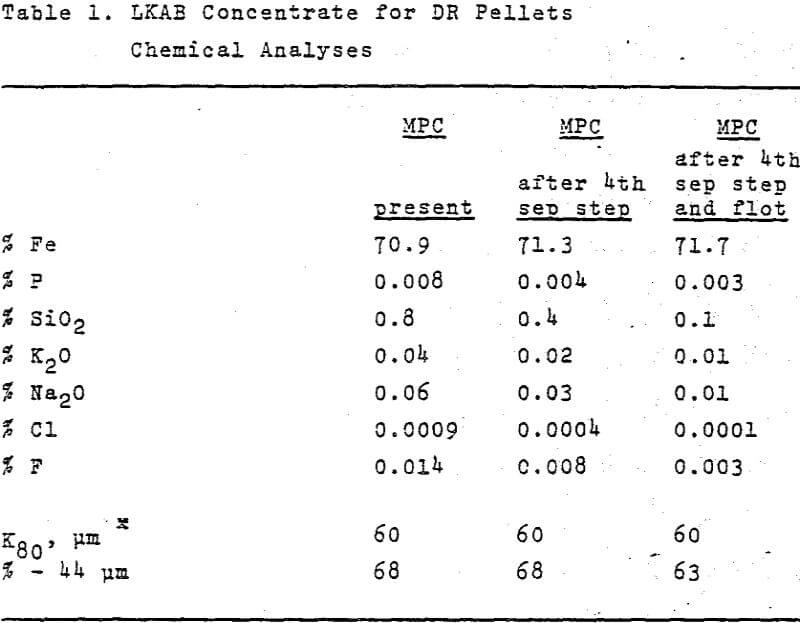
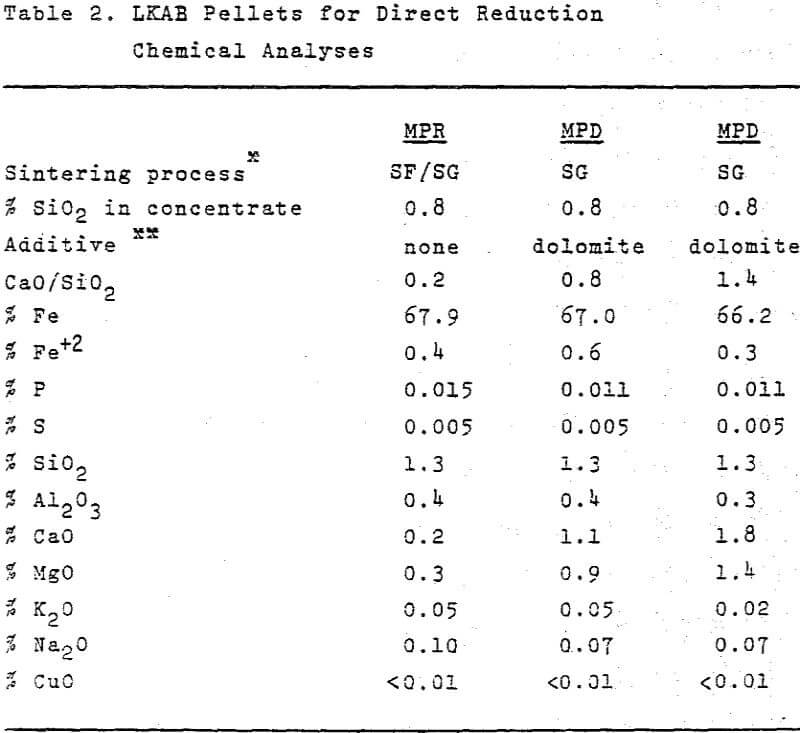
Table of Contents
The iron ores mined by LKAB in the north of Sweden have excellent beneficiation properties. This gives LKAB a favourable position as regards the production of low silica pellets for direct, reduction. LKAB have concentrated on developing and marketing grades of pellets for the Midrex-Purofer-Hyl process group, i.e. such D.R. processes which are carried out in a shaft furnace or a static bed using reformed natural gas as a reducing agent. The paper deals with properties and quality demands of iron ore pellets for such processes. Matters such as the effect of the residual magnetite in the fired pellets on the degree of metallization, the amount and composition of the slag phase in the pellets, the mechanism of the disintegration during reduction, the state of swelling of the pellets during the conditions prevailing in these processes, the importance of the porosity, the improvement of the reducibility and lowering of the sticking tendency of the pellets by small additions of suitable additives and the influence of the pellet size are discussed.
Properties and Quality Demands
LKAB have concentrated on developing and marketing grades of pellets that are principally suited to the Midrex-Purofer- HyL group of processes. In the following we shall discuss pellets for these processes only.
Since no melting takes place during reduction, the gangue remains behind in the reduced pellets. It is therefore essential that the input material contains only a small amount of gangue, since the gangue then has to be melted in the steel furnace, resulting in extra energy consumption and costs. Still, a certain modicum of gangue in the sponge iron is desirable to help build up slag in the steel furnace. The dephosphorizing capacity of steel-furnace slag depends on the quantity of basic components, CaO and MgO, that it contains, and it is generally considered that the basicity of the slag, defined as the ratio CaO+MgO/SiO2 + Al2O3 should lie between 1.5 and 2. Hence it would be an advantage if the composition of the pellets gangue was right from the outset. In the case of the HyL process, for instance, it is stipulated that the basicity of the pellets must not be less than 0.75 but optimal steel-furnace economy normally requires a basicity value of 1.1.
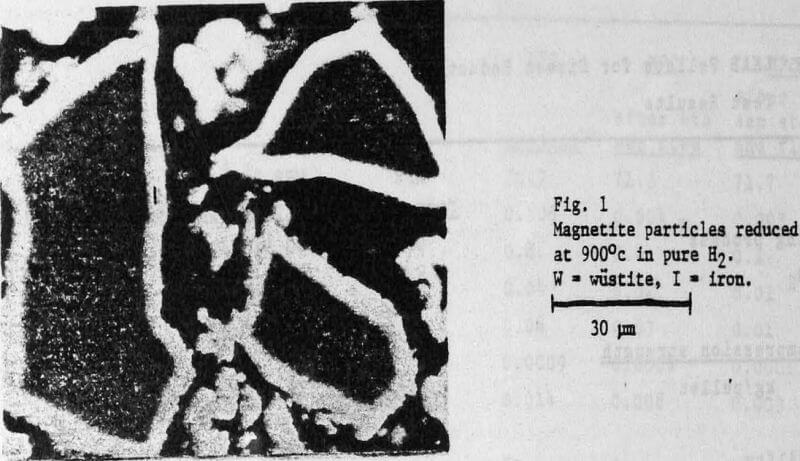
In direct reduction, as opposed to blast furnace operation, the entire reduction process must occur in the solid state. Thus it is important that the last trace of oxygen is removed within a reasonable time. It is true that the H2-rich atmosphere obtained in direct reduction, processes has a much stronger reducing effect than the CO-rich atmosphere of the blast furnace. But this does not always mean that complete reduction to metallic iron is achieved faster in H2 than in CO for all iron ore materials. In the case of magnetite the reduction process actually ceases before complete reduction is achieved. Paradoxically, this is due to the very strong reducing effect of hydrogen gas. In H2, metallic iron, nucleates very quickly on the wustite grains resulting in the rapid formation of an unbroken shell of iron over the entire surface of the wustite.
Therefore it is of great importance that pellets for direct reduction are completely oxidized. If the pellets contain residual magnetite, reduction , may cease before the desired degree of metallization is reached. Residual magnetite is found in the cores of large pellets which have not had time to be oxidized all the way through in the firing process.
A critical factor for the reduction of the pellets is therefore the rapidity with which the molecules of hydrogen or water vapor can be transported through the pore system of the pellet to and from the reactive surfaces – in other words, the rate of reduction is controlled by the rate of gas diffusion.
Given that there is no coke pillar to prop up the agglomerate and that the charge does not melt in the direct reduction shaft, it follows that the ore material has to withstand the full burden of its own weight. This means that in direct reduction, more than perhaps in the blast-furnace process, one needs to be wary of any tendency for the pellets to disintegrate during reduction. In the blast furnace, the permeability of the charge to the process gases is affected by the increase in the fines fraction due to reduction disintegration, but the coke is still there to maintain a certain degree of permeability. In direct reduction, operating difficulties are accentuated by large pressure drops, with lowered productivity as a result.
Metallurgical Test Methods
The test methods elaborated by LKAB hitherto for direct reduction pellets have been primarily oriented towards the Midrex process, but laboratory work is in progress with the object of making the tests applicable to the Purofer and HyL processes too. The underlying idea has always been that each relevant property should be determined separately by means of different tests under certain given, constant conditions.
Since direct reduction takes place entirely in the solid phase and preferably up to 95% – 97% reduction, LKAB has introduced the following concepts as measures of reducibility in isothermal reduction:
t95 = time required to reach 95% reduction, expressed in minutes
R40 = rate of oxygen loss at 40% reduction, expressed in % O/min
R92 = rate of oxygen loss between 90% and 95% reduction, expressed in % O/min.
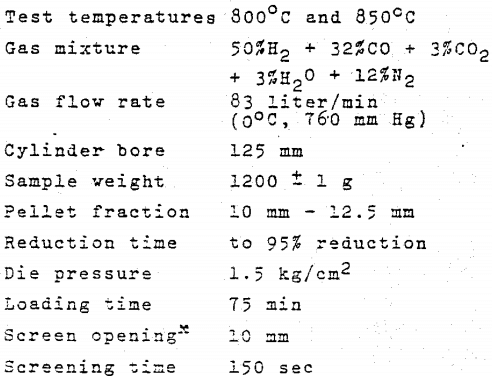
The sample container with the pellets (previously dried to constant weight) is lowered into the furnace and the pellets heated in nitrogen for 15 minutes to bring them to the required temperature. The nitrogen is then replaced with reducing gas, and the weight loss of the sample recorded continuously up to 95% reduction. When reduction is complete, the pellets are raised into the cooling zone and cooled in nitrogen for 20 minutes. The magnitudes of t95, R40 and R92 we determined.
The temperature cycle and the testing temperature inside the barrel are controlled and monitored by means of thermocouples The test comprises a heating phase with a reducing gas of composition matching the gas at the top of the direct reduction shaft, and an isothermal phase with a reduction gas matching the fresh gas that is blown into the shaft, i.e. the same as is used in the reducibility test. The test parameters are as follows:
The sticking properties are determined in an apparatus which is normally used for the reduction-under-load test for blast furnace pellets. When testing blast furnace pellets a bed of pellets is subjected to a constant load throughout reduction. In the case of pellets for direct reduction it has been shown in the laboratory that most of the sticking occurs in the wusite-to-iron stage.
LKAB Pellets for Direct Reduction
The chemical analysis and test data of LKAB’s present pellets grade for direct reduction, MPR, easily meet the requirements of today’s market. The quality will improve further when straight-grate production is started. The cold compression strength, reducibility and reduction strength will be improved and pellets exceeding 14 mm in diameter will be screened off. In the future, however, the desire on the part of direct reduction plants to increase their production by raising process temperatures will result in more stringent demands on the sticking properties of the burden. As we pointed out above, the sticking tendency of MPR can be substantially reduced by the addition of dolomite or limestone, which also leads to a further improvement in reducibility.
To sum up: the new pellets grades containing added dolomite offer the following advantages over the present MPR pellets produced in the shaft furnace:
- Greater handling strength
- Higher sticking temperature
- Improved reducibility
- Greater reduction strength
- Gangue composition more suitable for steel furnaces.