The principal characteristics of the process is that when applied in medium or in great degree, it only needs unelaborated raw materials such as sodium chloride and calcium carbonate, or of medium elaboration, as sulphur which is found in large quantity in the North of Chili however, the system may be also applied to small copper plants provided that low cost sulphuric acid is available as a substitute for sulphur, that is, in those plants of small capacity where large additional installations are not needed for the regeneration of the leaching solution.
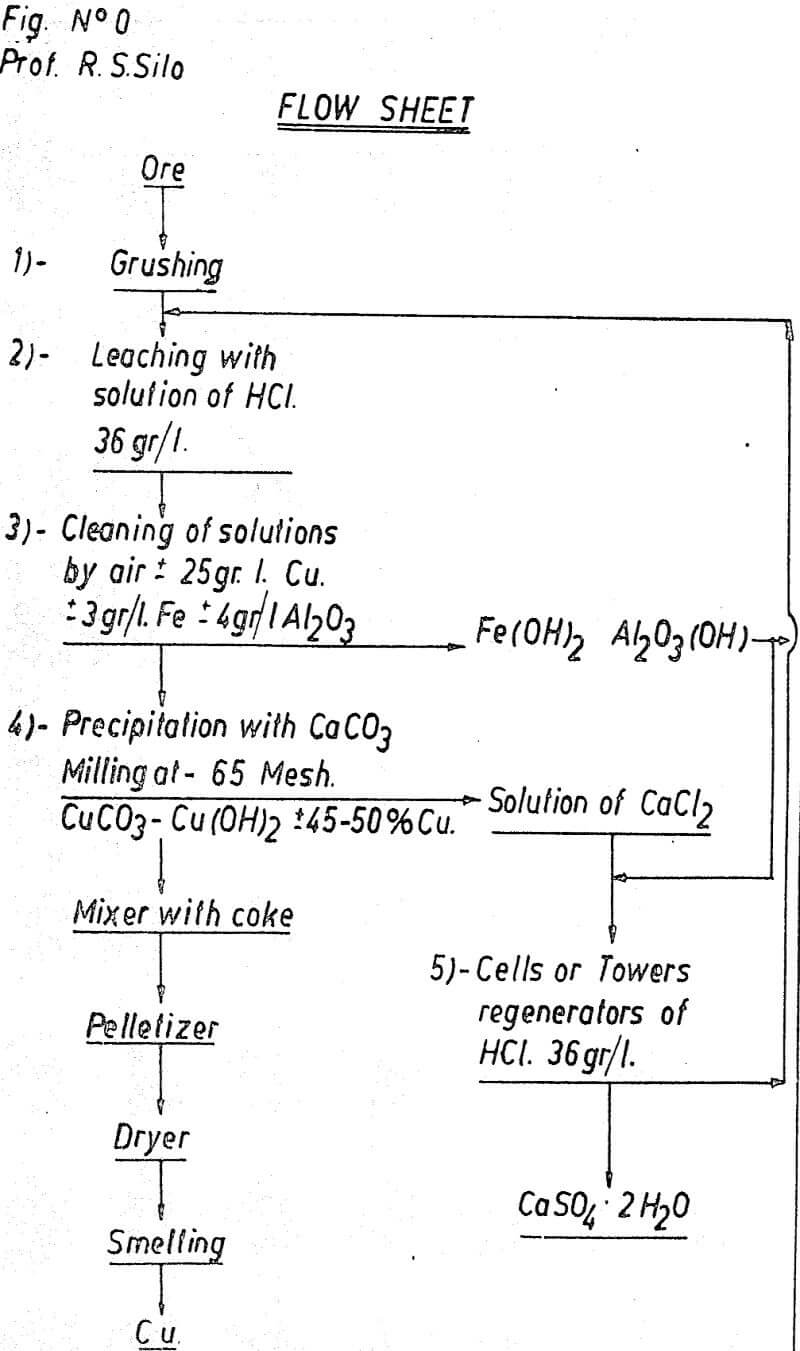
Leaching
The process requires the use of hydrochloric acid as leaching solution when treating copper ores, which may be produced in the plant itself. The operation is very simple, using sulphuric acid and NaCl as raw materials.
Our experiences have been done with solutions containing 36 grs. of hydrochloric acid per liter of solution in order to have a free acidity equivalent to 50 grs/liter of sulphuric acid which is the usual concentration of a leaching solution.
Hydrochloric solution has been prepared taking into account the stechiometric calculation of the reaction:
a). H2SO4 + 2 NaCl = 2 HCl + Na2SO4
The behavior of the leaching solution in front of copper specimens may be represented by the following systems of reactions:
b) CuO + 2 HCl = CuCl2 + H2O
c). Cu2O + 2 HCl = Cu2Cl2 + H2O
d). CuO·CuCO3·H2O + 4 HCl = 2 CuCl2 + 3 H2O + CO2
e). CuSiO3·2 H2O + 2 HCl = CuCl2 + 3 H2O + SiO2
f). Cu2(OH)3Cl + 3 HCl = 2 CuCl2 + 3 H2O
Gangues react in leaching in big or small degree, according to their content of Fe, Al2O3 or CaCO3:
g). Fe2O3 + 6 HCl = 2 FeCl3 + 3 H2O
h). CaCO3 + 2 HCl = CaCl2 + H2O + CO2
i). Fe(OH)2 + 2HCl = FeCl2 + 2H2O
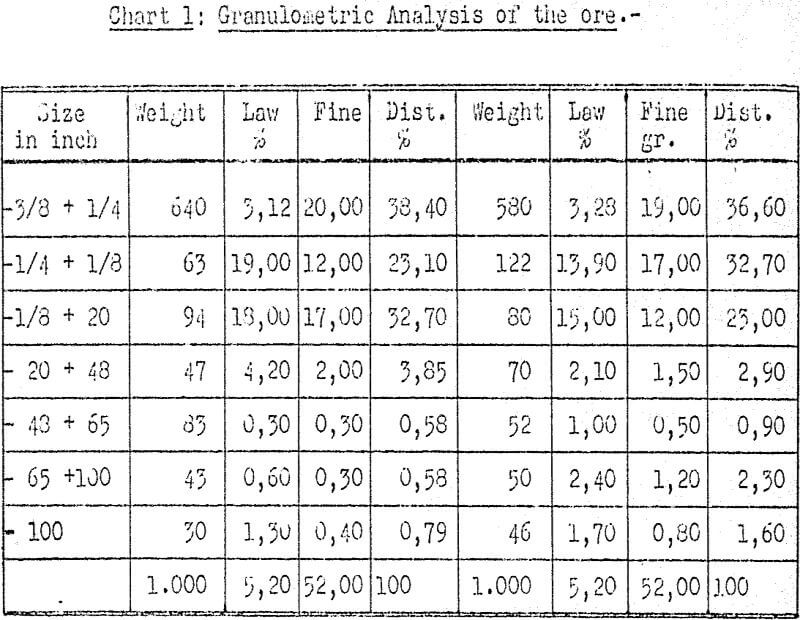
Percolation took place in 4 PYREX containers with a capacity of 15 liters, 11½” diam, 12″ high with semispheric bottom; each one having an additional wooden bottom with ¼” holes a plastic tube of ¾” and 10″ high was placed as air-lift. The air was generated at 6 pounds per square inch by means of a rotating laboratory compressor having a ½” outlet.
Time of treatment for each unit fluctuated between 72 and 80 hrs., including 2 washings with clear water to remove impregnations.
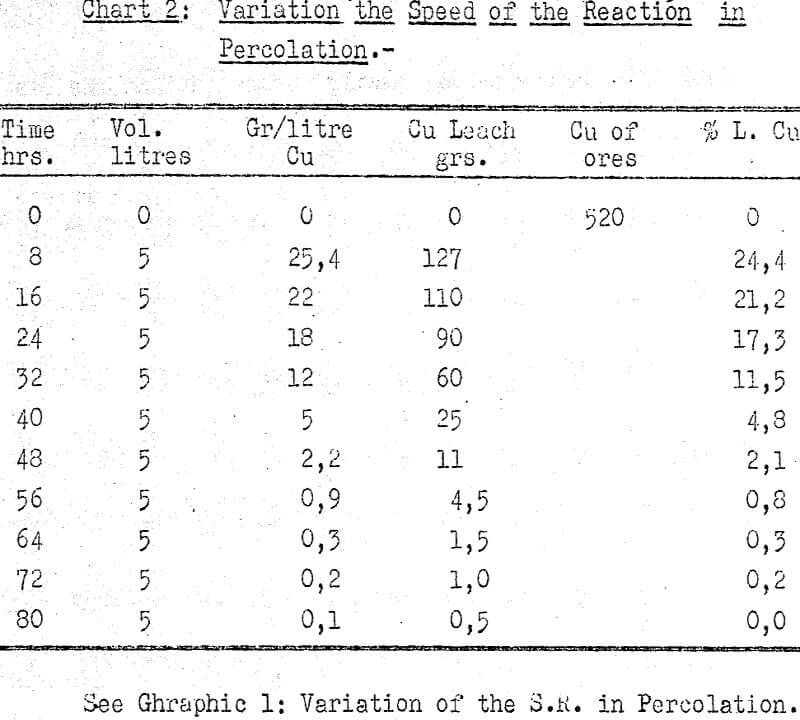
The copper contents in the solutions was from 22 to 26 grs/l. The iron contents in ferrous ions fluctuated between 2 and 5 grs/l, the highest figures corresponding to the starting solution.
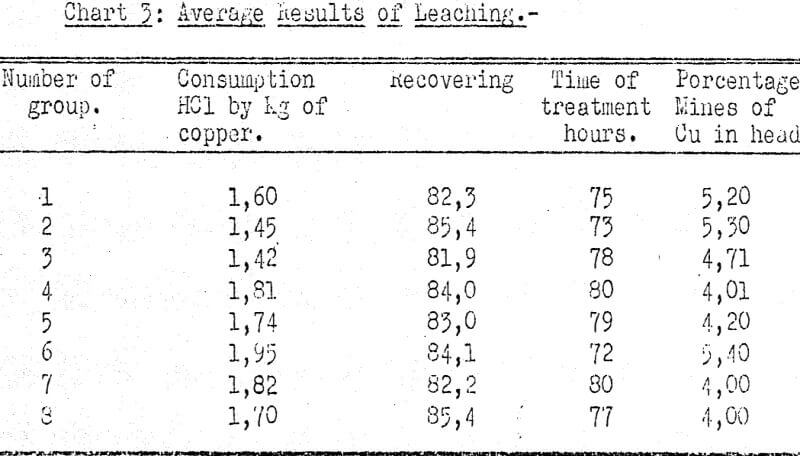
Washing of the Solutions
The solutions from the leaching process contain more or less Fe2O3 or Al2O3 which depends on the characteristics of the ore being treated. In our particular experiences the solution from percolation contained the followings amounts.
Since the precipitator used in the process is CaCO3, which displaces the copper, the alumina and the iron, it is necessary, to eliminate the Fe and the Al in order to avoid contamination of the cupric precipitate.
This operation is advisable in all those plants in which blister copper, or refined copper is wanted by fusion of the precipitate.
In all those plants which sell the precipitate directly without fusing, washing of the solutions is not necessary.
Precipitation
Reactions are violent and the precipitation of copper is total with a moderate agitation. An agitator with variable speed having a wooden rotor with 2 propellers on the axis was used.
Experiences were done in flasks of 15 liters of capacity using the principle of countercurrent.
Three precipitate units each containing 500 grs. of powdered limestone (small see shells) were used for the tests; starting from one of them countercurrent was established so as to obtain the exhausting of the Cu in the solution and of CaCO3 used as-precipitator.
Only a gentle agitation was needed to keep the limestone particles suspended.
The hardest step in the precipitation test was to find the ideal grinding degree of limestone in order to obtain its total exhausting during the reaction. In fact, as the precipitator was a solid it was to be expected that the particles were covered with a coat of precipitate which would interfere the progress of the reaction into the grain of CaCO3 consequently the products would contain a low per cent of Cu and a high per cent of limestone.
Regeneration of Leaching Solution
The residual solution treated with concentrated H2SO4 reacts quantitatively and instantaneously at ordinary temperature. To dose the H2SO4 it will do to titrate the calcium solution in its chlorine contents basing on the stachiometric calculation.
Chlorine losses all through the circuit are replaced by adding equivalent amounts of NaCl during this stage.
As stated at the beginning of this report, no excess of H2SO4, should be added during this step of the process to avoid the production of CuSO4, which reacts in presence of limestone producing gypsum in the precipitation process; this material besides contaminating the product causes serious difficulties in fusion step.
Since all of them are solid cupric salts mixed with gypsum, the inconvenience of this phenomenon is easily understood.
The clarified hydrochloric solution returns to the head of the cirduit to be recycled.