering. The anode was placed within a porous clay diaphragm. In runs 4 through 8, each deposit was weighed several times in the course of the experiment to determine the effect of nickel concentration on current efficiency as the nickel concentration was depleted from approximately 25 to 10 grams per liter. It was determined that current efficiencies of nearly 100 percent could be achieved if the electrolyte contained at least 20 grams of nickel per liter. The current efficiency declined sharply as the nickel concentration dropped below 20 grams per liter, and at a concentration of 10 grams per liter the current efficiency was on the order of 10 percent.
Subsequent runs employed apparatus in which the electrolyte was circulated and regenerated continuously. Figure 2 is a detailed flow diagram illustrating the use of the apparatus. The Nicaro basic carbonate regenerant was added at the rate of 6.25 grams per ampere-hour. The regenerated electrolyte entered the cell through a glass-cloth bag surrounding the anode at the rate of 500 to 1,200 ml. per hour; the higher rates were used with the higher current densities.
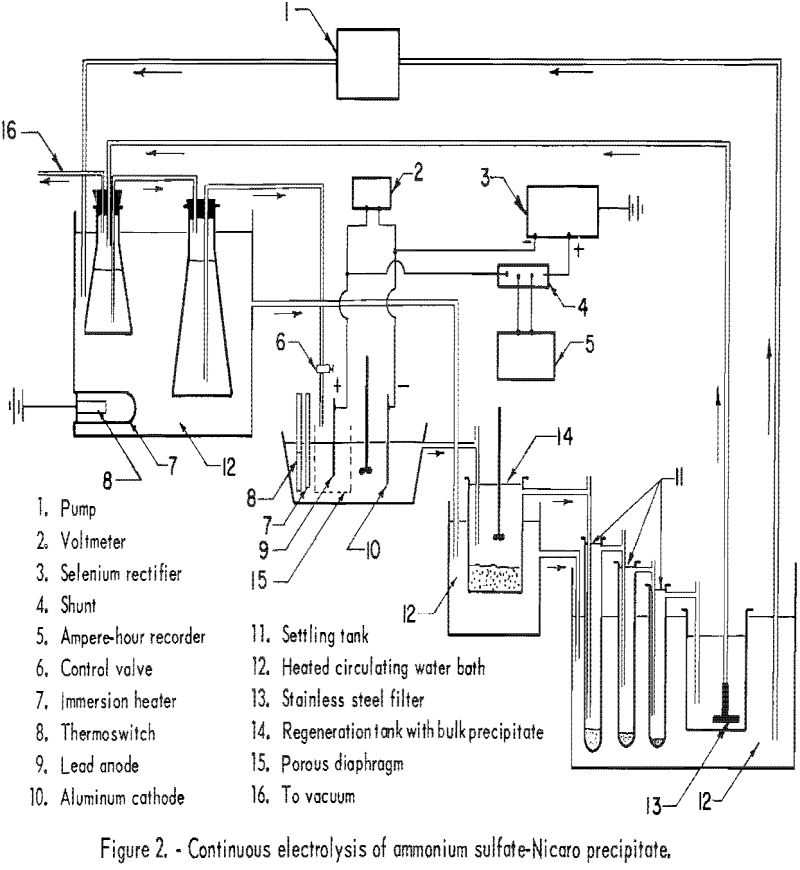
The second series of 7 runs was conducted at 50° C., and the third series of 6 runs was made at 75° C. The current density was varied at each temperature. At the lower current densities and higher temperature the cathode deposits were quite adherent, while at the higher current densities and lower temperature the deposits were coarse and sometimes flaked off.
During the runs a precipitate formed in the anode bag, which had an average nickel:cobalt ratio of 150:1. An insoluble residue was recovered from the regeneration tank, in which the nickel:cobalt ratio was 36:1. The chief impurities in the cathode deposits are shown in table 15.
The results for each series (summarized in table 16) indicate that only at low current densities and high temperatures was separation of cobalt from nickel realized by preferential dissolution of Nicaro bulk precipitate.
Nickel Sulfate – Boric Acid Electrolytes
Nickel sulfate – boric acid electrolytes were studied to determine if nickel and cobalt could be separated by differential dissolution of the two elements when Nicaro precipitate was used as regenerant. A total of 25 electrolytic tests was conducted in 3 series of experiments. In the first series the starting electrolyte contained no cobalt, and the effect of electrolyte pH on deposit composition was investigated. In the second series the starting electrolyte contained no cobalt, and the electrolyte pH was controlled by adjustment. In the third series the starting electrolyte contained cobalt, and the electrolyte pH was controlled without adjustment. The effect of the cathode and anode current densities on deposit composition was investigated in both the second and third series.
The electrolyte in all electrolytic tests was circulated continuously and regenerated with Nicaro precipitate to maintain the nickel concentration. At the end of every test the cathode deposit was stripped, washed, dried, compacted, and arc-melted, and a sample was obtained by drilling. The circuit consisted essentially of a feed tank, an electrolytic cell, an overflow container in which the electrolyte was regenerated, and two settling tanks. The final tank in the circuit contained a glass-wool filter and was connected to a pump controlled automatically by the level in the feed tank. In the electrolytic cell there was an aluminum cathode spaced 4 cm. from 2 lead anodes, an immersion heater, and constant mechanical stirring for uniform electrolyte composition and heat distribution. Figure 3 shows the equipment used, and figure 4 is a detailed flow diagram illustrating the same apparatus.
In the first series of experiments, 4 electrolytic tests were carried out at 51° C. with a starting electrolyte composed of 106 grams of nickel sulfate and 20 grams of boric acid per liter, but no cobalt. Pertinent data for this series are shown in table 17. The chief impurities in the cathode deposits are shown in table 20, column C. The best separation of nickel from cobalt was obtained at a pH of 4.7 to 5.2. The results of a previous experiment on the solubility of cobalt carbonate as a function of pH in a nickel sulfate solution indicated that cobalt carbonate had the lowest solubility at a pH of 4.75. The electrolyte of the following series of tests was maintained at a pH of 5.0±0.2 in attempts to separate cobalt from nickel and obtain Specification-grade nickel by electrodeposition. The same pH range was suggested in the literature for a conventional nickel sulfate – boric acid electrolyte.
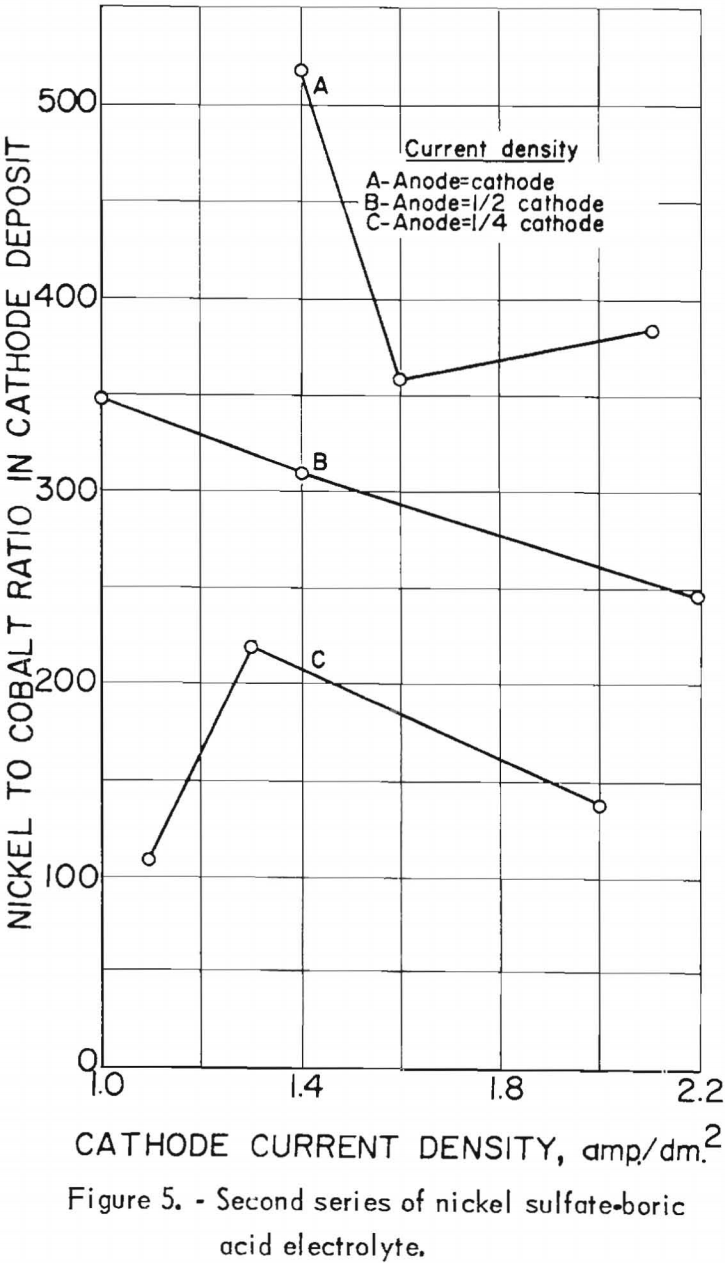
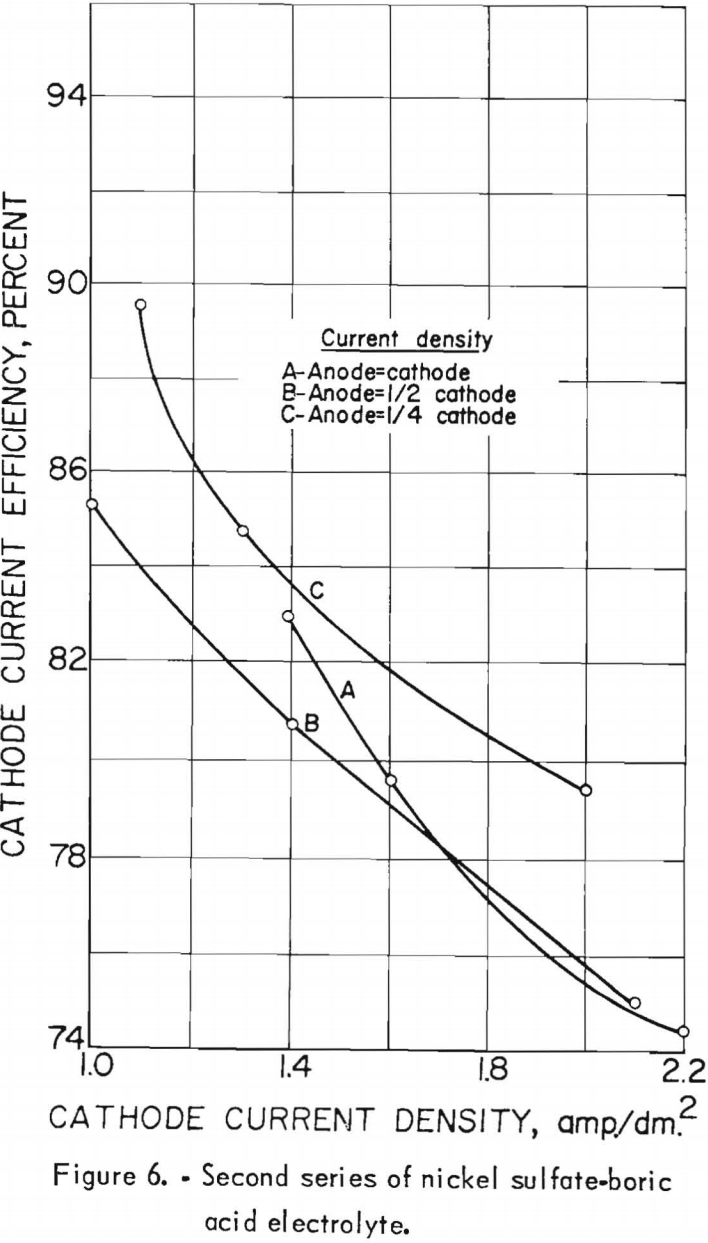
Nine electrolytic tests were made in the second series of experiments. The starting electrolyte was prepared from 106 grams of nickel sulfate and 20 grams of boric acid per liter but contained no cobalt. The pH of the electrolyte in the last settling tank was adjusted to 5.0±0.2 with sulfuric acid before being pumped to the feed tank. The effects of the cathode and anode current densities on deposit composition are shown graphically in figure 5. The best separation of nickel and cobalt appeared to result when the cathode and anode current densities were equal. Cathode and anode current densities also are plotted against cathode current efficiency in figure 6 for comparison. A slight increase in the current efficiency was noted at the higher anode current densities (curve C); otherwise, results are the same.
Pertinent data and the results of analyses are summarized in table 18. The results indicate that nickel and cobalt were separated through the differential solubility of the two elements when Nicaro bulk precipitate was used to regenerate the electrolyte. The chief impurities in the cathode deposits are shown in table 20, column D.
At the end of this series a large quantity of brown residue and some green basic carbonate remained in the regenerating tank. In an effort to convert all of this to the brown residue, an additional electrolysis was conducted without addition of fresh bulk precipitate. However, the pH of the electrolyte changed from approximately 5 to 2, causing dissolution of the brown residue and consequent increase of cobalt in the electrolyte from 0.11 to 0.33 gram per liter. A material balance indicated that, had the additional electrolysis not been performed, 44 percent of the total cobalt added as basic carbonate would have remained in the residue. The disposition of the remaining cobalt originally added as basic carbonate was as follows: 33 percent deposited with the nickel; 22 percent dissolved in the electrolyte; and 1 percent deposited at the anode. It should be noted that all of the nickel deposits were better than Specification grade, the average cobalt content being 0.4 percent. Power requirements calculated for a current efficiency of 90 percent are approximately 1.3 kw.-hr. per lb. of nickel.
In the third series of experiments, 12 electrolytic separations of nickel and cobalt were carried out. The starting electrolyte contained 0.47 gram of cobalt, 106 grams of nickel sulfate, and 50 grams of boric acid per liter.

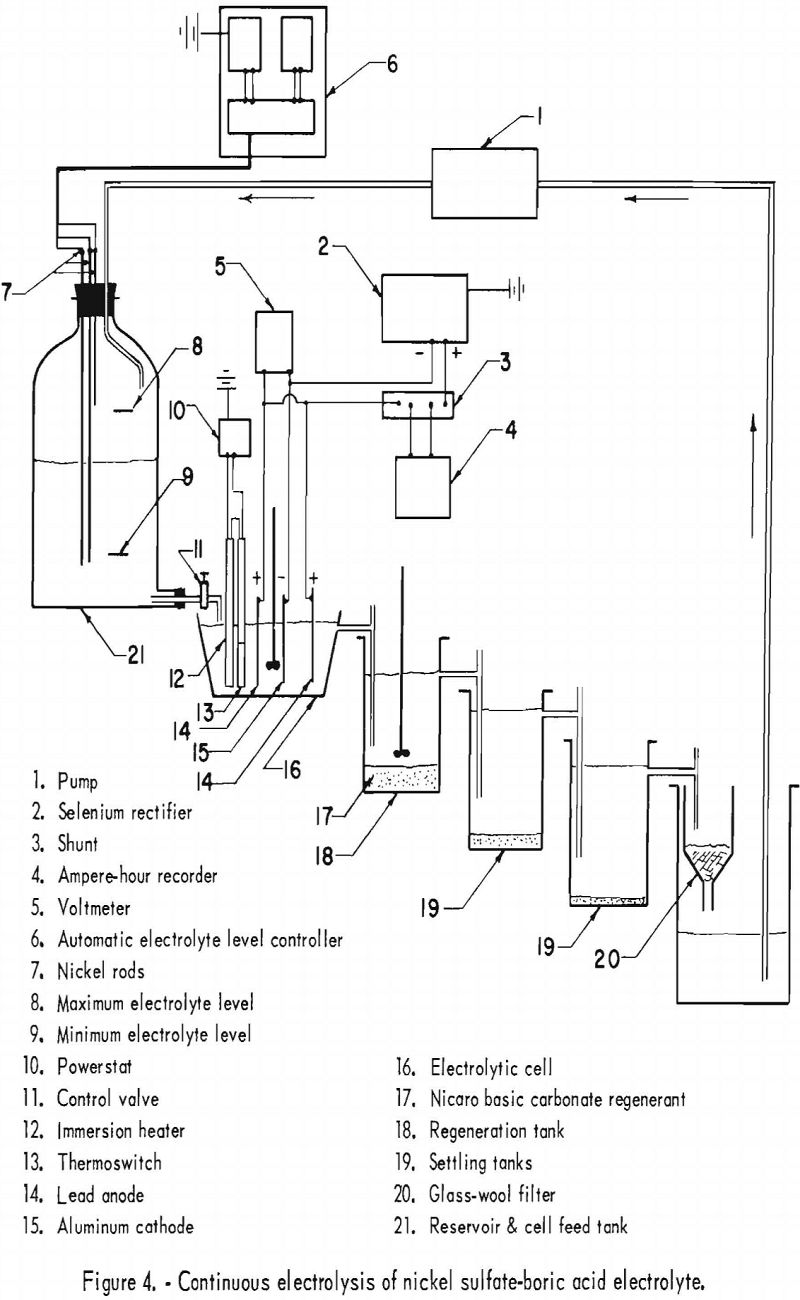
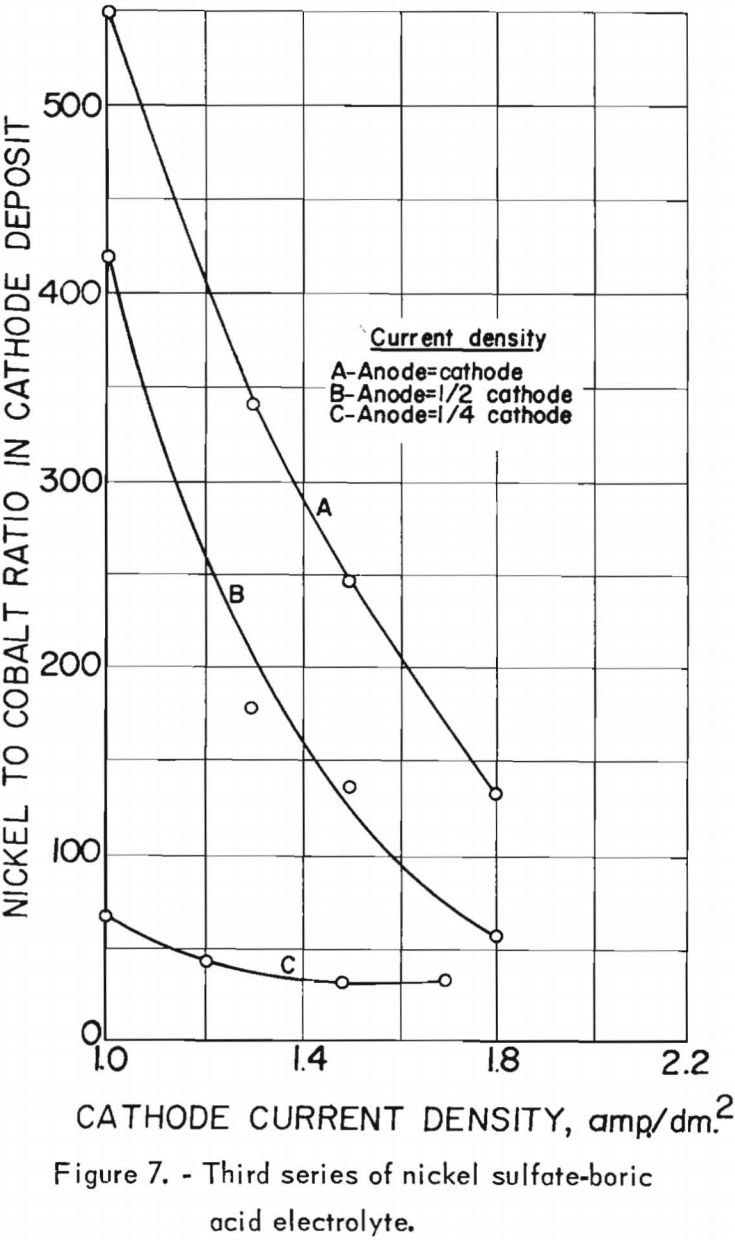
The effects of the cathode and anode current densities on deposit composition are shown graphically in figure 7. The best separation of nickel from cobalt appeared to result when the cathode and anode current densities were equal, which was also the case in the second series. Cathode and anode current densities also were plotted against cathode current efficiency in figure 8. A slight increase in current efficiency was noted at the lower anode current densities, whereas in the second series it had been noted at the higher anode current densities. The current efficiencies for this series were consistently higher than those of the second series, otherwise, results were similar. The chief impurities in the cathode deposits for the third series are shown in table 20, column E.
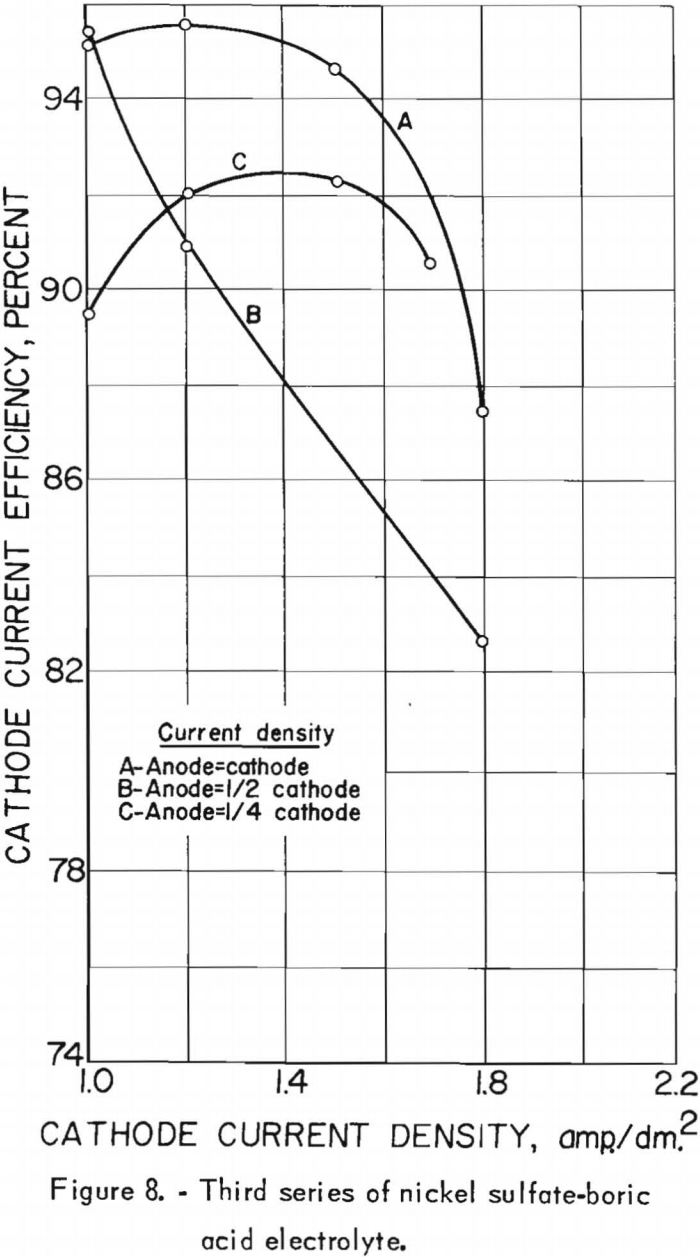
A brown residue accumulated in the regenerating tank, as in the second series. This appeared to result upon depletion of the fresh green Nicaro precipitate added as regenerant. Chemical analyses indicated that the nickel:cobalt ratio in the brown residue was 25:1, whereas in the undepleted green regenerant it was 71:1. The brown residue was soluble at the higher acidity (pH 2 to 3) of the spent electrolyte, but not at the lower acidity (pH 5.0±0.2) of the regenerated electrolyte. Although occasional removal of the cobalt-rich residue from the system should avoid the possibility of increasing the dissolution of cobalt, further investigation of methods to reduce the nickel:cobalt ratio of the residue while in contact with the electrolyte should be considered.
It should be noted that the third series was essentially a continuation of the second series. The final electrolyte of the second series was the starting electrolyte for the third series, with only the boric acid concentration increased from 20 to 50 grams per liter to buffer the regenerated electrolyte between a pH of 4.8 and 5.2 without adjustment. Although nickel and cobalt were separated by selective dissolution of nickel from an excess of Nicaro precipitate, some cobalt was also dissolved. This was verified, since the starting electrolyte of the second series contained no cobalt, while its final electrolyte (starting electrolyte of the third series) contained 0.47 gram of cobalt per liter.
The nickel:cobalt ratio in the deposits decreased below specification after the electrolyte had been used for a total of 549 ampere-hours, as indicated by the data summarized in table 19 for run 21 and thereafter. Whether this resulted from the electrolyte reaching a maximum cobalt concentration, which then deposited with the nickel, cannot be ascertained without further experimentation. That it resulted from the small increase of foreign ions or higher boric acid concentrations of the electrolyte is unlikely but should be also investigated. That it resulted from the formation of sulfur complexes through dissolution of Nicaro precipitate is doubtful, since a sulfate system was used, and, more important, the total sulfur of the electrolyte remained constant during electrolysis. As indicated above, further investigation is needed to determine if Specification-grade nickel can be obtained by continuation of the process. Further investigation should also determine the most favorable conditions of temperature and catholyte circulation as they affect concentration polarization and ionic mobility.
The cathode deposits for all three series generally were shiny and always adherent. The sources of cathode-deposit impurities can be traced by referring to table 20. Columns C, D, and E show the deposit impurities that originated from Nicaro precipitate added as regenerant (column A) and/or from the starting electrolyte (column B).
Selective Precipitation of Cobalt From Nicaro Liquors
Versene, or the tetrasodlum salt of ethylenediamine tetra acetic acid, reportedly complexes nickel preferentially to cobalt below a pH of 11. Three experiments were made to determine if nickel would be complexed and cobalt selectively precipitated by such a method.
In the first experiment a synthetic Nicaro solution was used with enough Versene added to complex the nickel completely at a pH of 9. In the second experiment the same Nicaro-Versene solution was acidified with sulfuric acid to a pH of 8. In both experiments the solution was evaporated to one-half of the original volume and the precipitate removed. This procedure was repeated three times, diluting the filtrate to the original volume each time. Analyses of the final filtrate and combined precipitates for each experiment are shown in table 21 (A) and (B). The results indicated that, although nickel was complexed and only 1 percent precipitated, early precipitation of cobalt was not favored, since approximately 46 percent remained in the filtrate.
The third experiment with synthetic Nicaro-Versene solution employed distillation instead of evaporation; the solution was distilled at a constant temperature of 95° C. to approximately one-third of the, original volume. The precipitate was separated from the mother liquor by filtration and washed, then the distillate, mother liquor, and washings were combined and redistilled. Distribution of nickel and cobalt shown in table 21 (C), indicated that the early precipitation of 99 percent of the cobalt definitely was favored. However, work on this system was discontinued, owing to the large amount of Versene needed to complex the nickel (26:1 by weight).
It has been reported that, if cobalt and nickel are precipitated as xanthates, the nickel xanthate can be dissolved in ammoniacal solutions and the cobalt xanthate collected by filtration. Synthetic and original Nicaro liquors were tested with separate solutions of potassium ethyl xanthate, potassium amyl xanthate, and potassium secondary amyl xanthate. In all instances, no separation of cobalt from nickel was obtained.
Sodium dibasic phosphate, monobasic ammonium phosphate, and dibasic ammonium phosphate were tested separately as selective precipitants for cobalt from a synthetic Nicaro solution. None of these precipitants was selective for cobalt.
As described in the beginning of this report, ammonium fluoride dissolved nickel carbonate preferentially to cobalt carbonate, probably by the formation of a fluoro complex. An experiment was conducted to determine if cobalt carbonate could be precipitated preferentially from Nicaro pregnant liquor by adding ammonium fluoride to complex the nickel, before evaporation to drive off ammonia and carbon dioxide. To three samples of Nicaro liquor, ammonium fluoride was added in amounts calculated to give ammonium fluoride-nickel ratios of 1:1, 2:1, and 3:1. The solutions then were evaporated to the point of incipient precipitation. In each case the precipitates contained both nickel and cobalt.
Selective Precipitation of Nickel From Nicaro Liquors
Versene the tetrasodium salt of ethylenediamine tetra acetic acid) reportedly complexes cobalt preferentially to nickel above a pH of 11. It was postulated that nickel could be precipitated by evaporation while cobalt remained in solution.
In the first experiment it was determined that sodium hydroxide would raise the pH of a synthetic Nicaro solution only slightly above the original value of 10. This was due to the adverse buffering action of the ammonium carbonate – ammonium hydroxide mixture. After addition of Versene the solution was evaporated under vacuum to one-third of the original volume. Since the precipitate contained 66.7 percent of the nickel and 47.4 percent of the cobalt originally in solution, no separation of the 2 elements was indicated at a pH of 10.
The second experiment employed evaporation of original Nicaro liquor to the point of incipient precipitation before concentrated ammonium hydroxide was added to raise the pH slightly above 11. By this method enough ammonia and carbon dioxide were driven off to prevent adverse buffering action. Versene was added and the solution evaporated to approximately one-third of the original volume. According to qualitative spectrographic analysis, the precipitate contained 1 to 5 percent cobalt; therefore, the precipitation of cobalt definitely was favored by the addition of Versene at a pH above 11.
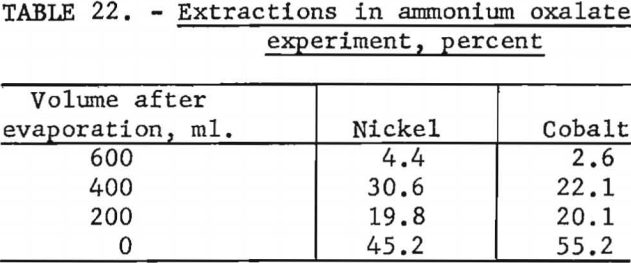
Ammonium oxalate was tested as a selective precipitant for nickel. One liter of cobalt-enriched Nicaro liquor containing 0.9 gram of ammonium oxalate was evaporated to the point of incipient precipitation and the precipitate removed. This procedure was repeated at successive 200-ml. intervals. Table 22 shows that ammonium oxalate does not appear to affect the precipitation of either nickel or cobalt, and no separation can be expected by this method.
Separation by Aeration
A series of tests was carried out to determine if cobalt could be oxidized to an insoluble colbaltic oxide by aerating a solution of nickel sulfate and cobalt sulfate. Temperature and pH were investigated as variables.
A solution was prepared containing 94 grams of nickel sulfate and 6 grams of cobalt sulfate per liter. A mechanical pump was used to circulate the solution through an aspirator; in this way, aeration was carried out for 1-hour periods at room temperature and at 80° C. at a pH of 4.2, 6.0, and 7.1.
No change in the solution was noted upon aeration at a pH of 4.2, either at room temperature or at 80° C. Traces of precipitates were obtained at both temperatures when the pH was raised to 6.0 and the solution aerated. Qualitative analyses of these precipitates indicated the presence of both nickel and cobalt. A considerable amount of precipitation occurred when the pH of the solution was raised to 7.1. Little additional change was noted upon aeration at room temperature; however, aeration at 80° C. produced a considerable amount of additional precipitation. Chemical analysis of the precipitate obtained at 80° C. indicated the presence of 38.0 percent of nickel and 11.5 percent of cobalt. These results indicated that no cobalt is separated from nickel by the above procedures.
In the next attempt to separate the 2 metals by aeration of a sulfate solution, 23 grams of sodium cyanide was added per liter in an effort to form the cobalticyanide complex. A heavy precipitation of green material, presumably nickel cyanide, was encountered, which was not affected by aeration for 1 hour at 80° C.
Another experiment was carried out in which 6 ml. of 30 percent hydrogen peroxide was added to a solution of nickel sulfate and cobalt sulfate. The solution then was aerated for 1 hour at 80° C. Since the precipitate contained both nickel and cobalt, no separation can be expected by this method.
