Table of Contents
Coal mine operators in the United States were required to make available to each underground coal miner a self-contained self-rescuer (SCSR). The regulations (30 CFR 75.1714) require that each person in an underground coal mine wear, carry, or have immediate access to a self-rescuer that provides an oxygen source. The oxygen self-rescuer will replace the filter self-rescuer (FSR) as primary escape equipment. FSR’s protect only against low levels of CO.
In an effort to compare performance of the six SCSR’s approved by the National Institute for Occupational Safety and Health (NIOSH), a breathing and metabolic simulator (BMS) was used to test the apparatus at a work rate equal to the average rate of a 50th percentile miner performing man-test 4 for 1 h as described in 30 CFR 11H. In addition to the six U.S. apparatus, six other oxygen self-rescuers from different countries and four prototypes developed for the Bureau over the past decade and stored since then were tested. All of the apparatus tested are listed in table 1, with their rated durations (service lives), country of origin, oxygen source, and weight.
It was not attempted to do an in-depth analysis of the design of each apparatus but merely to scrutinize performance behavior.
Description of an Oxygen Self Rescuer
All of the apparatus tested are of two types: chemical oxygen or compressed oxygen. Most of the chemical-oxygen apparatus tested use potassium superoxide (KO2) for both the oxygen source and the CO2 absorbent. One of the chemical-oxygen apparatus uses a sodium chlorate candle for an oxygen source and a separate chemical bed for CO2 absorption.
The compressed-oxygen apparatus use bottled oxygen under high pressure for the oxygen source with a separate chemical bed for CO2 absorption.
The two types of apparatus share many common parts, such as the mouthpiece, breathing hoses, CO2 absorbent bed, breathing bag, and relief valve. The compressed-oxygen self-rescuers need some extra parts not necessary with chemical-oxygen self-rescuers. These include a high-pressure storage bottle, a regulator to step down the high pressure, a constant-flow valve, and in most cases, a demand valve.
There are many variations possible in the size, shape, and placement of the various components necessary in any breathing apparatus, as will be seen in the separate descriptions of the apparatus tested that follow.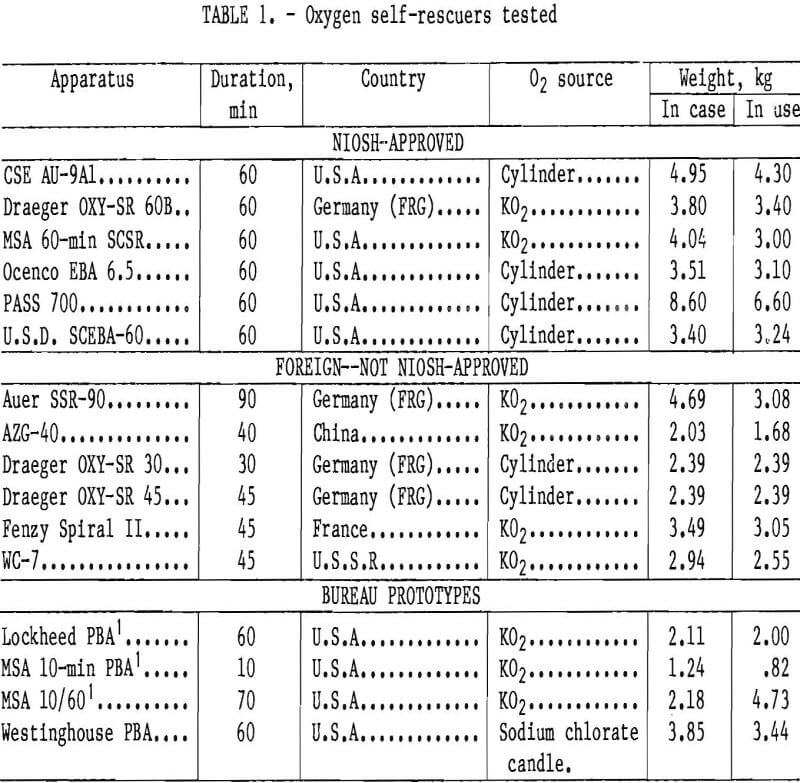
Niosh-Approved
CSE AU-9Al
The AU-9Al (figs. 1-2) is a compressed-oxygen self-rescuer with a throwaway steel bottle but otherwise reusable parts. It is field serviceable by trained personnel only. It has a bi-directional flow path, a constant O2 flow of at least 1.5 L/min, and a pressure-activated demand valve and relief valve. The cylinder contains 130 L of oxygen, and the CO2 absorbent is LiOH.


Draeger OXY-SR 60B
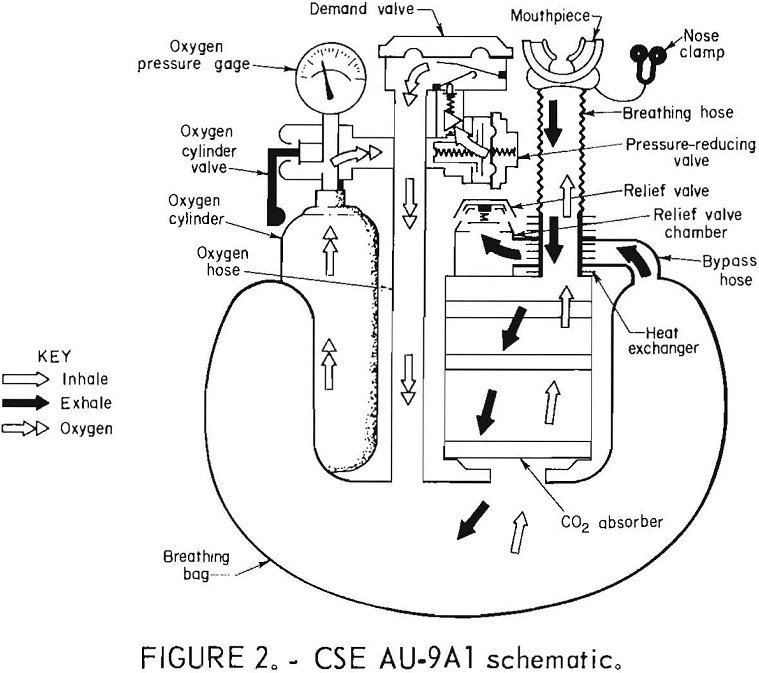
The OXY-SR 60B (figs. 3-4) is a chemical-oxygen self-rescuer which can be returned to the distributor, National Mine Service, for refurbishing. It has a unidirectional flow path through the KO2 (potassium superoxide) bed and a pressure-activated relief valve. A chlorate candle is provided for an initial spurt of oxygen until the KO2 bed is sufficiently activated by the user’s breath. The KO2 is pelletized.

MSA 60-Min SCSR
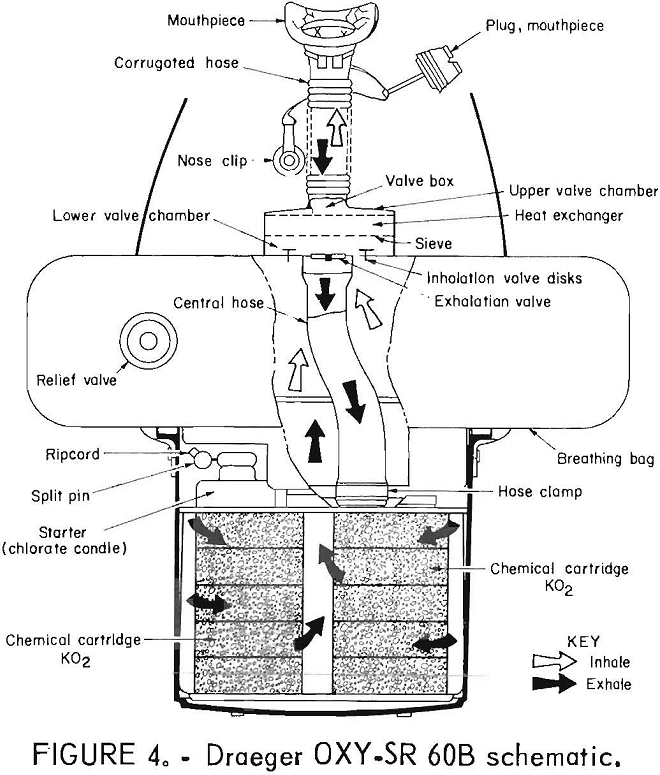
The MSA SCSR (figs, 5-6) is a chemical-oxygen self-rescuer which is entirely throwaway. It has a unidirectional flow path through the KO2 bed and a volume-activated relief valve. A chlorate candle is utilized for initial oxygen flow. The KO2 is in granular form.
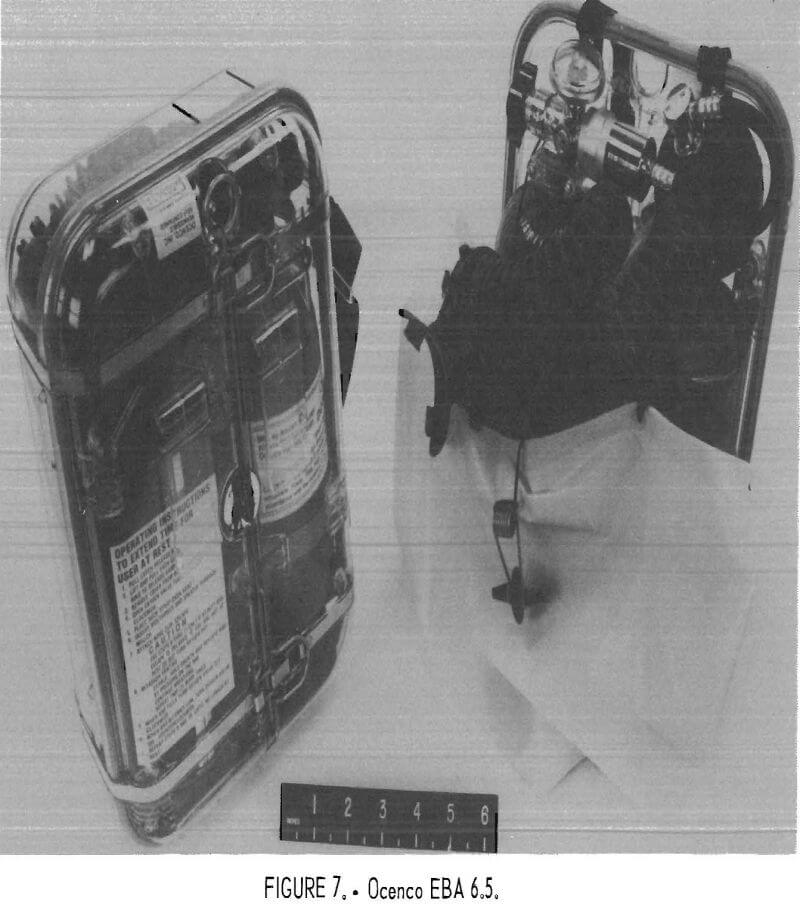
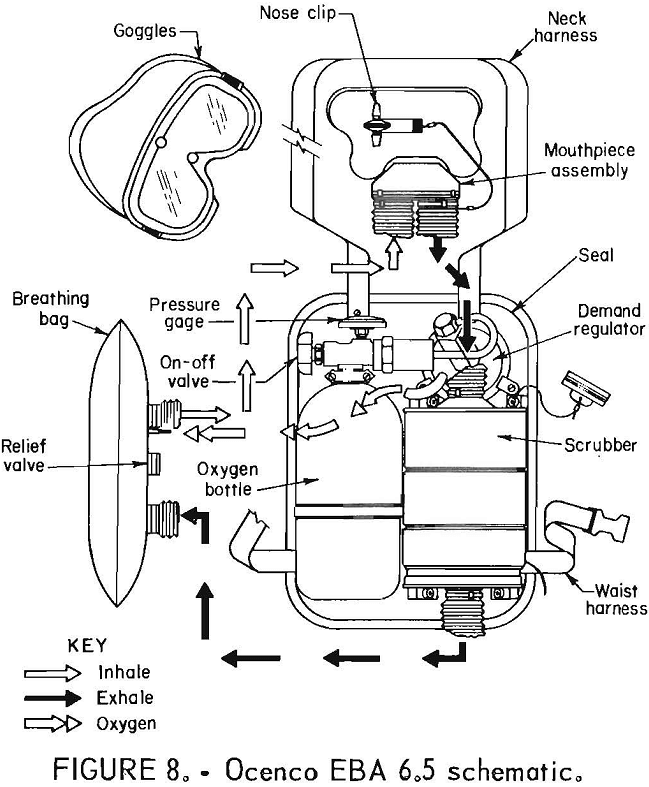
Ocenco EBA 6.5
The Ocenco EBA 6.5 (figs. 7-8) is a compressed-oxygen self-rescuer with a fiberglass-wrapped aluminum bottle which is reusable. The apparatus is refurbishable only by the manufacturer. It has a unidirectional flow path with directional check valves in the mouthpiece assembly, a constant flow of at least 1.5 L/min, and pressure-activated demand and relief valves. The cylinder contains 157 L of oxygen, and the CO2 absorbent is LiOH.

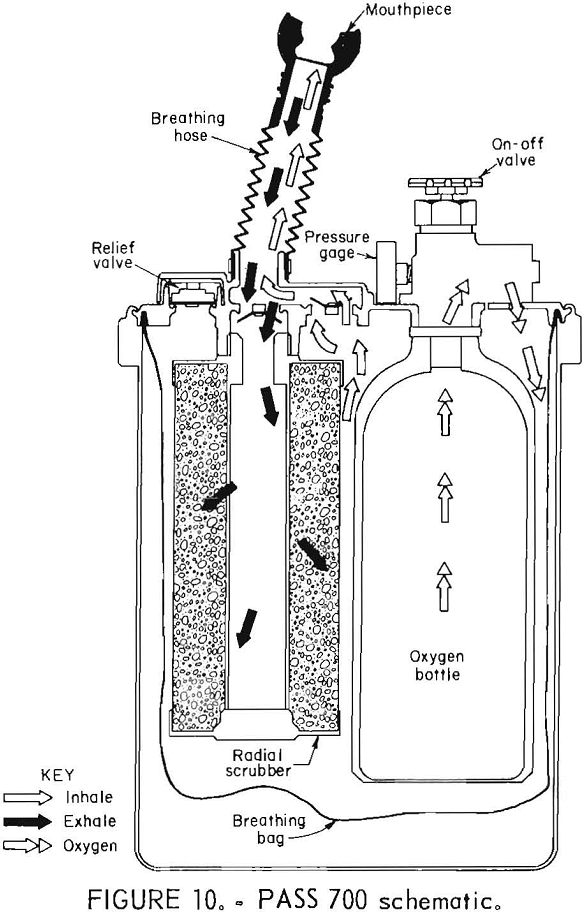
PASS 700
The PASS (Portable Air Supply Systems) 700 self-rescuer (figs. 9-10) is a compressed-oxygen system with an aluminum bottle which is reusable after refurbishing by the manufacturer. It has a unidirectional flow path through the CO2 scrubber, an enclosed breathing bag, no demand valve, a constant flow of oxygen of at least 3 L/min, and a pressure-activated relief valve. The cylinder contains 240 L of oxygen, and the CO2 absorbent is soda lime.

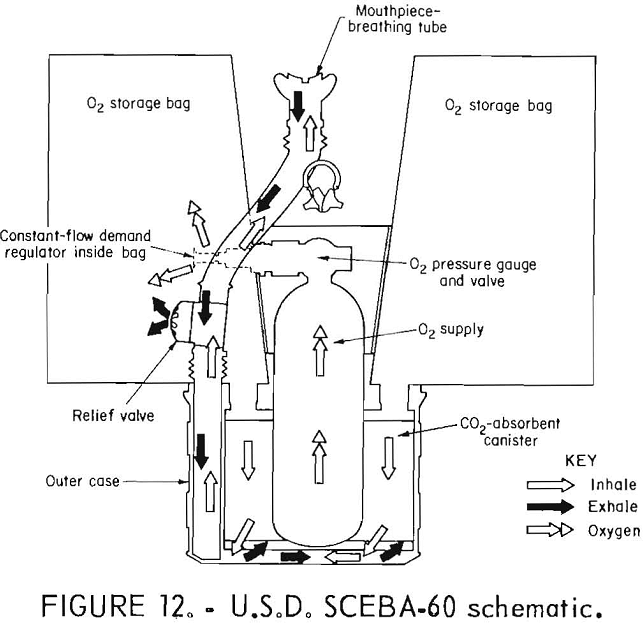
U.S.D. SCEBA-60
The U.S.D. SCEBA-60 self-rescuer (figs. 11-12) is a compressed-oxygen system which has some reusable parts but is not presently being commercially produced because it became available only after mine operators were required to have placed orders for their oxygen self-rescuers. It has a bidirectional flow path, a constant flow rate of oxygen of at least 1.5 L/min, and volume-activated demand and relief valves. The relief valve is triggered by bag volume but dumps from the breathing hose air that has not yet been scrubbed of CO2 or enriched with oxygen. It is available with a standard steel, throwaway bottle or a lightweight, fiberglass-wrapped aluminum, reusable bottle containing 130 L of oxygen. The CO2 absorbent is LiOH.

Foreign
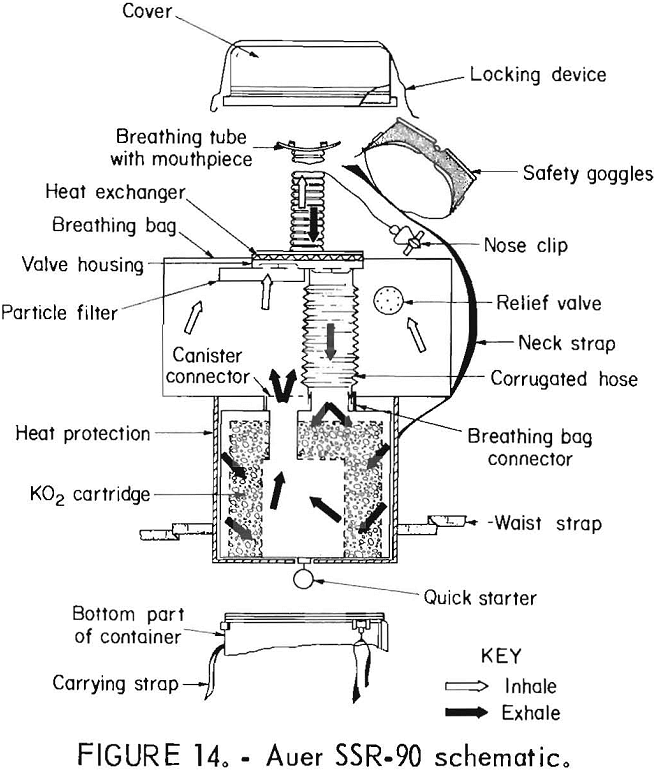
Auer SSR-90
The SSR-90 (figs. 13-14), manufactured in Germany by Auer, a subsidiary of MSA, is a chemical-oxygen self-rescuer which can be user-refurbished. It has a unidirectional flow path through the KO2 bed, a volume-activated relief valve, and a “quick starter” for initial oxygen flow.
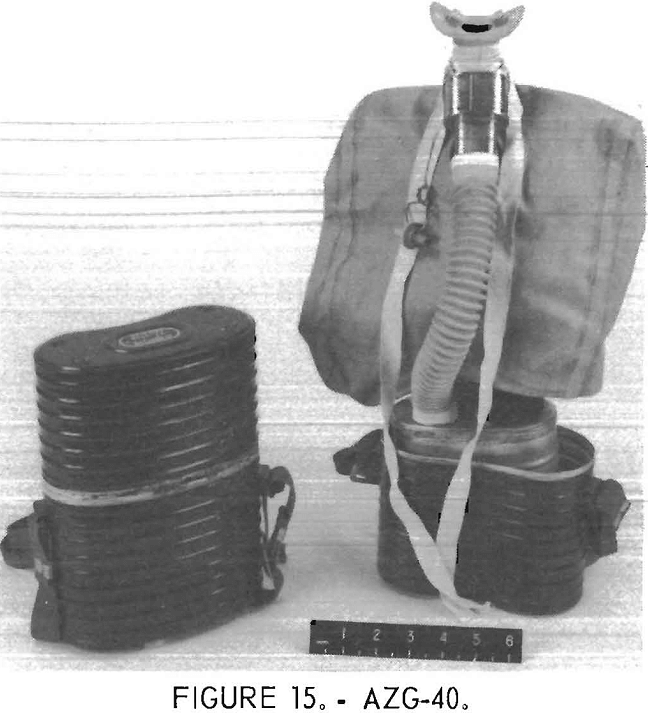
AZG-40
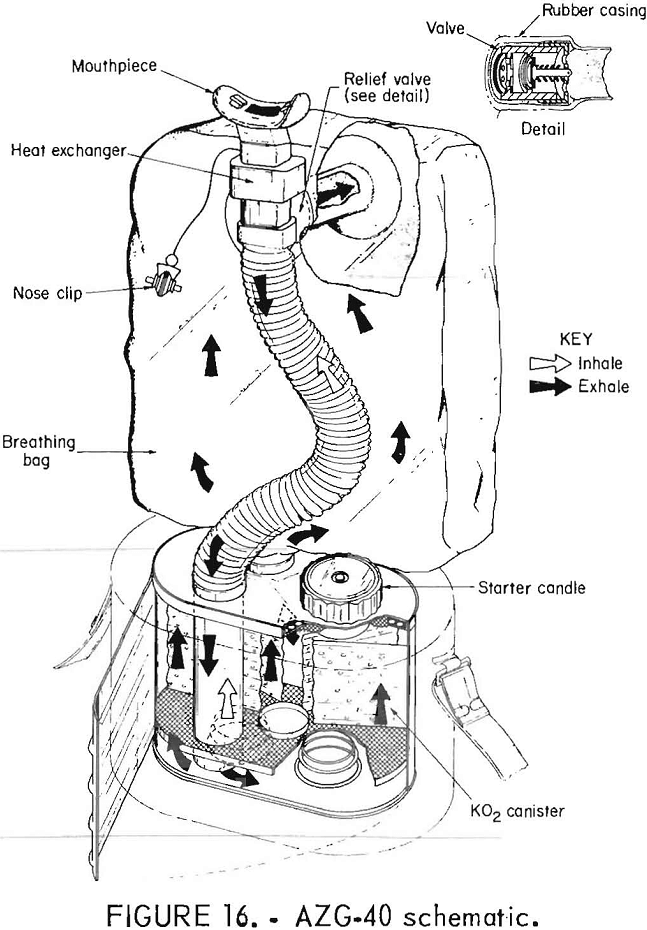
The Chinese AZG-40 self-rescuer (figs. 15-16) uses KO2 and is not reusable. It has a bidirectional flow path, a volume activated relief valve which vents from the breathing hose, a heat exchanger, and a quick-starting mechanism for initial oxygen flow.
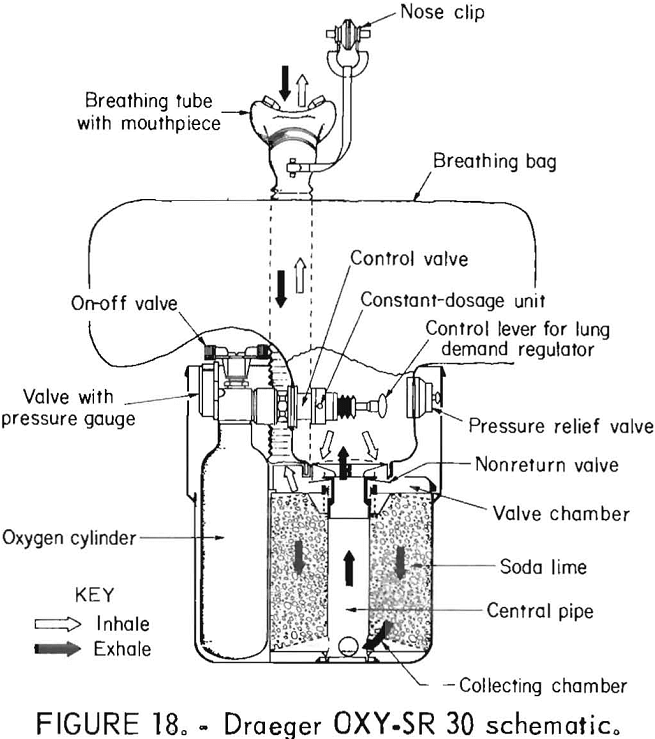
Draeger OXY-SR 30
The Draeger OXY-SR 30 (figs. 17-18) is a compressed-oxygen self-rescuer which is user refurbishable. It has a unidirectional flow path through the soda lime scrubber, a pressure-activated relief valve, a volume-activated demand valve, and a constant flow of oxygen of at least 1.5 L/min. The steel cylinder contains 64.5 L of oxygen.
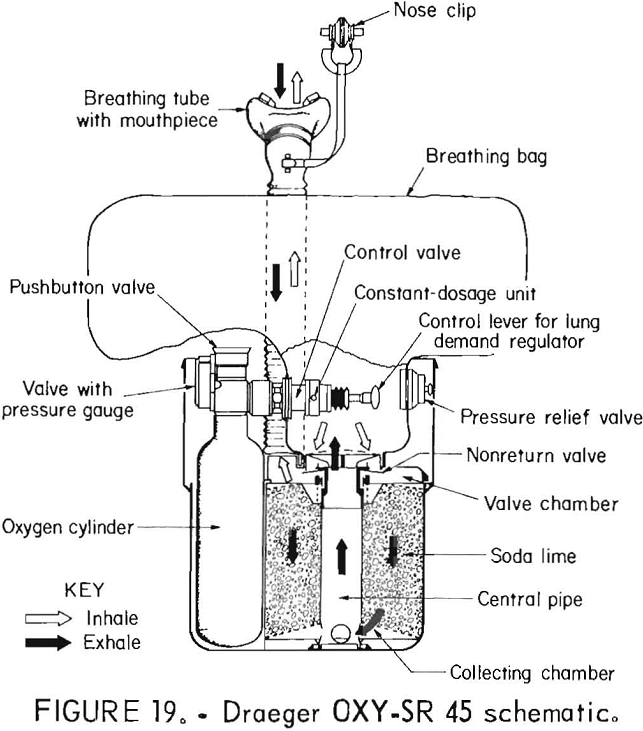
Draeger OXY-SR 45
The Draeger OXY-SR 45 is nearly identical to the OXY-SR 30 with the following differences: The constant flow rate is only 1.2 L/min, and the oxygen flow cannot be turned off once it is activated. Figure 19 shows the apparatus schematically diagrammed.

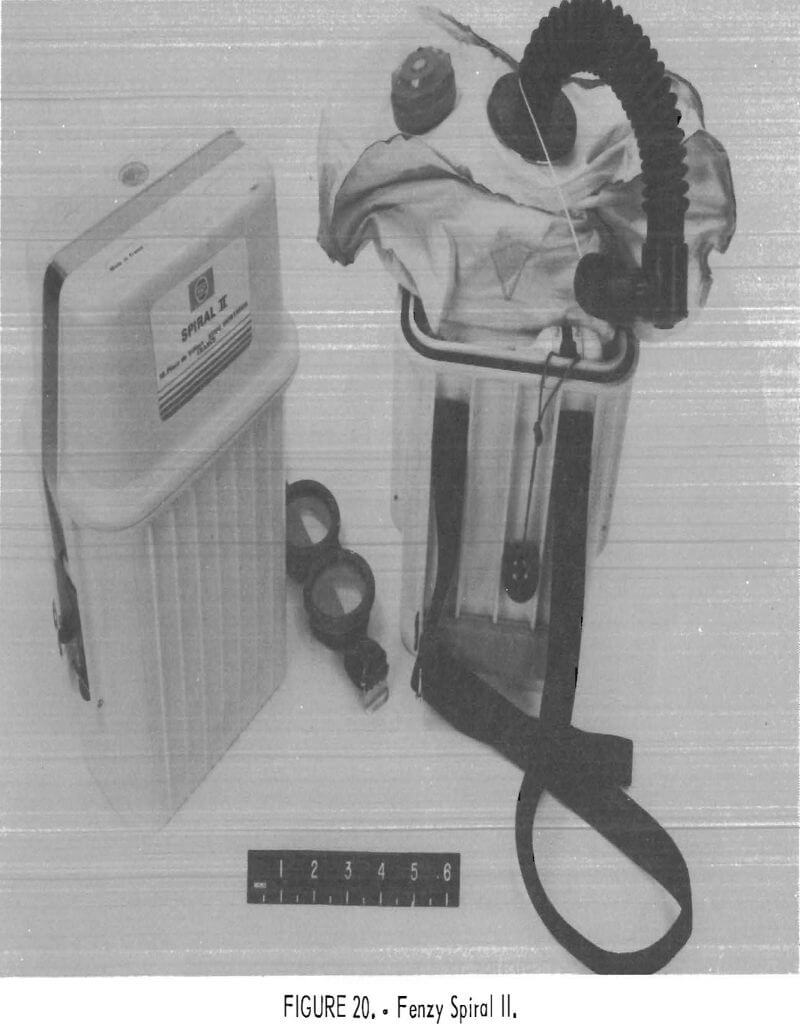
Fenzy Spiral II
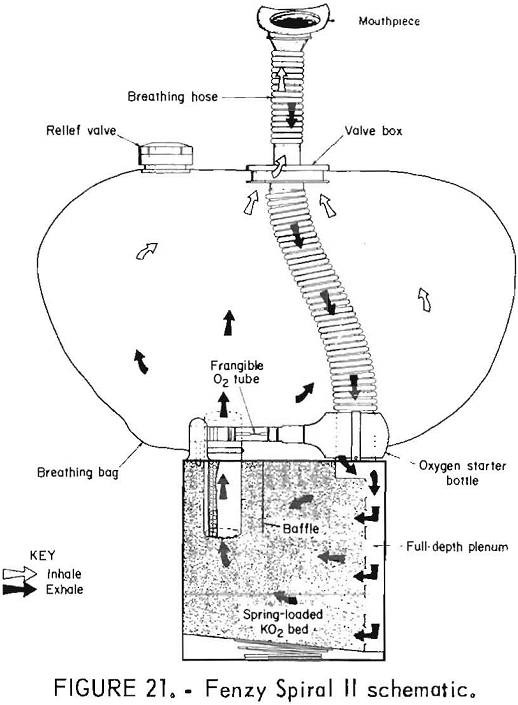
The Spiral II (figs. 20-21) is a chemical-oxygen self-rescuer which is user serviceable. It has a unidirectional flow path through the KO2 bed and a pressure-activated relief valve. A very small compressed-oxygen bottle is utilized for initial startup. The bottle is yanked upward by pulling on a plastic ball connected to the bottle; this breaks a metal seal, rapidly releasing the contents into the system.

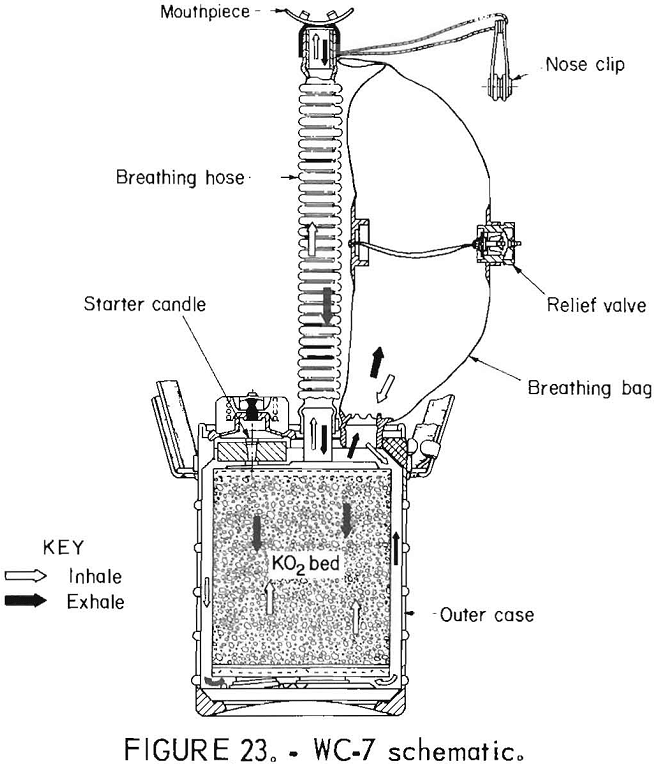
WC-7
The Soviet WC-7 self-rescuer (figs. 22-23) uses KO2 as the oxygen source and is throwaway. It is a bidirectional flow path device with a volume-activated relief valve. The starting device utilized delivers 6 L of oxygen within 30 s.

Bureau Of Mines-Developed Prototypes
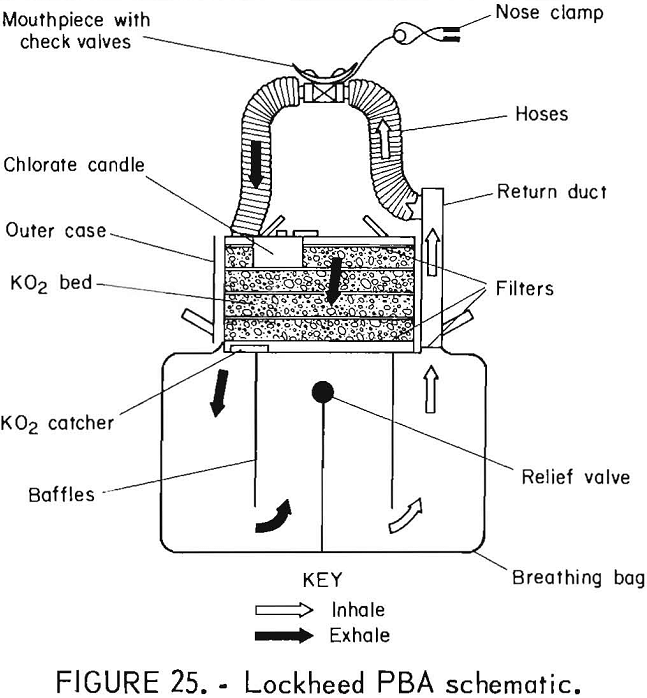
Lockheed PBA (Personal Breathing Apparatus)
The Lockheed PBA (figs. 24-25) is a NIOSH-approved prototype. The apparatus were manufactured in 1974 and stored in warehouses until tested for this study. These chemical-oxygen self-rescuers were intended to be throwaway devices. The flow path is unidirectional with check valves in the mouthpiece. The device has a pressure-activated relief valve and a chlorate candle for initial oxygen flow.

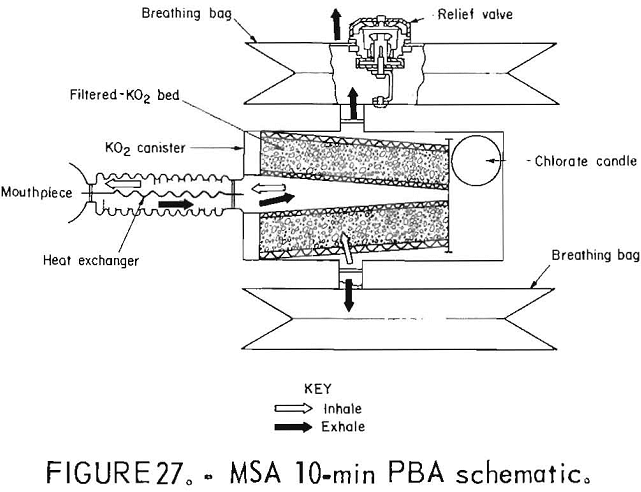
MSA 10-Min PBA
Another NIOSH-approved prototype, the MSA 10-min PBA (figs. 26-27) was also manufactured in 1974 and similarly stored in warehouses until being tested. The 10-min PBA is a one-use, chemical-oxygen self-rescuer with bidirectional flow, a chlorate candle, and two breathing bags, one of which contains a volume-activated relief valve.
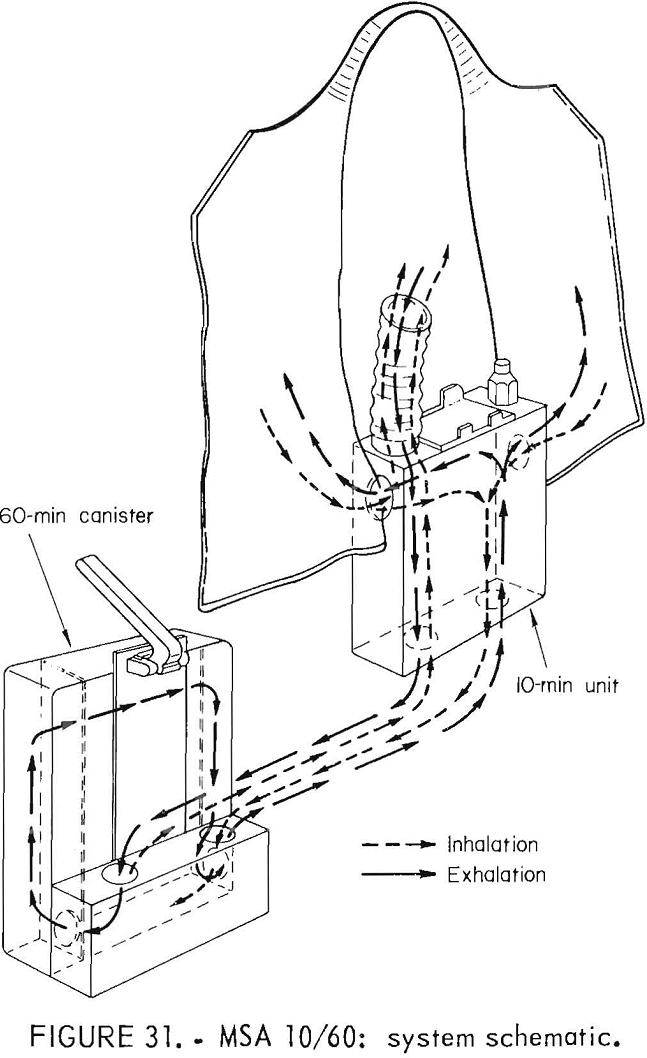
MSA 10/60 Oxygen Self-Rescuer
These NIOSH-approved prototypes were built in 1979 and were stored until being tested. The chemical-oxygen 10/60 was designed so that the 10-min apparatus

could be belt-worn with the 60-min canister being stored. In an emergency, the 10-min device is donned and the user proceeds to the storage place of the 60-min canisters, which are then attached without the need to remove the mouthpiece. The oxygen source in both portions is KO2. Chlorate candles are provided on both portions of the device; the candle on the 60-min portion is automatically activated when the device is attached to the 10-min portion. The entire apparatus is one-use only. The 10-min apparatus has a bidirectional flow path, but when the 60-min canister is attached, the flow path is changed to unidirectional flow. Figure 28 shows the 10-min apparatus in the case and deployed, and the 60-min
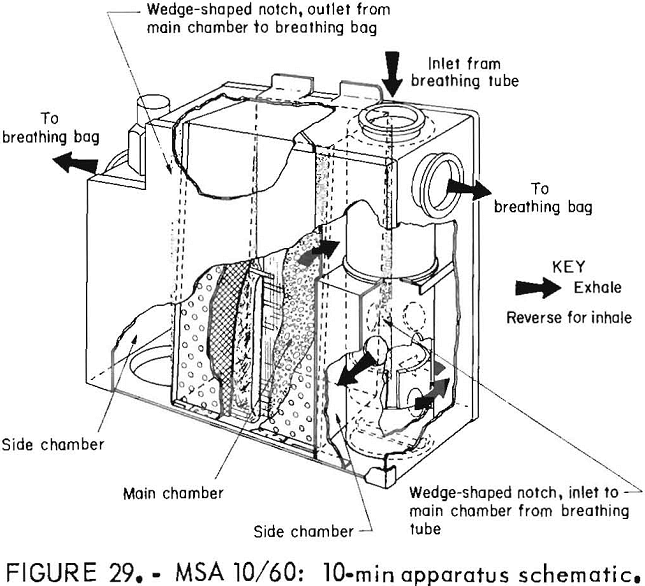
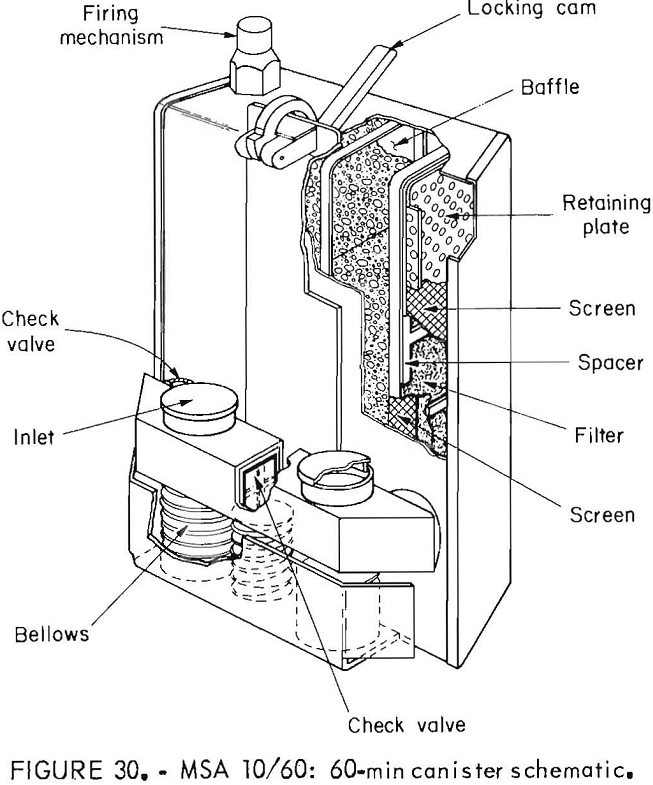
canister. The 60-min canister does not have its own case but is stored in a plastic bag. Figures 29, 30, and 31 are schematics of the 10-min apparatus, the 60-min canister, and the combination flow scheme.
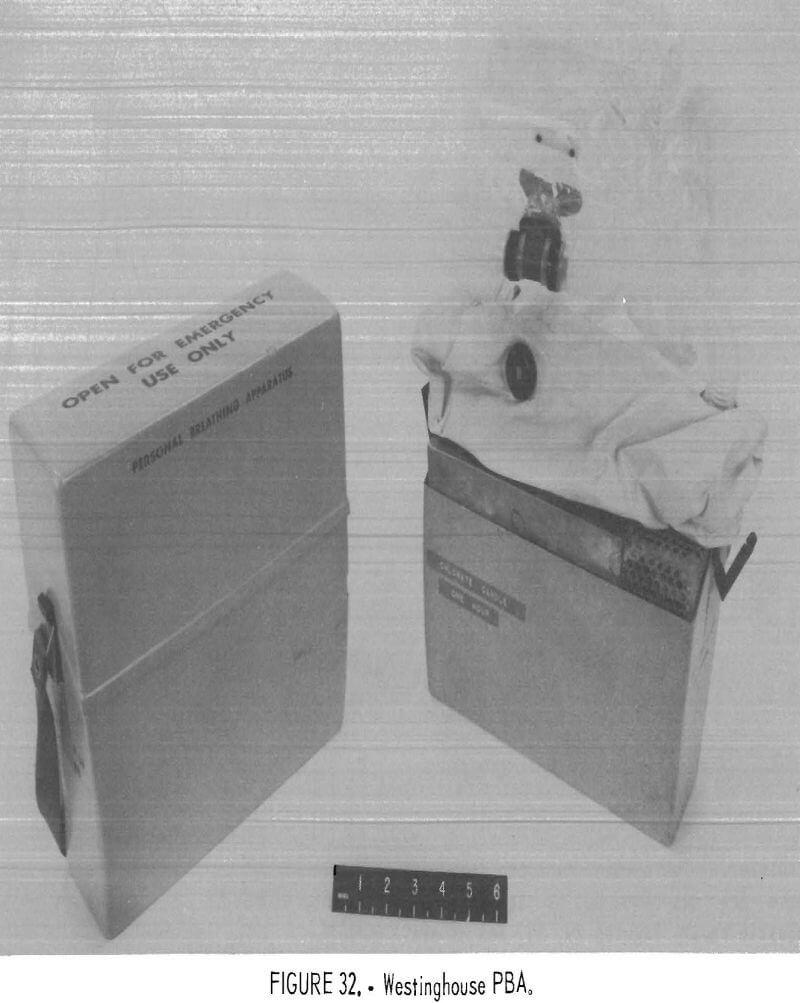
Westinghouse PBA
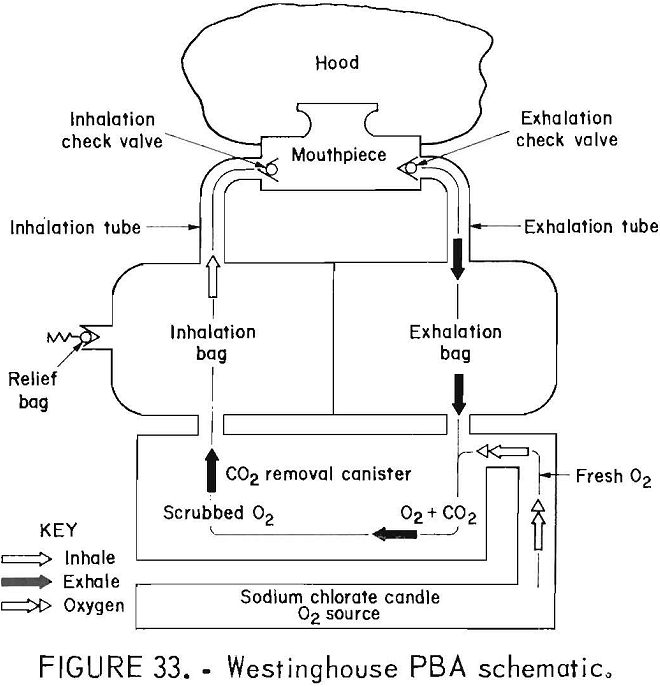
The Westinghouse PBA (figs. 32-33) was the first Bureau prototype ever developed. The apparatus tested were manufactured in 1971 and were not certified by NIOSH. The flow path is unidirectional with a two-chambered breathing bag, one chamber for exhalation and one for inhalation, with a pressure-activated relief valve on the inhalation side of the bag. The oxygen source is a large, L-shaped, sodium chlorate candle which provides at least 3 L/min of oxygen flow continuously, regardless of usage rate. The CO2 scrubber uses LiOH. The original directive for this contract, included face protection, and the design included a combination hood-lens-noseclip-mouthpiece. Only three of these proto-types remained for testing in this study.
Test Apparatus and Experimental Procedure
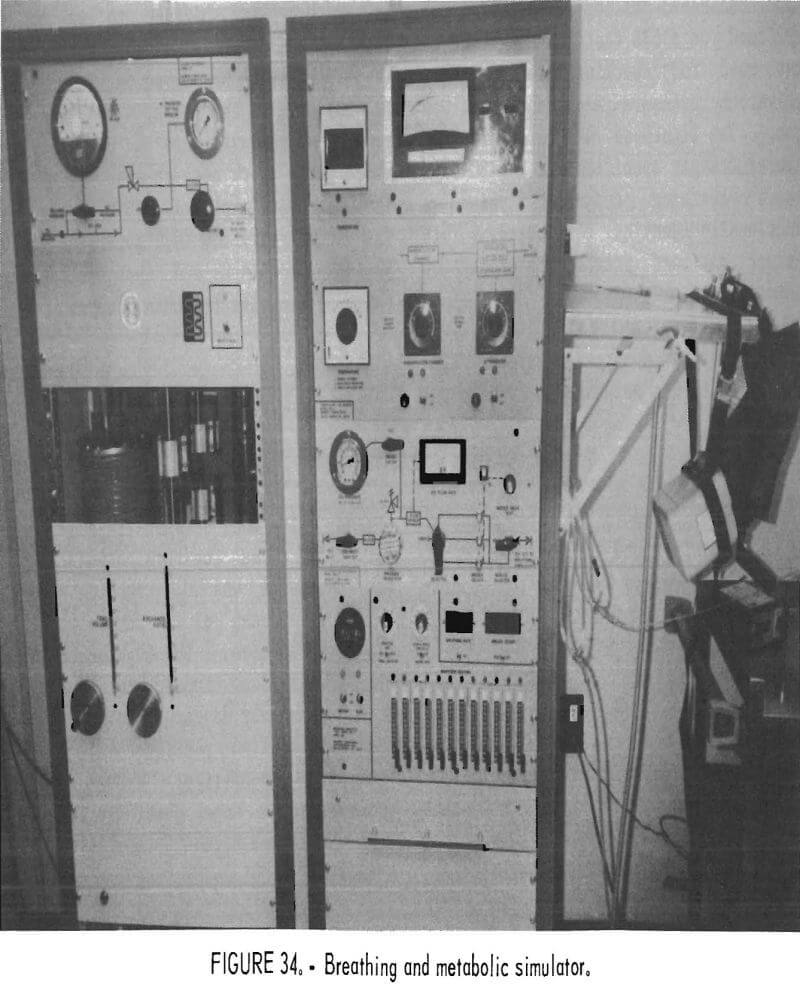
A breathing and metabolic simulator (BMS) was used for testing the apparatus in this study (fig. 34). The BMS elicits a constant and repeatable metabolic demand on the breathing apparatus; human subjects cannot be depended upon to do this. Also, human subjects would not have tolerated the high temperatures and breathing resistances encountered during some of the tests.
The BMS has a unidirectional flow path, unlike a human being. The air movement is effected by a bellows moved through a mechanical linkage to a motor. A smaller bellows withdraws a varying percentage of the volume of the main bellows, depending upon the O2 concentration, simulating O2 consumption. Another small bellows injects CO2 and N2 into the system, replacing the O2 consumed and the N2 removed in the process. The gas inhaled into the BMS is also heated and humidified (in this study, to approximately 37° C and 80 pct relative humidity).
The metabolic parameters used in this study follow (all volumes at standard temperature and pressure, dry):
Vo2(oxygen consumption) = 1.35 L/min
Vco2(CO2 production) = 1.30 L/min
Ve (ventilation) = 31.9 L/min
V+ (tidal volume) = 1.21 L/breath
Fr (respiratory freq) = 26.5 breath/min
Qpeak (peak flow rate) = 126 L/min
The performance parameters measured were inhaled concentrations of O2 and CO2, inhaled gas temperature, and both inhalation and exhalation breathing resistances. The inhaled gas concentrations were measured in a box just inside the inhalation port of the BMS where the inhaled gases are mixed, called the inhalation mixing box. Measurement at this point has the advantage of including effects of dead space volume, such as that of the breathing hoses which have bi-directional flow paths. Breathing resistances and temperature were measured at the mouthpiece. Factors determining test termination were CO2 higher than 4 pct, O2 lower than 15 pct, or low bag volume, indicating an expired O2 source.
Breathing circuit tightness was determined by pulling a negative pressure of 70 mm H2O at the mouthpiece of an apparatus and measuring the time required to rise to -60 mm H2O. Times longer than 60 s are considered passing. This test was performed on a CSE test stand, which is also used for checking relief valve activation pressure and constant flow rate. The tightness standards are the same as Draeger specifies for the BG-174A rescue breathing apparatus using a functionally identical test stand. This has not yet been related to protection factors.
When testing compressed-oxygen self-rescuers, it was necessary to purge the BMS breathing loop of most of the ambient nitrogen. Otherwise, the small amount of oxygen in the apparatus breathing loop would be so diluted owing to the large volume of the BMS breathing loop as to result in such a low concentration of oxygen that the test would have to be cancelled. If used on a person, with a much smaller volume in the breathing loop, this would not occur in most cases. This situation would arise only when the constant flow rate of O2 was less than the O2 consumption rate (Vo2) and when there was a substantial amount of N2 in the breathing loop, if one forcefully exhaled into a breathing apparatus from ambient, for example.
To start the test, 10 inhalations on the BMS were taken from each compressed oxygen self-rescuer with a demand valve, elevating the O2 concentration to 60 pct or greater. If the apparatus termination mode were low volume in the breathing bag due to an empty bottle, the amount of O2 used for the 10 breaths was added on in minutes to the end of the test. Ten breaths equals approximately 14 L at ambient, which would work out to about 9 min at the usage rate employed. This procedure was unnecessary for KO2 apparatus since they overproduce O2 and thus continually purge N2 from the breathing loop.
Results and Discussion
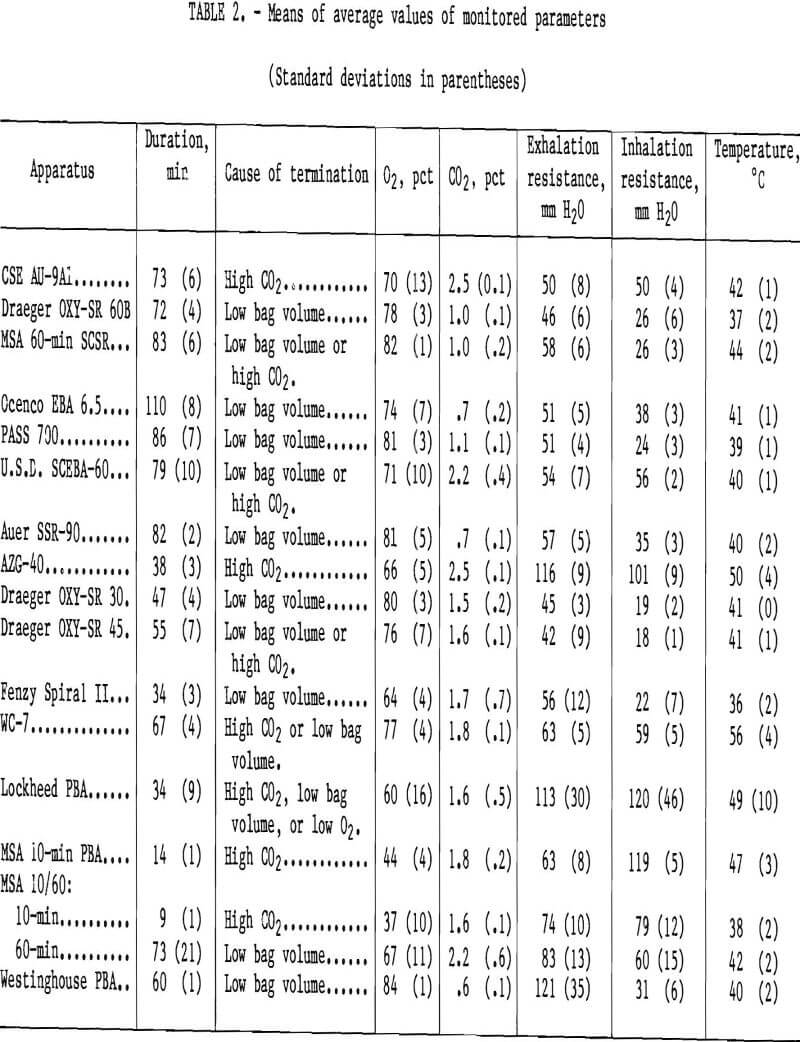
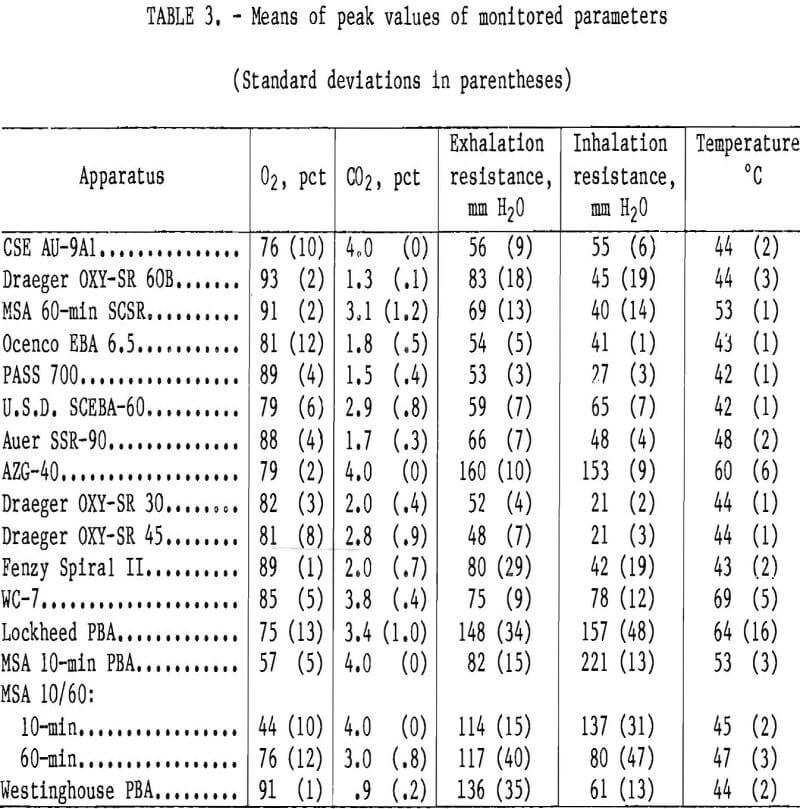
See tables 2 and 3 for the means of both the average and the peak values of monitored parameters for all the apparatus tested. Discussions of individual apparatus follow.
CSE AU-9Al
Of the six NIOSH-approved, commercially available apparatus, it can be seen that the two with bidirectional flow paths (CSE and U.S.D.) have the highest average CO2 levels. The inhalation resistance necessary to elicit 30 L/min from the demand valve was between 28 and 40 mm H2O. The cause for termination in all the CSE tests was CO2 reaching 4 pct, which was chosen as a physiologically appropriate value. The CO2 scrubber in the AU-9Al does appear to be rather small. The only danger in this is that a user may have no positive indicator of apparatus end-of-life. Rather, a user may experience some physiological symptoms due to the high CO2 inhaled, such as increased tidal volume and mild discomfort, which will eventually force him or her to slow the pace.
All of the CSE’s tested passed the negative pressure test on the CSE test stand, proving them to be very tight units, which is assumed equivalent to a high protection factor.
Breathing resistance and temperature remained steady throughout the tests. CO2 steadily rose until test termination at 4 pct. O2 varied depending upon the regulator constant flow rate. It slowly fell if the flow rate was less than the usage rate (approximately 1.55 L/min ambient temperature and pressure, saturated) and vice versa.
Draeger Oxy-SR 60B
The OXY-SR 60B was the coolest, NIOSH- approved, commercially available apparatus, with an average inhalation gas temperature of only 37° C. The termination mode was consistently an empty bag, indicating that oxygen production had stopped. This is considered a positive indicator of termination, one that can be easily detected by the wearer. The breathing loop proved to be a tight one, passing the negative pressure test on the CSE test stand.
The apparatus behaved typically for a KO2 unit with the chlorate candle firing causing the inhaled oxygen to rise to approximately 30 pct within a few seconds, level or drop for a minute, and then rise at a steady pace to a peak of approximately 93 pct at 40 min or so. It would take less time to reach the peak on a human subject since the BMS has a much greater volume in its flow loop. Temperature rose, while CO2 remained fairly steady throughout the tests. Breathing resistance also remained fairly steady until about 50 min, when exhalation resistance through the KO2 bed started to increase dramatically, signaling the beginning of the end of the test. On the average, though, the breathing resistance is one of the lowest tested.
MSA 60-MIN SCSR
This apparatus differs markedly from the OXY-SR 60B in appearance, but, in actuality, is remarkably similar in its functional flow path. This can be seen by comparing the schematics of each apparatus. The major differences in performance are largely attributable to the different forms of KO2 used. Draeger uses a pelletized form, while MSA uses a granular form, which offers more surface area for the H2O-O2-CO2 reaction to take place. As a result, approximately 970 g KO2 in the MSA provides for a longer service life than does 1,150 g in the Draeger. The trade-offs are the higher exhalation breathing resistance and in-haled gas temperature, as can be seen from table 2.
The MSA 60-min SCSR’s had a tight breathing loop and passed the negative pressure test with ease.
Breathing resistance and CO2 remained steady or slowly rose throughout the tests. Temperature rose throughout, the first half of the tests and then leveled off for the duration of the tests. Oxygen concentration behaved similarly to that with the OXY-SR 60B.
Ocenco Eba 6.5
One of the lightest NIOSH-approved apparatus, the EBA 6.5 had the longest duration of any self-rescuer tested, ending with an empty bottle. The ample CO2 scrubber enabled the unit to achieve the lowest average CO2 concentration of any commercially available apparatus. The demand valve required a very high negative pressure (in the vicinity of 195 mm H2O) to elicit 30 L/min of flow. A user would have to adapt to this high resistance to flow, to say the least.
The EBA 6.5 apparatus rose from -70 to -60 mm H2O on the negative pressure test in 7s, as an average. Representatives from Ocenco claim that any leaks are to be found on the exhalation side of the breathing loop, and, thus, would not result in any in leakage of toxic gases. This was not confirmed.
Breathing resistance remained steady throughout the tests, as did temperature after an initial warmup period. CO2 rose at a constant pace during the tests but never reached the termination point of 4 pct. O2 concentration behaved like that of the CSE AU-9Al, varying depending upon the setting of the particular O2 regulator.
PASS 700
This apparatus had one of the lowest inhalation breathing resistances measured. This can probably be attributed to its radial-design CO2 canister, which offers a small bed depth. Without a demand valve, utilizing a one-way, on-off mechanism and an enclosed breathing bag, this is probably the most foolproof and damage-resistant self-rescuer. The trade-offs are its lack of flexibility (no demand value) and size and weight.
This apparatus, like the Ocenco, is a comparatively leaky unit, with only 9 s elapsing between negative pressures of 70 and 60 mm H2O on the CSE test stand. It was not attempted to determine the source of these leaks. The breathing resistance remained steady throughout the tests, as did the inhaled gas temperature and C02 concentration. The oxygen level rose to its peak within 20 min and remained steady for the duration of the tests.
U.S.D. SCEBA-60
The U.S.D. SCEBA-60 is not yet being commercially produced, and only prototype units, which varied widely in certain performance parameters such as duration and breathing loop tightness, could be tested. The duration average was 79 min with a standard deviation of 10 min. Some apparatus were very tight and passed with ease the 60-s, CSE negative pressure test. Others rose from -70 to -60 mm H2O in 18 s. Presumably the apparatus will become tighter rather than leakier once production techniques are perfected.
The termination mode for the SCEBA-60 was usually an empty oxygen bottle, but in one case termination was due to high inhaled CO2. The negative pressure required to elicit 30 L/min of O2 from the demand valve varied widely, depending upon the position of the breathing bag collapsing on the tilt-lever demand valve. The range of negative pressures was from 13 to 80 mm H2O, with most values near 25 mm H2O.
The oxygen concentration during the tests varied depending upon the setting of the constant-flow valve. The CO2 concentration steadily rose from approximately 1.5 to 2 pct at the beginning of the tests to approximately 2.5 to 3 pct by the end of the tests, except for one test which went 93 min when CO2 reached 4 pct. The breathing resistance and temperature remained steady throughout the tests.
AUER SSR-90
From the test results and a comparison with the NIOSH-approved apparatus, it appears that the AUER SSR-90 could pass NIOSH testing and receive certification. The termination mode in all cases was low bag volume due to an empty oxygen bottle. The breathing loop was not tight, as evidenced by the negative pressure test which showed rises from -70 to -60 mm H2O in less than 5 s in four of five cases.
Oxygen concentration followed a pattern similar to that of other KO2 apparatus, rising to a maximum of 80 to 90 pct and then holding steady or slowly falling until the end of the tests. CO2 held steady for the first two-thirds of the test at approximately 1 pct and then rose to around 2 pct at the end.
Breathing resistance remained steady throughout the tests, and temperature increased steadily from ambient to approximately 48° C at the end.
AZG-40
This was a surprisingly lightweight and small unit which would provide adequate protection if not stressed too much. The trade-offs that permit its low size and weight are its high breathing resistance and inhaled gas temperature as well as its lesser duration. The average peak inhaled gas temperature would not likely be tolerated by a human being. This and the high resistance would force a person to slow the pace. The impact of carrying the apparatus in normal activities is, however, minimized by its weight and size.
The termination mode was high CO2, which is not preferred. The breathing loop proved to be very tight, easily passing the negative pressure test in four of five cases. The fifth apparatus was defective and failed the test. The connection between the bag and the canister was not secure and leaked. This was repaired before the BMS test was performed.
On two of the five apparatus, the starter candles did not go off until the 18th and 19th minutes of the tests, even though they were started according to instructions like the rest. As a result, O2 concentration initially rose slowly from ambient and jumped when the candle finally went off, which caused venting of the bag and a slight drop in temperature. It appears from this problem and the aforementioned defect that more attention needs to be paid to quality control and possibly to design.
During the tests, O2 concentration rose during the first half and fell during the last half, while CO2 rose throughout the test from approximately 2 pct to the termination point of 4 pct. Breathing resistance and temperature also continuously rose throughout the tests.
DRAEGER OXY-SR 30
The performance testing of this unit revealed no surprises nor any unusual behavior. The termination mode in all cases was an empty oxygen bottle. All units passed the negative pressure test. The negative pressure required to elicit 30 L/min of oxygen flow from the demand valve ranged from 16 to 64 mm H2O, depending upon the position of the breathing bag collapsing on the demand valve.
Oxygen concentration remained steady throughout the tests, while CO2 was either steady or gradually increased from around 1.5 pct to 2.5 pct. Breathing resistance stayed level throughout the tests, while inhaled gas temperature leveled off after warming up.
DRAEGER OXY-SR 45
The performance of this apparatus was, of course, very similar to that of the OXY-SR 30. Since the regulator is set at a lower rate than that of the OXY-SR 30 and since that is lower than the BMS oxygen consumption rate, the oxygen concentration would be expected to drop, as it did in most cases. The termination mode was usually an empty oxygen bottle, but was high CO2 in one case. All units passed the negative pressure leak test. The negative pressure required to elicit 30 L/min of O2 flow from the demand valve was similar to that of the OXY-SR 30.
CO2 concentration gradually increased throughout the tests from around 1.5 pct to 3 pct and higher. Breathing resistance and inhaled gas temperature were the same as with the OXY-SR 30.
Fenzy Spiral II
One thing about the Fenzy Spiral II that makes it different from any other KO2 device is that when the starter cord is pulled, the mouthpiece should be in the mouth, or in this case, connected to the simulator. The reason for this is that the oxygen is stored in a small compressed gas cylinder and pours into the circuit very rapidly, unlike a chlorate candle, and if the mouthpiece is not already connected to the simulator, it escapes.
Another interesting thing was that approximately half the KO2 in the bed was not utilized at the end of the tests. When a used unit was taken apart in order to determine the flow path of the apparatus, a sizable quantity of the KO2 was still yellow in color, indicating that It was not utilized. This explains the short duration of the apparatus considering its size and weight. Owing to the design of the bed, there is apparently not a good flow path, resulting in incomplete utilization of the KO2.
The termination mode in all cases was low bag volume. The apparatus is tightly constructed and passed the negative pressure leak test in most cases.
The performance characteristics were typical of KO2 apparatus with oxygen concentration rising as nitrogen is pushed out of the flow loop owing to the overproduction of oxygen. CO2 stays steady throughout the test. Breathing resistance stays steady until the end, when it increases dramatically. Inhaled gas temperature continually rises throughout the tests but probably would have leveled off if the tests had not terminated early owing to incomplete utilization of the KO2.
WC-7
Of the five Soviet apparatus tested, three were built in 1978 and two were built in 1973. All of these held up very well with no visible signs of aging. One unit built in 1973 (not tested) already had the starter activated or, at least, was not intact. This could be detected by shaking the apparatus, which produced a rattling sound. Also, the mouthpiece lugs were missing. The product literature says that the apparatus can be stored for as long as 2 years and thereafter must be checked in batches, segregated by their ages, every 6 months to validate their collective integrity.
The apparatus are not permitted to be stored on any operating machinery. This precaution is presumably the result of experience with oxygen self-rescuers, the first types of which were produced in 1958. This particular model was first produced in 1968. The miners are not required to wear the self-rescuer at the workplace if they keep it within 3 m. They are required to wear the unit if they travel.
The manual states clearly “Do Not Run!” This reveals a design philosophy which willingly compromises performance capability to achieve size and weight savings. This philosophy is presently under discussion in the United States.
The termination mode in four of five cases was high CO2, with one case ending due to low bag volume. Four of the five apparatus also passed the negative pressure leak test.
The performance characteristics were typical of KO2 apparatus, with CO2, inhaled gas temperature, and breathing resistance all showing a continuous rise and O2 rising and falling from beginning to end, peaking between 80 and 90 pct. It can be seen from the mean tables that the inhaled gas temperature was very high, with one apparatus reaching a peak of 75° C. A person wearing the apparatus would have been forced to slow the pace long before this point. This exemplifies one trade-off for the light weight and small size of the apparatus.
Lockheed Pba
These apparatus, which had been stored since 1974, had problems with aging of parts. The major problem was that the breathing hoses deformed so badly that, when used, either one hose or the other was crimped off such that both hoses could not present an open airway at the same time. This occurred in most of the apparatus tested. When this occurred, the hoses had to be cut off and replaced with plastic tubing. In some apparatus, the breathing bag was stuck together and was difficult to unfold. Also, some units emitted smoke from the mouthpiece when the candle was started. Ten units were tested.
As can be seen from the duration column in table 2, there was some decay in KO2 efficiency, assuming that the apparatus did once have at least a 60-min capacity. The breathing resistance and temperature were rather high, also. Since we have not tested new such apparatus under these precise conditions, it is not known whether these undesirable test parameter values are due to aging or to design.
The multiple termination modes can probably be attributed to the variability in manufacture of prototypes. This also would explain why approximately half of the apparatus passed the negative pressure leak test while the other half did not.
The performance characteristics varied widely from test to test, possibly depending upon the condition of the apparatus. In most cases, breathing resistance remained steady. Temperature rose throughout the tests in all cases, and in some cases rose steeply. Oxygen behaved typically for a KO2 device. CO2 rose in all cases, steeply in some, but recovered in two cases.
Msa 10-Min Pba
These prototypes were manufactured in 1974 and held up well except for gnarled breathing hoses, which seriously impaired their performance towards end-of-life. Breathing resistance became so high that the breathing hoses partially collapsed during inhalation. The durations all were longer than 10 min with termination due to high CO2, but the breathing hoses began to collapse before this, although the pressures were really not very high (approximately 221 mm H2). In fact, they were In the range of what is necessary to elicit 30 L/min of O2 from the Ocenco EBA 6.5.
Four of five apparatus held tight on the negative pressure leak test.
Oxygen concentration behaved typically for a KO2 apparatus. Temperature rose constantly and rapidly throughout the tests. Breathing resistance remained steady until the hoses began to collapse which caused inhalation resistance to increase dramatically. CO2 rose slowly until near the end, when it rose rapidly.
Msa 10/60 Oxygen Self-Rescuer
The MSA 10/60 prototype units were 4 years old, and as with other old prototype units, there was a wide range of performance. Some failings were defective goggles, a 60-min canister that would not start, and few apparatus passing the negative pressure leak test. There were also high standard deviations in monitored parameters, as seen in tables 2 and 3.
The termination mode was high CO2 for the 10-min portion in all cases and low bag volume for the 60-min canister in all cases. The 60-min canister was connected to the 10-min apparatus when the CO2 reached 4 pct.
CO2 concentration was steady and then rose quickly in the 10-min portion but rose very gradually in the 60-min canister. O2 concentration rose and fell quickly in the 10-min portion. The rise and fall were more gradual in the 60-min canister. Breathing resistance behaved similarly.
Westinghouse PBA
The Westinghouse PBA prototypes had been stored since produced in 1971 and, as might be expected, had suffered some damage from decay. Of the three tested, one had a leak in the breathing flow loop, while another had a decayed O2 delivery flow tube from the chlorate candle to the breathing loop. This was repaired during a test. The third apparatus was intact. In general, the apparatus functioned rather automatically without much variation. This can be seen by the low standard deviations exhibited in the monitored parameters of duration, O2 and CO2 concentration and temperature. The large standard deviation noted in the exhalation resistance was due to one test with a high value. The other two tests had similar values (98 and 105 mm H2O).
The termination mode in all three cases was low bag volume, indicating the candle was expended. Only one apparatus passed the negative pressure leak test.
Oxygen concentration rose quickly and stayed steady at near 90 pct throughout the tests until the candle was expended. CO2 concentration remained at a low, steady point throughout the tests. The inhaled gas temperature stayed steady throughout the tests after an initial heating-up period. Breathing resistance also remained steady throughout the tests.
Conclusions
The apparatus exhibited an amazingly wide range of designs. Performance also ranged over a large spectrum, as can be seen from tables 2 and 3.
The two self-rescuers from the U.S.S.R. and China both evidenced a design philosophy of light weight and small size, sacrificing comfort in the areas of breathing resistance and inhaled gas temperature.
The prototype apparatus, which had not seen routine production, had a high variation in performance parameters, reflecting their handmade construction. This was seen most readily In the U.S.D. SCEBA-60, the Lockheed PBA, and the MSA 10/60.
Of the apparatus that were stored for some time, some had aged better than others. The prototype Lockheed and Westinghouse apparatus had problems with badly deteriorated rubber parts, which required extensive repairs before they could be tested. However, some of the WC-7 apparatus were built 10 years ago and performed perfectly.
The Ocenco EBA 6.5 required a great amount of negative pressure to open the demand valve—four to five times more pressure than for the same flow rate with the CSE or the U.S.D. apparatus. Also, the breathing loop was not a tight one, and all failed the CSE negative pressure tests, as did all PASS 700’s. Of the foreign apparatus, the Auer was also leaky. For the prototype units, results were inconsistent with some passing and some failing.
To investigate its low durations, a Fenzy Spiral II was dissected; the KO2 beds were only half used, which indicates a poor bed design.
There are many design philosophies utilized in the apparatus tested. Ideal performance would include an obvious termination mode, such as an empty bag. Other parameters, such as CO2, temperature, and breathing resistance, would be low so as not to interfere with normal respiratory and metabolic functions. An ideal device would give such performance and also be small and lightweight. Trade-offs are, of course, necessary. This paper, shows how different manufacturers have made different trade-offs and the results of their philosophies.
