Table of Contents
Apatite, the most abundant crystalline phosphate mineral, is found in igneous rocks and probably is the primary origin of all other phosphates, whether mineral or organic. Its chemical formula may be expressed as Ca5(Cl,F) (PO4)3. The chlorine may be replaced almost entirely by the fluorine. It is found in large or small hexagonal prisms, usually of a vitreous luster and a green or red color, but also violet, white, or yellow. It is found largely as an accessory mineral in granitoid rocks. Its hardness is 4.5 to 5 and its specific gravity is about 3.2.
There are many other phosphate minerals—for instance, amblygonite, autonite, collophane, lazulite, monazite, pyromorphite, torbenite, triphylite, turquoise, vivanite, wavellite—with base elements of calcium, magnesium, iron, aluminum, manganese, lead, cerium, lanthanum, lithium, or several of these in combination and with various amounts of water of crystallization. However, these phosphate minerals have but little value save as specimens for mineral collections, the exception perhaps being turquoise.
Uses of Phosphate
Without phosphates, plant life cannot survive. With insufficient phosphates, plant life cannot thrive. Before the time of man, the phosphate in all vegetation traveled a cycle, it went from the soil into the plant and when the plant, or its parts, decayed and died, the phosphate returned to the soil from whence it came. Where soil was deficient in phosphate, little vegetation grew. Where there was soil rich in phosphate, dense forests and undergrowth flourished, other necessary conditions for plant life prevailing.
When, in the process of agricultural evolution, the products of the soil were removed to cities and other areas far from the land where they were grown, impoverishment of the phosphate content of the soil was a natural consequence and, in order to maintain fertility, it became necessary to add phosphate to the land. It was not until near the end of the eighteenth century that some farmers began to use ground bones, or bone meal, as an application to the soil to replace the phosphate that was removed by harvested crops shipped to other places.
Manufacture of Products
Superphosphate is manufactured by acidulating finely ground phosphate rock with sulphuric acid. The result is a conversion of about 80 pct of the tricalcium phosphate Ca3(PO4)2, to monocalcium phosphate CaH4(PO4)2, and about 15 to 17 pct to dicalcium phosphate Ca2H2(PO4)2, the remainder of the tricalcium phosphate being unchanged or having changed to insoluble iron and aluminum phosphates.
Monocalcium phosphate is soluble in water, dicalcium phosphate is soluble in ammonium citrate, and together they are considered “available” phosphates for plant food in the United States and Canada. The calcium thus removed from the tricalcium phosphate is still in the superphosphate but it is there as hydrated calcium sulphate (gypsum) and really dilutes the final product, superphosphate. For example, one long ton of phosphate rock, 74 pct BPL, containing 33.9 pct P2O5, will make almost two short tons of superphosphate containing only 20 pct P2O5. Therefore, the superphosphate plants must be close to the fertilizer-consuming centers, to avoid rail haulage.
In the larger superphosphate plants about one half of the fluorine in the phosphate rock is recovered and used in the manufacture of fluosilicates, valuable for insecticides, concrete hardeners, and other uses.
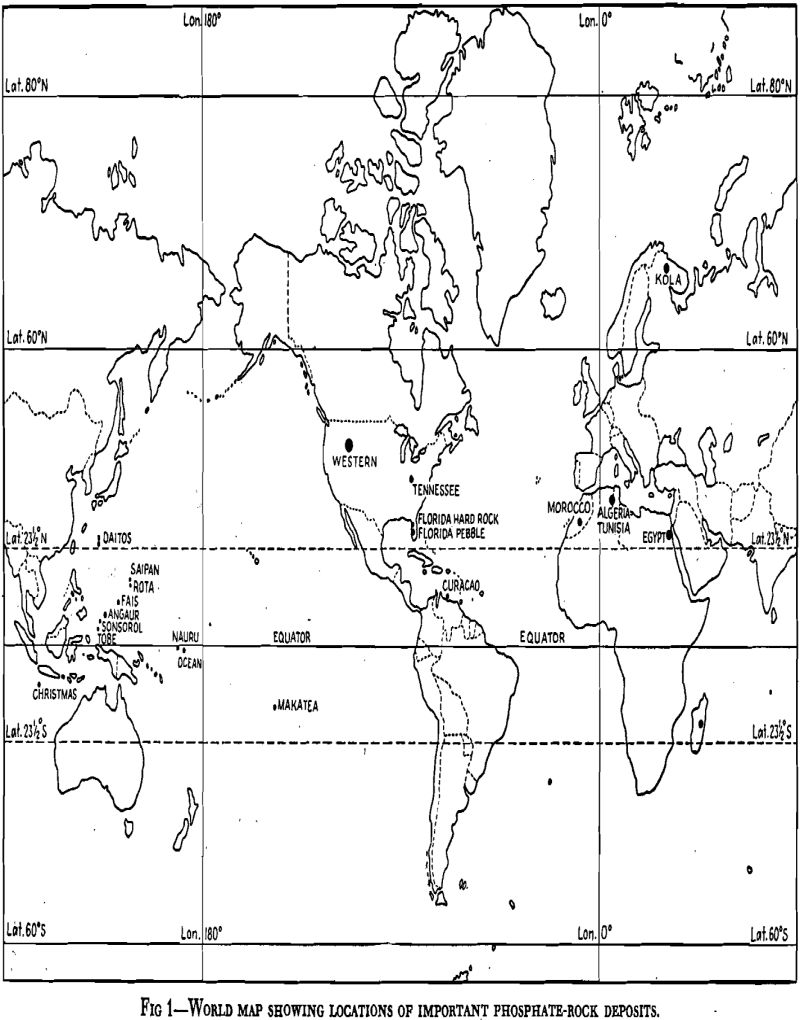
Florida Pebble Field
The Florida pebble field, accounting for about 70 pct of the total American production, is in the western part of Polk County and parts of other counties adjacent thereto. It is about 50 miles by rail from Tampa and Port Tampa, on the Gulf Coast, where special tidewater terminals are maintained by two large railroad systems for loading phosphate pock for seaborne shipments.
The pebble phosphate is found in alluvial deposits of Pliocene age mixed with sand and clay. This bed is called locally the “matrix.” It varies in thickness from a few feet to about 25 ft and underlies an overburden of about twice its thickness, though this varies considerably. The overburden is composed of sand and clay, and in places a bit of “hard-pan” in thin beds. The phosphate pebble in the matrix varies in size from pebbles of about 2 in. in diameter down to micron sizes, and generally the finer the pebble, the higher its grade in BPL.
This pebble came from the erosion of the Hawthorne marl of Miocene age. The detritus from this erosion apparently settled out in basins or depressions underwater together with the sand and clay from other near-by formations, forming what is today called the matrix, containing from 10 to 35 pct phosphate pebble. The phosphate rock is now covered with a barren overburden.
Seven mining companies are operating in the field, with a total of about 15 mines, each with its large dragline and its own ore-dressing plant, locally called a “washer,” from the early days when only the coarse pebble was recovered by screening.
At most of the mines, the overburden, from 10 to 50 ft thick, and the matrix below it, from zero to 35 ft thick, containing the phosphate pebble, are now excavated by large draglines. These excavators usually are of the “walker” type, electrically driven, with booms varying from 150 to over 200 ft in length and carrying buckets that vary in capacity from 10 to more than 20 cu yd. These draglines are capable of removing overburden at the rate of from 600 up to about 1500 cu yd per hour and excavating the matrix at the rate of from 300 to 1000 cu yd per hour.
On arrival at the washer plant, the slurry is passed through trommels to remove debris and large clay-balls. The disintegration of the smaller clay-balls is accomplished by log washers and Hardinge mills. The clay slime (including the unrecoverable phosphate of the micron sizes), is removed by various types of deslimers, including Dorr, hydroseparators, deslimers and Allen tanks. Plus 5-mesh pebble is recovered by vibrating screens; minus 5-mesh, plus about 20-mesh, is recovered by screens or Fahrenwald sizers, from the first two or three compartments of which the pebble is free of silica sand. The maximum size of the silica sand varies between 18 and 20-mesh. The pebble from the remaining compartments is diluted with silica sand of about the same size and this is the feed to the agglomeration tables or the flotation cells, or both. Spirals may replace tables.
The sized products in the washer bins are known as “wet rock” and are transported by rail to the central drying plants of the several operating companies. As a rule, very little storage capacity is provided at the washers, as the railroads spot enough empty wet-rock cars every day for the following 24 hr of production. These cars are bottom-dump gondolas: The haul from the washers to the central drying plants varies from about one mile up to 25 miles. Wet-rock shipments contain from 10 to 20 pct moisture.
Tennessee Field
The Tennessee field is in the central basin of Tennessee. Three types of phosphate are recognized—brown, blue, and white. The brown phosphate represents almost all of the phosphate rock produced in Tennessee today. It consists of residual deposits derived from several formations of Ordovician age. They lie on an irregular floor of limestone and in the depressions have accumulated the less soluble phosphates. These deposits range up to as much as 50 ft in thickness in the deeper depressions.
Draglines, much smaller than those used in Florida, remove the overburden and excavate the phosphate matrix, which grades 35 to 60 pct BPL. After treatment in the washer, however, it will have been concentrated to about 60 to 72 pct BPL. It is then dried and shipped to manufacturers of superphosphate and to electric-furnace plants for production of phosphorus. Some matrix is used directly as a furnace burden.
Other Deposits in United States
Other phosphate deposits in the United States have been mined and at one time were relatively large and important producers, such as those in South Carolina and the river pebble deposits of Florida. Still others of minor importance have been worked at times in a small way, such as those in Kentucky and Arkansas. In the central part of Kentucky brown phosphate deposits have been found in Fayette, Woodford, Scott, Jessamine, Franklin, and Clark Counties. Some phosphate was produced there prior to 1927. In the northern part of Arkansas, in Stone, Izard, Searcy, Marion, Baxter, Newton, and Independence Counties, phosphates occur in late Ordovician shales as original sedimentary deposits. Some mining was carried on between 1900 and 1912.
Morocco
The deposits in Morocco are in sediments between the Atlas Mountains to the east and the Atlantic coast to the west, traversing an area in the rolling plain for a distance of about 200 miles. These sediments are of the Eocene period and are composed of shales, limestones, and sandstones, usually only moderately folded. The phosphate being mined is an altered limestone, probably of marine origin, in the form of gently dipping beds 6 to 10 ft thick in these sediments. There are other parallel phosphorite beds, which are less attractive from the mining viewpoint.
Algeria-Tunisia
Along the border between the Province of Algeria and the Regency of Tunisia, starting about 75 miles south of the Mediterranean, there extends a series of phosphate deposits 125 miles southward to Metlaoui. These deposits, which are found on both sides of the border, are phosphorites in sediments similar to those in Morocco, but are of lower Eocene age. With a few exceptions, all mining is underground, the beds have a small angle of dip, and are opened by means of main adits similar to those in Morocco. The mining methods are also similar. The phosphate beds vary in thickness up to about 10 ft. Some beds break into very fine fragments while others produce large fragments mixed with fines.
In Algeria, the important operation is at Djebel Kouif, about 25 miles north of Tebessa, and belongs to the Cie. des Phosphates de Constantine (known as “Constantine”). This mine has produced for many years but reserves are sufficient for only a few years more. Therefore, Constantine has developed another deposit about 60 miles south of Tebessa, called Djebel Onk, which will continue the supply of phosphate rock from this company. Transportation is over cableways and a narrow-gauge railway to Tebessa, from which the state railway takes it to the port of Bone on the Mediterranean. Production from the operations of Constantine has been generally over 500,000 tons annually.
Russian Deposits
Phosphate production in Russia started about 1885. There are many deposits of phosphorites in European Russia, among which are those at Podolia and Bessarabia. Those at Podolia have been used for many years for the manufacture of superphosphate. From the Kostroma and Viatka phosphates, low-grade superphosphate (12 pct P2O5) has been made for a long time. Low-grade phosphorites at Smolensk, Koursk, Yoronech, Saratoff, Simbirsk, and Voleyda have been ground finely and used for direct application to the soil.
About 1930, in the northern part of European Russia, within the Arctic Circle, a huge deposit of igneous apatite was discovered, and its development into one of the largest phosphate producers was begun by the Soviet Government. It is in the Kola Peninsula, about 100 miles by rail south of the port of Murmansk, which is on a wide river flowing to the Barents Sea. It is about 700 miles by rail north of Leningrad.
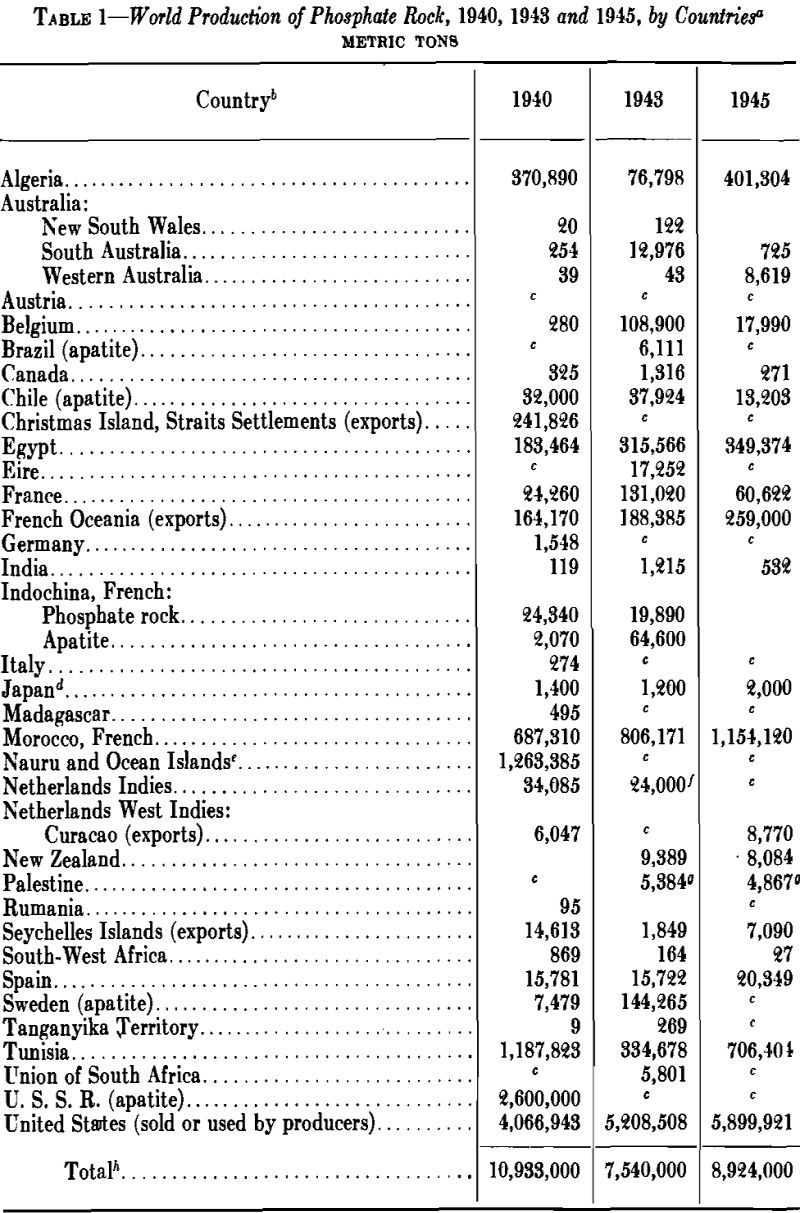
World Production of Phosphate Rock
World production of phosphate rock will continue to increase as world populations increase, because man’s civilization seems to require about half of his number living in large cities and being fed by the rest. Phosphate-rock production would double today if the farmer were to put back into the soil each year the phosphate that is lost to the soil by cropping. It would probably treble if, in addition, he gave the soil that which is lost by erosion and for other reasons.
Today, even in countries proud of their intelligence and their productive ability in agriculture, the phosphate lost to the soil by cropping is not being replaced. In 1944, for instance, the 25 states west of Michigan, Pennsylvania, West Virginia, Kentucky, Tennessee, Mississippi and Louisiana, removed more P2O5 from the soil by cropping than was returned to the soil in the fertilizer applied. The other 23 states not only put back the P2O5 lost by cropping but, in addition, more than times as much more. It is interesting to note that if the 25 states to the west had replaced only the P2O5 lost by cropping in 1944, they would have applied the equivalent of over 3,500,000 tons of 72 pct BPL phosphate rock instead of the equivalent of only about 1,000,000 tons that was actually applied to the soil. In addition, if times more in excess were applied, as was done in the other 25 states, they would have applied the equivalent of almost 9,000,000 tons of 72 BPL phosphate rock. This would have brought the 1944 consumption of phosphate rock in the United States up to about 12,000,000 tons as compared with a consumption slightly over 5,000,000 tons.