Table of Contents
A laboratory smelting study was conducted on a copper ore containing chalcocite and native copper to test the technical feasibility of non-pyritic smelting (low pyrite). The results of this investigation led to the following conclusions, based on sulfur and copper recoveries and matte compositions:
- The concentrate can be effectively smelted when pyrite is replaced by sulfur or gypsum.
- The copper content of the matte can be substantially increased by substituting gypsum for pyrite.
- The addition of small amounts of metallic iron to smelting charges containing sulfur or gypsum increases the recovery of both sulfur and copper.
Also, if metallic iron is used in conjunction with gypsum, it is possible to eliminate limestone from the charge. The gypsum-metallic iron combination then serves as the exclusive matte- and slag-forming ingredient providing both sulfur and lime.
Copper metal is normally recovered from copper sulfide ores or mineral concentrates by a pyrometallurgical process of smelting, converting, and refining. In the first stage, iron and copper sulfides plus fluxing materials are melted in a reverberatory furnace. This vessel is a long, shallow furnace consisting of a hearth, side and end walls, and a roof. The furnace is heated by burners placed in one end wall, and the products of combustion escape at the other end. Gas, fuel oil, or pulverized coal is used to produce a long flame which heats the material on the hearth by radiation. Consequently, the reverberatory is essentially a melting furnace, and there are no extensive reactions between furnace gases and the charge on the hearth. The principal, chemical reactions are between the constituents of the furnace bath.
The essential function of the reverberatory furnace is to melt the charge and permit the formation of matte and slag. The matte is a molten solution of two sulfides, Cu2S and FeS. It may range in grade from 20 to 80 percent copper, and the combined copper, iron, and sulfur will usually exceed 95 per-cent of the matte. The matte is denser than the slag; consequently, the liquid matte and slag segregate into two layers which serve as the basis for their separation from the furnace.
The matte is withdrawn from the reverberatory furnace and transferred to a converter where it is blown with air which oxidizes the FeS. By adding a siliceous flux the FeO is slagged and removed. Further blowing oxidizes the sulfur preferentially from the Cu2S, leaving metallic copper. The product, which is called blister copper, is porous and brittle, and contains small amounts of impurities. The blister copper is refined to improve its physical properties by electrolytic or pyrometallurgical methods.
The smelting process appears to have been designed originally for iron-bearing copper sulfide minerals containing an excess of sulfur, such as chalcopyrite (CuFeS2). Hence, when non-iron-bearing copper minerals with a deficiency of sulfur, such as chalcocite (Cu2S), are treated, both iron and sulfur must be added to the furnace charge to produce a comparable copper-iron-sulfate matte. Usually, they are added to the smelting furnace in the form of pyrite (FeS2) or chalcopyrite (CuFeS2), depending upon the cost or availability of each. These raw materials are relatively expensive in the Midwest, because they must be transported great distances.
The purpose of this Bureau of Mines investigation was to explore and to evaluate various nonpyritic sulfur- and iron-bearing materials as replacements for the pyrite currently used in the matte smelting of chalcocite concentrates. The substitute sulfur-bearing materials tested were elemental sulfur and gypsum. The substitute iron-bearing materials were either fine-size metallic iron or magnetic taconite concentrates. Various combinations were also evaluated.
Raw Materials
Included in the complement of raw materials were chalcocite flotation concentrate, pyrite, sulfur, gypsum, magnetic taconite concentrate, metallic iron, limestone, and granulated reverberatory slag from commercial copper operations. The chemical compositions of the various raw materials are presented for comparison in table 1. About 10 percent of the copper content of the concentrate was native copper. In addition to the materials shown in the table, Kentucky bituminous coal containing 55 percent fixed carbon, 33 percent volatile matter, and 2.0 percent ash was used in several tests.
Equipment and Procedure
A 35-kva induction furnace, shown in figure 1, was used to heat the smelting charges. It was fitted with a graphite cylinder insulated from the copper induction coil by sheet mica and lampblack. The graphite cylinder was lined with stabilized zirconia to prevent excessive oxidation. Standard fire-clay crucibles (250-ml capacity) were used to contain the furnace charge. The slag temperatures were measured optically.
The general test procedure consisted of combining selected proportions of chalcocite concentrate, smelter slag, and the desired flux and matte-forming constituents to maintain a smelting charge of the following composition: 13 to 16 percent Cu, 4 to 10 percent Fe, 4 to 12 percent S, 14 to 16 percent CaO, 9 to 12 percent Al2O3 , and 29 to 34 percent SiO2. The test mixture was thoroughly blended, and a 300-g charge in a fire-clay crucible was then placed in the induction furnace and heated to 1,300° C. After remaining at temperature for either 2 or 3 hours, the crucible was removed from the induction furnace and cooled to room temperature, and the products were separated, weighed, and chemically analyzed. This testing program was designed to compare the effect of various sulfur- and iron-bearing materials on the smelting characteristics of chalcocite-native copper concentrates, as measured by chemical analyses of the slag and matte products; therefore, to determine the reproducibility of the results, tests were conducted in duplicate, and the average results were reported.
The selection of 1,300° C as the fixed operating temperature was based primarily on previous research experience smelting a wide range of reverberatory compositions. The temperature selected had to be high enough to yield a fluid slag, which promotes a rapid and sharp separation of the matte layer, so that the measured responses would be due to the controlled variations in the experiments rather than to the sluggishness of the system.
The impact of temperature alone was reconfirmed in a series of tests conducted as previously described but on a fixed charge analyzing 15.0 percent Cu,

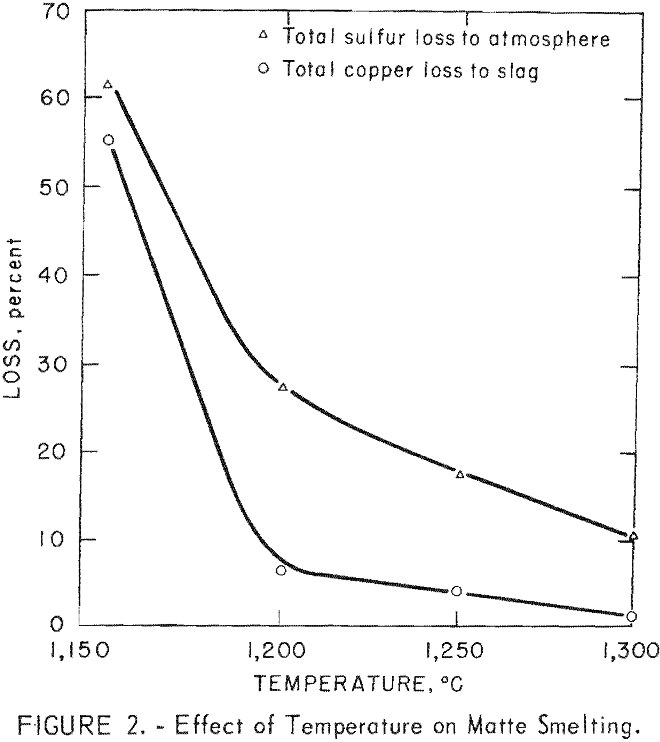
6.0 percent S, 7.5 percent Fe, 13.0 percent CaO, 6.5 percent Al2O3, and 35.0 percent SiO2. The results are plotted in figure 2; the data show that losses of both copper and sulfur diminished as the temperature was raised. The behavior of sulfur may seem to be anomalous, but the authors feel it is not. At all temperatures both evolution and reaction are occurring, but at higher temperatures the matte-forming reactions have accelerated to such an extent that there is apparently less opportunity for sulfur volatilization.
Results
Nonpyritic Smelting With Normal Sulfur
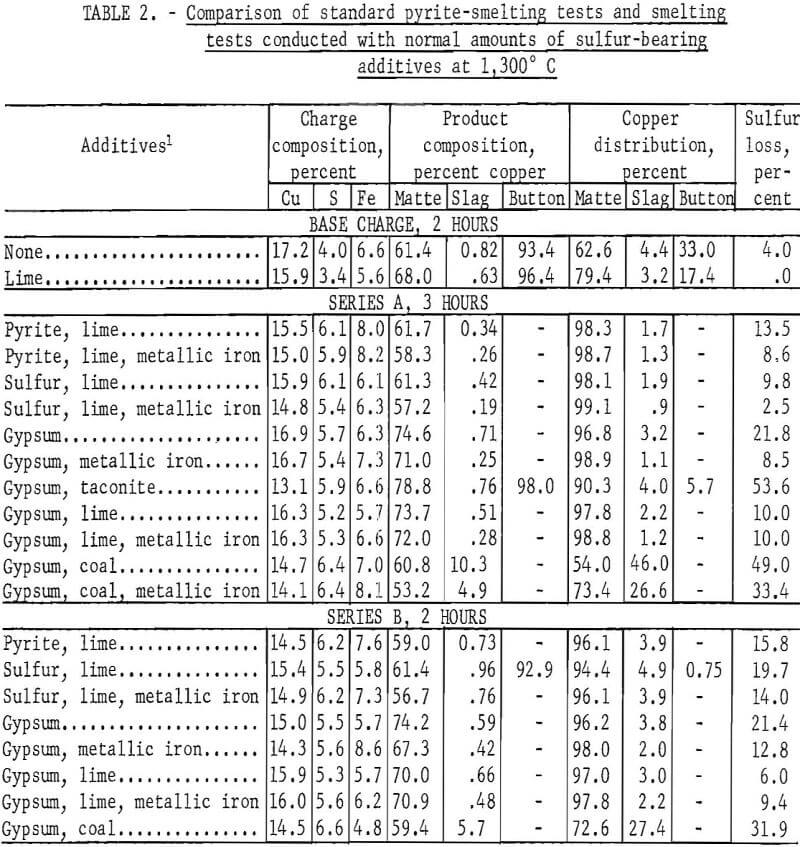
Two series of tests were conducted with sulfur contents of 5.2 to 6.4 percent, which is normal in the sense that one would expect this level in the smelter charge. In series A, 12 tests were each smelted at 1,300° C for 3 hours. Elemental sulfur and gypsum, in conjunction with various iron-bearing additives, were the potential pyrite substitutes. In some tests, coal was added to decompose the gypsum and free additional sulfur for matte formation.
The results are tabulated for comparison with standard pyrite-smelting tests in table 2. From these data it appears that either elemental sulfur or gypsum in combination with metallic iron or limestone can be substituted for pyrite. The addition of metallic iron significantly reduced the amount of copper entrained in the slag. Conversely, the addition of iron oxide in the form of magnetite increased the loss of copper. Also, the use of coal to assist in the decomposition of gypsum was harmful. The matte produced using gypsum contained much more copper than comparable mattes made with pyrite or sulfur.
Test series B was conducted with similar materials and test procedures but for 2 hours only in order to determine if the trends established at the 3-hour exposure time would remain constant if the smelting conditions were changed. This was a rather conservative deviation from the original procedure, but it was effective in increasing the amount of copper reporting to the slag. Despite the increased copper losses to the slag, the results of the B series tests were comparable with the results of, and tended to support the trends established in, test series A.
Nonpyritic Smelting With Excess Sulfur
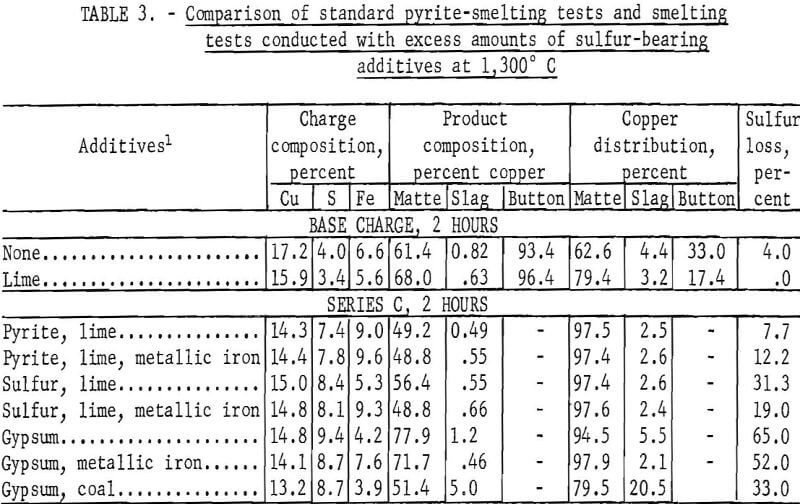
Test series C was conducted with an excess of sulfur-bearing additives; the sulfur content of the charge ranged from 7.4 to 9.4 percent. Each of the seven individual tests was smelted for 2 hours. Elemental sulfur and gypsum, individually and in conjunction with metallic iron, were evaluated as potential pyrite substitutes. The use of coal to create calcium sulfide by decomposition of gypsum was also investigated. The results of this investigation, which are tabulated for comparison with standard pyrite-smelting tests in table 3, complement the results obtained in test series B with which they are directly comparable.
Nonpyritic Smelting With Deficient Sulfur
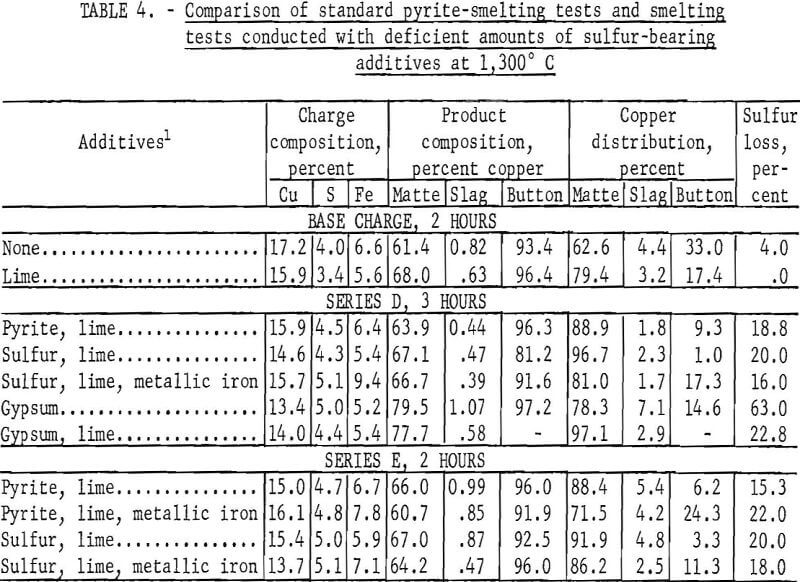
Test series D and E were conducted to investigate the effects of matte smelting with a deficiency of sulfur ranging from 4.2 to 5.1 percent. Series D was composed of five tests, and series E included only four. As in series C, sulfur and gypsum, individually and in conjunction with metallic iron, were investigated as potential pyrite substitutes, but the results were inconclusive. The data are presented in table 4. Owing to the overall sulfur deficiency of the charges, the native copper present in the chalcocite concentrate formed a metallic button after smelting. Under the rather artificial operating conditions of these two test series, none of the matte-forming materials demonstrated any clear advantage.
Discussion of Results
Not surprisingly, the action of elemental sulfur was similar to that of pyrite in smelting. It reacted with iron in both ore and slag to create iron sulfide which ultimately entered the matte. When operating within the so-called normal-sulfur-content range, there was a slight tendency for some of the native copper to remain unreacted with sulfur and to form a button. Also, within the normal-sulfur range volatilization was not serious, and sulfur losses were no greater than with pyrite.
With excess sulfur in the charge, the evolution of SO2 on a percentage basis was much greater than with pyrite. The incorporation of metallic iron in the charge mitigated the sulfur losses but diluted the grade of the matte. Otherwise, in terms of copper recovery and matte quality, the use of sulfur was completely satisfactory.
Gypsum as a Pyrite Substitute
Gypsum was evaluated as a nonpyritic smelting aid individually and in combination with several other materials. This investigation was more extensive than the sulfur study because of gypsum’s availability, low cost, and potential for serving as a fluxing material as well as for supplying sulfur.
The average value of crude gypsum mined in the United States in 1965 was $3.72 per ton.7 Gypsum mines in Michigan and Iowa are conveniently located for use by the Michigan copper industry. Assuming a sulfur content of 18 per-cent, the mine price of gypsum per ton of sulfur is $20.67, compared with $28.50 for dark elemental sulfur f.o.b. Gulf ports. Also, there is waste-product chemical gypsum available which could probably be used to replace mineral gypsum in matte smelting and thereby effect an additional savings in both money and natural resources.
At the temperatures reached in matte smelting, the decomposition of gypsum without a reducing agent takes place rather slowly. However, nearly all of the sulfur added as gypsum either reported to the matte or was volatilized. Traditional reduction agents, hydrogen, carbon monoxide, or carbon, were not present in the test charge; consequently, other materials assisted in the decomposition. One ingredient was, of course, the native copper in the concentrate; another was metallic iron which was incorporated in some of the batches. It has also been reported that compounds such as silica, ferric oxide, and alumina accelerate the rate at which gypsum decomposes. Apparently, the various materials present in the charge combined with the lime to form silicates, aluminates, and ferrites, and the sulfur reacted with the copper and iron in the matte or was volatilized as SO2.
The gypsum-smelting tests produced results substantially different from those obtained in comparable pyrite or sulfur tests; in particular the matte was very rich in copper. In fact, it was not possible to produce mattes of equal copper content with pyrite or sulfur under any of the test conditions. One of the principal reasons for conducting test series D and E with a deficiency of sulfur was to produce a copper-rich matte, but it was not possible with pyrite or sulfur. The composition of the highest grade matte obtainable, Cu2S, is approximately 79.8 percent Cu, and with gypsum it was possible to approach this composition. With pyrite or sulfur, the maximum copper content of the matte was only 67 percent; in some instances, copper buttons were produced, and the matte contained only 61 percent Cu. Unfortunately, not only the mattes but also the slags produced when smelting with gypsum and a deficiency of sulfur contained a greater amount of copper than when smelting with pyrite or sulfur so that the overall recovery was lower.
With gypsum as the exclusive matte- and slag-forming ingredient, recoveries were generally inferior to comparable tests which employed pyrite and limestone. However, the gypsum smelting charges included considerably less iron than the standard charges; to compensate fox this difference, additional experiments were conducted with metallic-iron powder or magnetic-taconite concentrate added to the charge. The addition of the taconite concentrate, which is essentially magnetite (Fe3O4), proved to be a detriment; both the sulfur loss and the amount of copper reporting to the slag increased. Also, a metallic copper button was formed. However, the addition of metallic iron was found to be beneficial; both the sulfur loss and the amount of copper reporting to the slag were reduced. In general, the results obtained with gypsum and metallic iron were superior to those obtained in the standard-smelting test in which pyrite and limestone were used.
The primary reason for the behavioral difference between taconite and metallic iron is that Fe3O4 is a stable oxide and will not combine with the sulfur or the silica; conversely, the metallic iron is highly reactive. The following chemical equations suggest possible means by which metallic iron could react with gypsum to make sulfur available for matte formation:
4Fe + CaSO4 → CaS + 4FeO
3CaS + CaSO4 → 4CaO + 2S2,
2Cu + S2 → 2CuS,
and
2FeO + ½ S2 → 2FeS + SO2
When gypsum is added to a smelting charge in conjunction with an independent fluxing agent such as limestone, the results are superior to comparable tests using gypsum exclusively. Based on a comparison of sulfur and copper recoveries and matte composition, smelting with the gypsum-limestone combination was equal or superior to comparable smelting in which the standard pyrite-limestone combination was employed. However, the use of limestone in conjunction with gypsum was not as effective as the use of metallic iron. The use of limestone in smelting tests employing gypsum in combination with metallic iron did not improve the results.
The use of gypsum and a carbonaceous reductant to smelt copper-bearing furnace slags has been described and patented. Consequently, it was anticipated that the addition of coal would assist the decomposition of gypsum in smelting ores of greater copper content. However, the technique was not successful. The copper reporting to the slag and the sulfur loss to the atmosphere were excessive. An analysis of the smelting products revealed that a crust of coal had formed on the top of the slag. Several clusters of matte were attached to this crust and contributed heavily to the copper content of the slag. A layer of fine coal particles had deposited between the matte and the crucible which may have acted as a thermal insulator, lowering internal temperatures and contributing to the poor results. In addition the coal particles owing to their low specific gravity functioned as a buoyant trap transporting particles of matte to the crust above the slag and increasing its copper content.
