Table of Contents
The Bureau of Mines Twin Cities Metallurgy Research Center is engaged in a study to uncover a cleaner, more direct pyrometallurgical process for winning copper from sulfides. The .initial target is low-sulfur concentrates containing primarily chalcocite (Cu2S). Development work, still in progress, points to a new and, perhaps, improved smelting procedure in which the following sequence of events occur: desulfurization, metallization, melting. The first step, which sought to produce a calcine analyzing less than 1.0 percent S, is discussed in this text; the others will comprise subsequent reports.
The copper industry has experienced a progression of growth with one expansion program after another, but continues to give support to a batch smelting process which is a significant contributor to air pollution. Most copper produced in the United States is derived from ores in which the metal occurs in chemical combination with sulfur, iron, and occasionally other elements. After mining, the ores are beneficiated to reject much of the host rock and concentrate the values into a smaller bulk, At the smelter, pyrometallurgical practices remove most of the remaining unwanted elements to yield metallic copper. Finish refining is usually carried out electrolytically.
In a broad sense, sulfide copper pyrometallurgy is a batch sequence in three separate vessels; that is, reverberatory furnace to yield “matte copper,” a converter to produce “blister copper,” and another reverberatory furnace to produce fire-refined copper. Matte-smelting is relatively unchanged since it became universal practice some 50 to 60 years ago. It is now serving a wide range of smelter feed materials, but many concentrates are not immediately amenable to the matte-smelting process. There may be either an excess or a deficiency of essential matte-forming elements, so adjustments must be made to the furnace charge. Often when smelter feed carries a sufficient excess of sulfur, byproduct manufacturing of elemental sulfur or H2SO4 is incorporated into the metallurgical flowsheet. Other copper ores and concentrates require supplemental sulfur and sometimes other elements if smelted to produce matte and a satisfactory discard slag. Relatively little sulfur is recovered from matte smelting. From the standpoint, of pollution alone, development of a smelting technique for low-sulfur concentrates not requiring the addition of sulfur would be a significant gain.
In the ferrous industry, response to the basic oxygen process and advances in blast furnace metallurgy have created a virtual revolution in steelmaking. A comparable measure of technological success has not been realized in copper metallurgy. This is not to imply a lack of activity because, as noted before, the industry has experienced tremendous growth. Furthermore, there are a number of reports of innovations and systems which deviate from standard practice. For example, oxygen and oxygen-enriched air are being employed to accelerate reactions, to develop higher temperatures, and to promote increased efficiencies.u Oxygen-enriched air is permitting the diversion of some sulfide copper concentrates directly to the converters.5 In one form or another, flash smelting is making inroads in Canada, Finland, Japan, and the U.S.S.R. These techniques are said to combine two of the three smelter operations into one, thus making possible economies in heat utilization and the evolution of favorable sulfur gases for byproduct manufacture. Soviet metallurgists have under study a continuous converter and a cyclone smelting system. In Australia, the Worcra process promises to provide a flow-through smelting system from concentrate to blister copper to replace the batch system involving three furnaces and three product transfers. These developments, as well as many in early stages of study, indicate widespread interest and a desire to upgrade the metallurgy of copper.
Partial roasting of sulfide copper minerals was practiced industrially for many years as a practical means of adjusting sulfur content to appropriate levels for matte-smelting. The development of fluid-bed systems has introduced possibilities of selectively roasting one or more mineral components in the same ore by judicious regulation of roast temperature and atmospheres.
With regard to chalcocite, there have been a number of research studies which divulged the kinetics and mechanism of roasting synthetic Cu2S or selected mineral specimens. From these, and their own work, Wadsworth and coworkers concluded the oxidation of Cu2S to yield CuO may be divided into three general stages (all of which may occur simultaneously): (1) Primary oxidation to Cu2O, (2) secondary sulfate formation, and (3) sulfate decomposition. Individual steps during the oxidation resulted in the sequential appearance (and disappearance) of the following phases: Digenite (approximately Cu1.8-S), Cu2O, CuSO4, CuO·CuSO4 and CuO. Lower temperatures were favorable to the formation of sulfates; above 800° C the sulfate layers were absent. A rather interesting observation at 850° C was that the presence of 50 to 70 percent SO2 in the atmosphere accelerated the oxidation of Cu2O to CuO.
Unpelletized Concentrate
Raw Materials
The desulfurization program has centered about chalcocite concentrates (from a large, integrated mining operation) which were essentially minus 100 mesh, with about 90 percent minus 400 mesh. Chalcocite (Cu2S), together with minor amounts of cuprite (Cu2O), covellite (CuS), chalcopyrite (CuFeS2), and bornite (Cu5FeS4), accounted for about 90 percent of the copper in the concentrates; the remaining 10 percent was present as native metallic copper. The major gangue mineral was quartz but there were significant quantities of kaolinite and sericite as well.
The initial sample, a flotation concentrate (sample 1) which was somewhat lower than usual in copper and sulfur content, was the focus of some laboratory research, particularly in the unpelletized condition. It had the following analysis in percent: Cu-26.1, Fe-4.6, S-5.9, CaO-0.88, Al2O3-8.9, SiO2-37,6. Its specific gravity was 3.48, the average particle size was 5.3 microns, and the specific surface area was 3,260 cm²/g. Table 1 presents chemical and sizing analyses of two higher grade concentrates (samples 2 and 3) which are probably more characteristic of present mill practice. These were pelletized and constituted feed materials for tests in the small kiln and 4-inch fluid-bed reactor.
Cold Tube Fluidization
The initial attack on the problem of desulfurization was made in a fluid-bed reactor. A number of tests were made beforehand in a 4-inch-diameter by 54-inch-long Lucite tube to define the conditions under which the concentrate could be fluidized. Segregation tendencies as well as the drift of airborne fines were visible to a depth of 50 inches. Air requirements, furnished by a plant compressor system, passed through a moisture trap before being regulated by flowmeter to the base of the cold tube. The latter was fitted with a precisely matched steel ball and cone inlet.
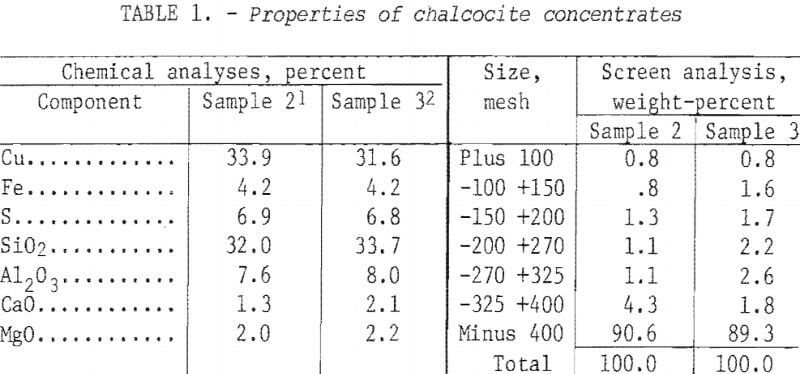
Batch tests of 1 hour each with a constant bed weight (8 lb) were run with a variable airflow and the data are plotted in figure 1. Note that satisfactory fluidization occurred from 0.50 to 0.70 scfm higher airflow rates caused severe channeling, or slugging, as a result of the coalescence of numerous small air bubbles into large bubbles. The latter caused irregular and unsatisfactory operation of the fluid bed.
Hot Fluidization
Roasting experiments were carried out in a fluid-bed reactor (fig. 2) at elevated temperatures. This apparatus was basically a 4-inch-diameter by 108-inch-long type 316 stainless steel column encompassed in an 18-inch-square flue. The flue was low-thermal-conductivity brick supported by a steel angle-iron frame. The base of the flue was open and the top cover connected to an exhaust stack system. The reactor was heated by the combustion of natural gas in four flame nozzles set in a gas ring at the base of the reactor. Temperature within the reactor was measured by five thermocouples located along the vertical axis. An automatic temperature controller was connected to the No. 3 thermocouple located 24 inches above the base of the reactor tube, Fluidizing air, after passing through a moisture trap, was regulated by flowmeter through a 1-inch ceramic ball check valve inside the base of the reactor tube. An overflow discharge pipe attached to the reactor base plate and extending into the reactor column established fluid-bed depth which could be varied from 12 to 52 inches.
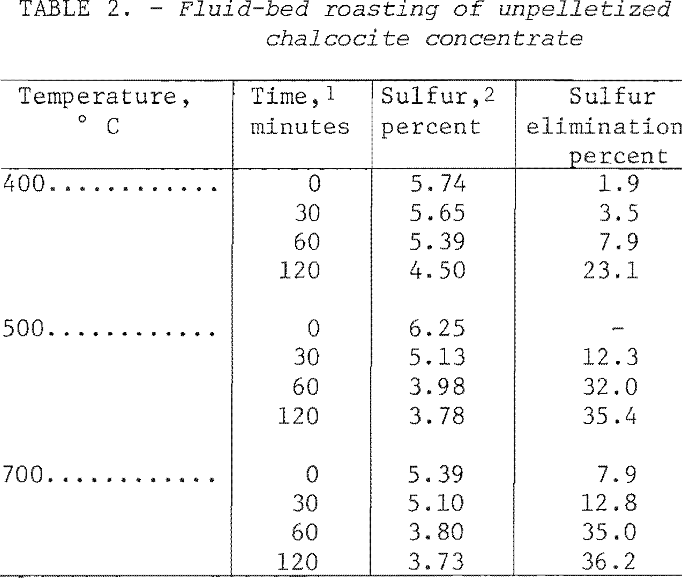
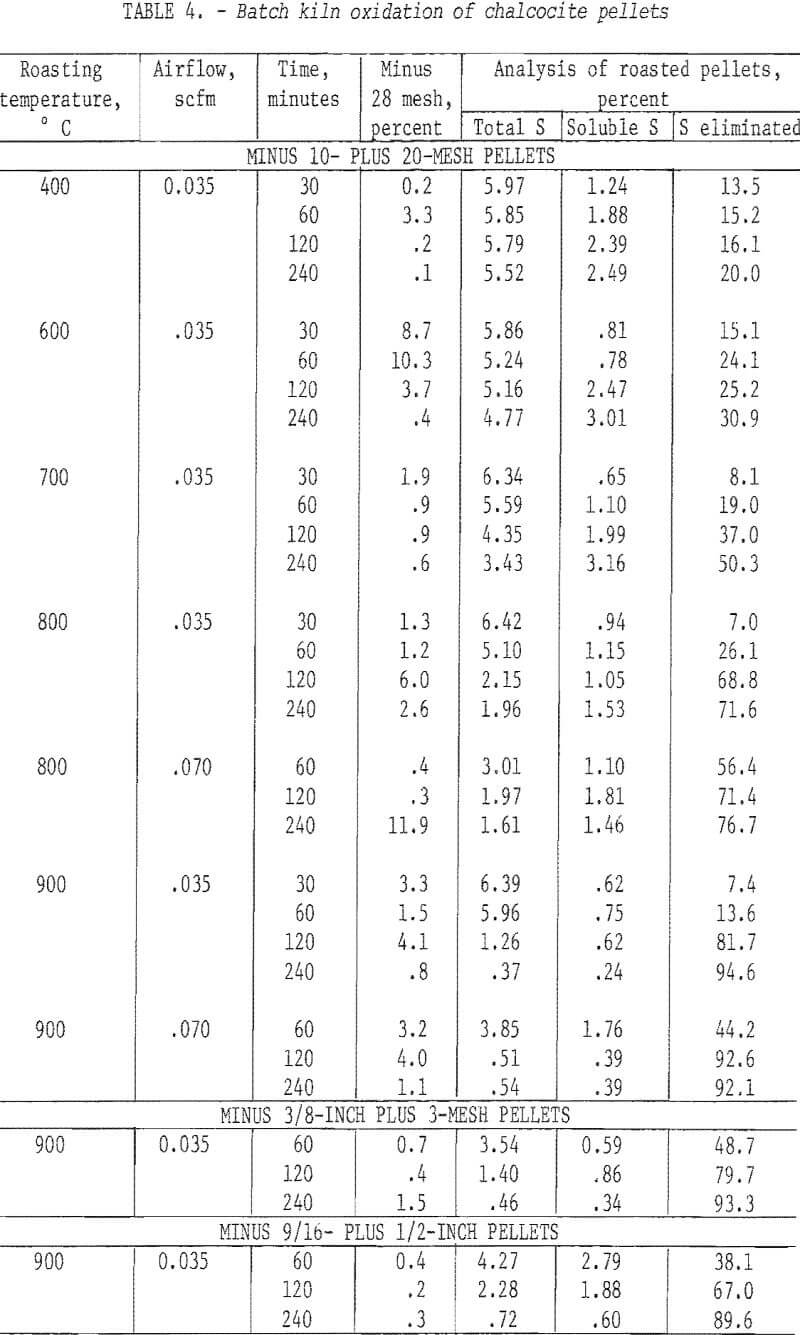
Utilizing a 0.50-scfm airflow rate a series of oxidizing roasts was conducted on chalcocite concentrate with variations in time and temperature. The procedure was as follows: An 8-lb batch of dry concentrate was placed in the reactor, fluidized with nitrogen, and preheated At the desired temperature, oxidation roasting was initiated by switching from nitrogen to air. Sampling was accomplished by momentarily opening the bottom drawoff valve. The data, presented in table 2, show that from 400° to 700° C, little more than a third of the sulfur was evolved at best after 2 hours Petrographic examination revealed the remaining sulfides were well coated with oxides and sulfates. Penetration through these layers was apparently especially difficult. It appeared that higher temperature, prolonged roasting, and a greater oxygen content in the bed or some combination of these was essential to a reasonably complete oxidation of the sulfides.
Tests at 800° C were totally unsatisfactory. Concentrate grains showed an accentuated tendency to adhere to each other or to the reactor, so that agglomeration and defluidization occurred. At the usual low airflow rates, fusion of the bed occurred frequently. At moderate airflow rates, a goodly proportion of fine solids was carried out of the system as dust. This cut retention time to a minimum, with the result that only token quantities of sulfur were removed from these fractions.

Experimental Work—Pelletized Concentrate
Pelletizing
The above experience with dry concentrate did not encourage further endeavor. The sintering at high temperature might have been alleviated by introduction of a diluent but airflow rates could not be raised substantially without ejecting too much of the charge from the reactor. Use of air-oxygen mixtures could restrain the space rates, but our limited experience with this course indicated it would encourage fusion of the bed.
The solution was to agglomerate the concentrate into minipellets prior to roasting. Even with very small pellets, the airflow rate required in a fluid-bed system would be substantially higher than for concentrate, but it could be kept within bounds by limiting the pellet size. However, by purposely making larger pellets the use of other vessels such as a rotary kiln was made possible.
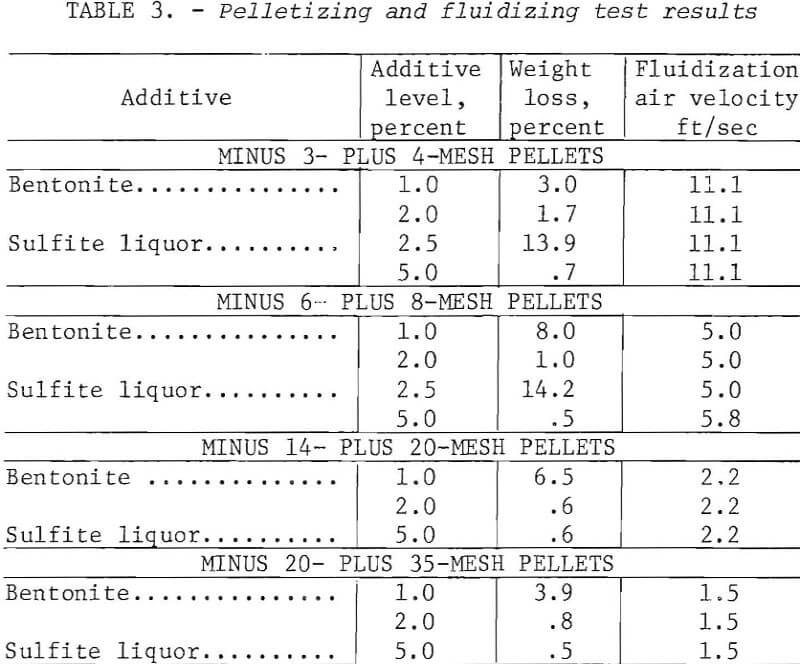
The chalcocite concentrate was pelletized as follows: Batches of 500 g including binder were blended to a moisture content of 8 to 10 percent and slowly hand-fed into a 16-inch-diameter by 5-inch-deep unlined steel drum rotating at 50 rpm. The contents of the drum were removed periodically and screened to separate the desired size; the unconsolidated material was recycled. When a sufficient quantity of sized pellets had been collected, they were returned to the drum alone and rerolled for a few minutes to improve the quality. Pellets ranging in size from 35 mesh to ½ inch were prepared using either bentonite or sulfite liquor as binders.
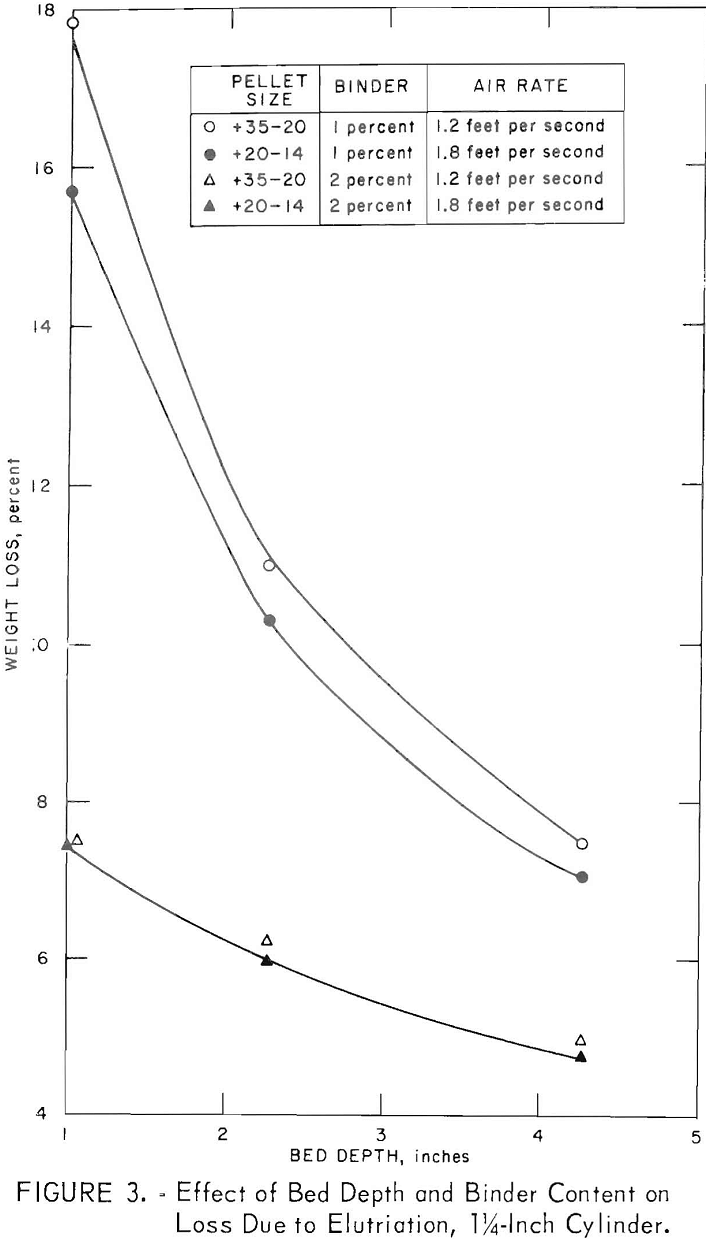
Table 3 gives the properties of some small pellets produced as above and dried at 105° C. For pellets smaller than 3 mesh, the usual criteria for evaluating unfired pellets (for example, green and dry compression strengths, drop number) were meaningless. An abrasion and degradation test was developed using a 1-¼-inch-diameter cold tube as a test vessel. Small quantities of dried minipellets were fluidized for 4 minutes at 150 percent bed expansion, then dust losses and degradation were determined. Longer tests (30 minutes) were carried out to check the influence of bed depth as well as binder content. These data, presented in figure 3, show that deeper beds were a factor in preserving integrity of the pellets.
In general, the pellets were somewhat slow to nucleate and grow but satisfactory pellets were achieved. The rate of growth, quality, and sphericity were a function of the binder level, and 2 percent bentonite proved to be superior to 1 percent. Good pellets also resulted from the use of 5 percent sulfite liquor.
Drum Kiln Tests
A 4-inch-diameter by 6-inch-long stainless steel drum was used to simulate kiln roasting. The vessel was supported on its horizontal axis by a heavy steel pipe which served as a hollow shaft; a screw cover closed the front end. A gas and thermocouple line passed through the pipe shaft into the drum and adequate seals were provided in the system to assure complete atmospheric control. The small rotary kiln and drive mechanism (fig. 4) were mounted on a steel frame provided with casters to facilitate introducing and removing the kiln from the electric muffle furnace used to furnish heat for close control of roasting temperature. As a temporary substitute for the regular muffle door, two firebricks, in equal halves, closed the front of the muffle around the drum shaft.
Three sizes of pellets, minus 10 plus 20 mesh, minus 3/8 inch plus 3 mesh, and minus 9/16 inch plus ½ inch, were prepared with 2.0 percent bentonite and were desulfurized in the small kiln. The tests were limited to small-scale experimentation in which pellet size, temperature, time, and airflow through the vessel were variables. The charge weight, 200 g, was constant.
Table 4 presents the data from drum roasting. The column headed “minus 28 mesh” resulted from screening the product pellets to gage degradation. Generally, the pellets held up well; only two batches degraded more than 10 percent and there were no obvious reasons, other than normal experimental error, to account for these two. Adequate desulfurization was accomplished only at 900° C and most remaining sulfur was water-soluble. This was surprising in view of the temperature and indicated that the calcined material contained CuSO4 or a basic sulfate.
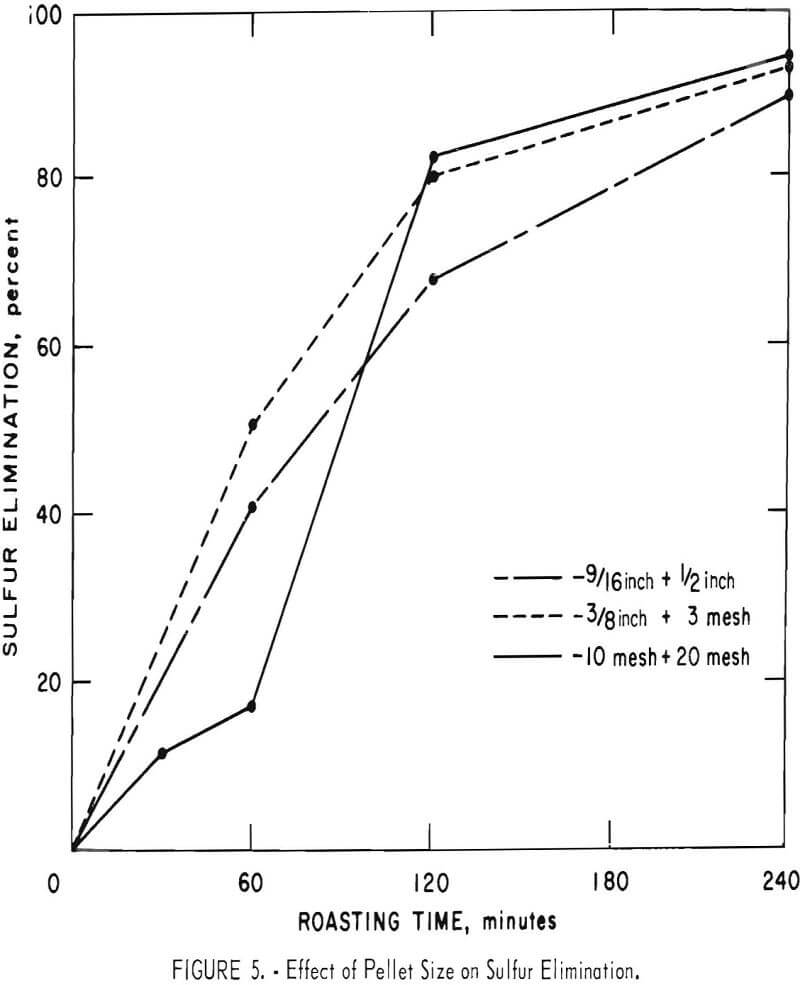
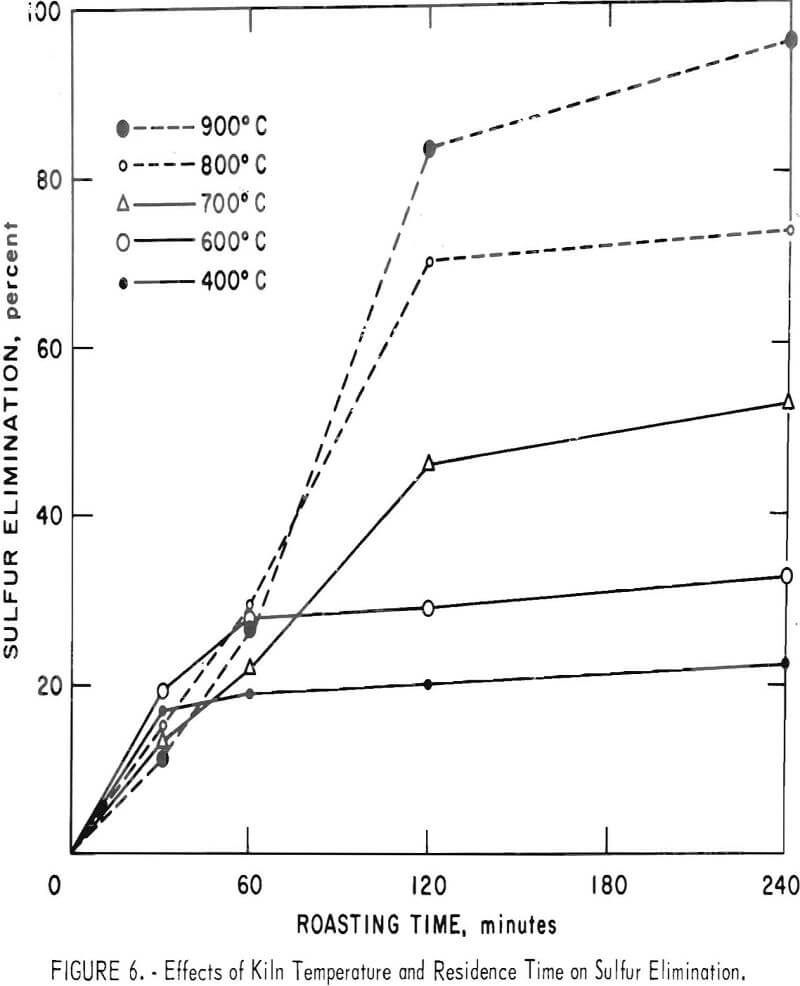
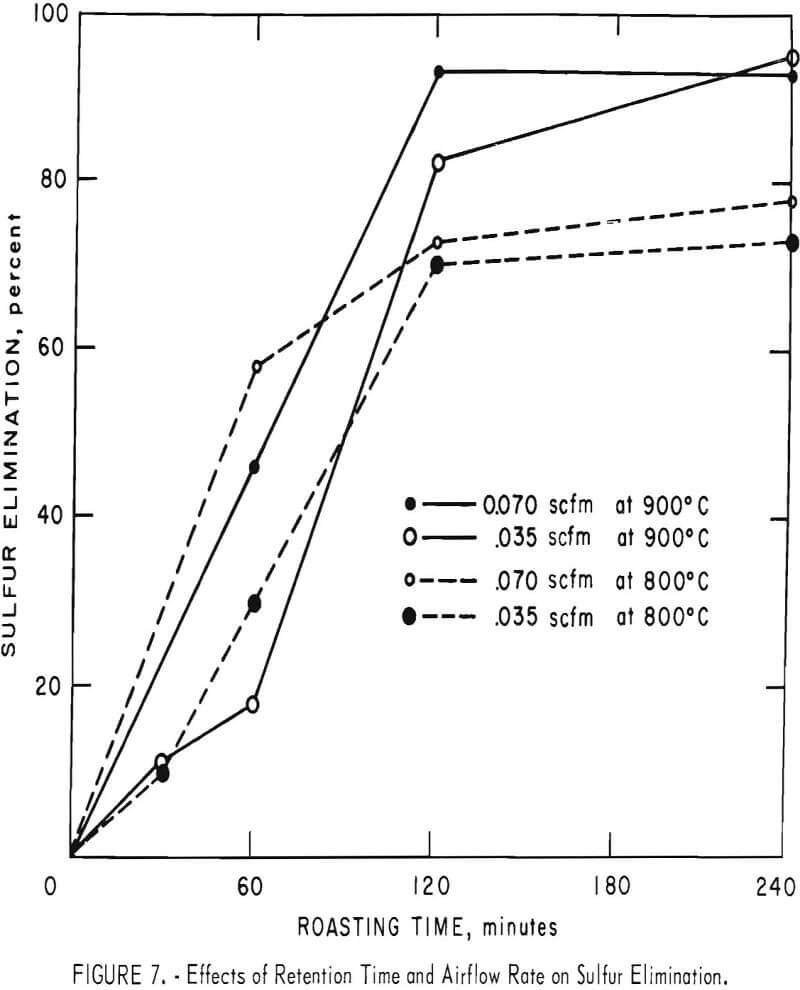
Some of the data of table 4 have been plotted to portray graphically the impact of pertinent variables. From figure 5, it appears that the ½-inch pellets were a bit too large for reasonably complete desulfurization, but there was no significant difference in the behavior of the two smaller sizes. For a kiln process, pellets of about ¼-inch diameter would combine the attributes of convenience in manufacture, reactivity and durability. Figures 6 and 7 display the effects of temperature and airflow and it is seen that only at 900° C were the results satisfactory. There is an indication that a substantial excess of air will accelerate the reaction somewhat. By comparison with data presented later it will be noted that much less air was admitted to the kiln than that blown into the fluid-bed reactor. This reflects only the additional air required for fluidization. Below 800° C much CuSO4 was in evidence and longer time spans merely resulted in conversion of Cu2S to CuSO4 or CuO-CuSO4 with little evolution of SO2.
Continuous Fluid-Bed Roasting
Although the results of the drum kiln tests at 900° C attained a low level of product sulfur, it was felt the potential advantages of fluid-bed operation made it worthy of further investigation. The fluid-bed concept lends itself equally well to both oxidation roasting and reduction roasting, providing for both control accuracy and transport convenience. Therefore, desulfurization followed by reduction to metallic copper could provide two satisfactory stages of the proposed recovery process.
With the experience gained in the earlier experiments, it was possible to proceed directly to round-the-clock operation of the 4-inch fluosolids reactor on minipellets. Measured quantities of chalcocite flotation concentrates, binder, and water were blended in a 36-inch-diameter paddle-type mixer and pelletized in the 36-inch-diameter stepped disk shown in figure 8. Most of the desulfurization roasts were made on minus 10- plus 20-mesh pellets
bonded with 1 to 2 percent bentonite. A double-deck mechanical shaking screen was used in sizing test lots for pilot plant studies. Screen undersize was returned to the pelletizer as seed, the oversize was recycled by way of the mixer and then back to the pelletizer, thereby closing the circuit. The small, moist balls were dried at 105° C.
To top-feed the system, a pipe was welded to the reactor tube at a suitable inclination, above the surface of the teetering column. New feed dropped through this pipe to the top of the fluid bed. Roasted product was drawn off through a valve at the bottom of the reactor. An air-lock table feeder was used for bottom feeding. Solids to be reacted were entrained with air, then carried through the ball check valve and into the fluid column. New feed thus forced an equal volume of roasted materials into the overflow pipe. During continuous runs feed was introduced and product discharged at specified time intervals.
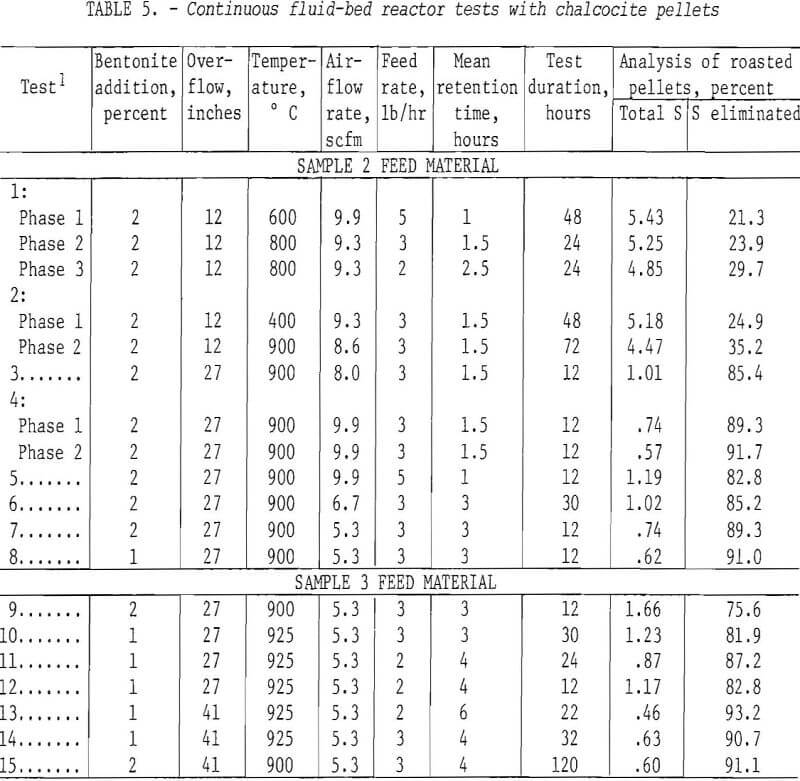
A number of continuous runs were made within a wide range of temperatures varying the feed rate and bentonite content of the minipellets. The campaigns ran from 1 to 5 days. The system reached equilibrium within relatively few hours, so in the lengthier campaigns, it was possible to complete several individual tests. The data have been gathered in table 5. As indicated in table 1, pelletized samples 2 and 3 were the feed materials for tests 1-8 and 9-15, respectively.
One of the earliest experiments in the 4-inch reactor, that of top feeding, was completely unsuccesful. Introduction of chalcocite pellets into the hottest zone plus the exothermic oxidation of the copper sulfide caused rapid fusion of the bed even at excessive airflow rates. Bottom feeding, on the other hand, was completely trouble-free. Evidently, this permitted some time for the pellets to reach maximum temperature during which the heat of reaction was absorbed into the system. An endeavor to achieve more complete elimination of sulfur by two-stage roasting had the opposite effect. Typical data
are the runs of tests 1 and 2 in table 5 where the first stage was operated at 400° or 600° C and the second at 800° or 900° C. The operation was very smooth mechanically, but the sulfur content of the products proved only that normal and basic copper sulfates formed at lower temperatures are extremely difficult to decompose, even at 900° C. Examination of typical products by X-ray disclosed only tenorite (CuO) and dolerophanite (CuO·CuSO4). The microscope revealed very small amounts of copper sulfides chalcocite (Cu2S) or digenite (Cu1.8S) and cuprite (Cu2S) as well, but no CuSO4. Chemical analytical evidence proved that soluble sulfates of copper were present in pellets calcined at 900° C. This was contrary to the findings of Wadsworth and coworkers, who found only traces of CuO-CuSO4 above 850° C, but their roasting time extended beyond 12 hours as compared to less than 4 hours (usually) in the present study.
All the succeeding campaigns, which ranged from 12 hours to 5 days, were conducted at 900°-925° C. Judging from the sulfur analyses of the product, which was the principal criterion of success, the objective was achieved. Sulfur contents well under 1.0 percent were achieved, and the results could be duplicated within the limits of experimental error.
Concerning the major operating variables, after temperature, residence time is most important. Retention was a function of three factors: feed rate, airflow, and the height of the central overflow pipe. The average retention ranged from 1 to 6 hours, but these are strictly operating estimates. Furthermore, the fluid-bed system is analogous to a stirred-tank reactor so that while some feed grains exit almost immediately, others remain for many hours Feed rate and overflow height exercise the most direct influence on time. Three pounds per hour was established as a good workable feed rate but, in retrospect, it is likely that 4 or 5 lb/hr could have been managed. Similarly, the 12-inch pipe did not use enough of the reactor, but 27-inch and 41-inch overflows were satisfactory.
Airflows determine the bed expansion, so the dilution factor also regulates residence time. In the early runs at lower temperatures, perhaps because of the rather indifferent desulfurization, there was a tendency to operate with a great air excess. This persisted into the 900°-925° C campaigns but was gradually brought down to about half the original space rate. This was still well above the fluidization velocity and supplied more than enough oxygen to the system. It is known that excessive oxygen to dilute the SO2 evolving from the solids is not essential to insure continued desulfurization. We have discovered, as did Wadsworth and coworkers, that chalcocite and oxygen will react in the presence of large percentages of SO2. However, a certain air volume is essential to dissipate reaction heat and inhibit agglomeration and possible defluidization.
As noted earlier, there was a marked quality difference between 1- and 2-percent-bentonite minipellets which, in the 4-inch reactor, was reflected in the dust load. The last two campaigns of table 5, those of tests 14 and 15, were quite similar in other respects than bentonite content. Based on weights of end products only, the dust in test 2 comprised 27.5 percent while that of test 15 was only 8.0 percent. A dust load of 10 percent or less can be conveniently redirected to the pelletizer circuit with fresh concentrate. These data emphasize the importance of structurally sound pellets for fluosolids roasting of chalcocite. The copper oxide pellets which survive are strong and porous and can be readily reduced to metallic copper in the second step contemplated in this envisioned process. For example, in subsequent fluid-bed tests, about 90 percent of the copper in roasted pellets was converted to metal by reduction with hydrogen at 800°-900° C.
Summary and Conclusions
As one phase of development research to improve the metallurgy of copper smelting, roasting experiments were conducted on commercial chalcocite concentrates. This material (26-34 percent Cu and 6-7 percent S) was so fine (less than 5 microns average particle diameter) that it could not be desulfurized in the natural state without severe mechanical difficulties. Preparation of small pellets with 2 percent bentonite made it possible to use either a small rotary kiln or a 4-inch fluid-bed reactor to achieve more than 90 percent sulfur elimination. Pellets of minus 10 plus 20 mesh, minus 3/8 inch plus 3 mesh, and minus 9/16 inch plus ½ inch were kiln-roasted at 900° C in air to sulfur contents of 0.37, 0.46, and 0.72 percent, respectively. Minipellets (minus 10 plus 20 mesh) were continuously desulfurized (at 900°-925° C) in a 4-inch fluid-bed reactor in campaigns up to 5 days’ duration to yield roasted material with 0.60 percent S. Surprisingly, considering the high temperature, most of the residual sulfur was water-soluble.
The objective was to make a copper oxide which would adapt readily to the next processing step, namely gaseous reduction to the metal. In this respect, the fluid-bed product was especially good, being strong, porous, and readily reducible, so the two-stage fluidization process from concentrate to metal seems technically attractive.
