To have a complete text for a Method for the Determination of Phosphorus we must first discuss the interaction with other elements:
The Effect of Arsenic.—A question involving the temperature of precipitation of ammonium phospho-molybdate, which was brought to my attention some time ago, led to the following experiment:
A known quantity of arsenic was added to a steel solution, and the yellow salt was precipitated at varying temperatures, with the following results:
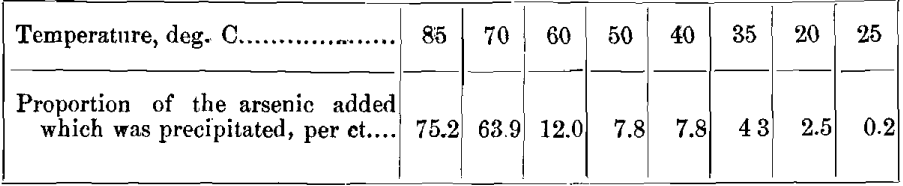
These results show the possibility of contamination of the phospho-molybdate by arsenic if the temperature exceeds 25° C. While differing degrees of dilution, etc., seem to affect the percentage of arsenic precipitated, at 25° C. I have never found it to exceed the limits of error of a phosphorus determination.
The Ratio of Phosphorus to Molybdic Acid in the Ammonium Phospho-Molybdate.—A quantity of ammonium phospho-molybdate was carefully prepared at 25° C. by the addition of microcosmic salt to an ammonium molybdate solution. This was carefully washed by decantation with water, until the final decantation contained less than .00001 per cent, of the original solution, then transferred to a porcelain evaporating-dish and dried at 85° C. to 90° C. for three days, with no contamination by organic matter. The result was a fine pulverulent yellow salt, with no tendency to caking, showing no sodium, and completely soluble in ammonium hydrate to a clear, colorless solution.
The phosphorus was determined:
- By direct precipitation of the ammoniacal solution with magnesium mixture.
- By Hundeshagen’s method of freeing the solution from molybdic acid before precipitation of the ammonium magnesium phosphate, by the saturation of the ammoniacal solution with H2S, acidulation with HCl, and filtering from the precipitated MoS. After this treatment, less than 0.02 per cent, of molybdic acid remained in the solution. The resulting determinations of phosphorus were as follows:
By method (1), 1.622 and 1.625 per cent.; by (2), 1.626 and 1.631 per cent.; average of all, 1.626 per cent.
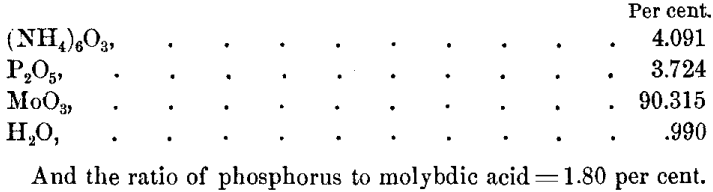
The molybdic acid was determined by precipitation of lead molybdate PbO,MoO3 and lead phosphate Pb3,(PO4)2 by lead acetate in an acetic acid solution, washing by decantation with boiling water, filtering on a weighed paper, and drying at 100° C. to 120° C. The final decantation showed no trace of lead, proving the mixed salts to be insoluble in boiling water. The resulting determinations of molybdic acid were 90.334 per cent, and 90.287 per cent.; average, 90.315 per cent.
The yellow salt, dried at 150° C., lost 0.99 per cent, of water. Further drying for fourteen hours, at 180° C., gave no additional loss, and, notwithstanding the ammoniacal solution was slightly blue in color, titration with a dilute solution of K2Mn2O8 showed that no perceptible reduction of molybdic acid had taken place.
These results would give the phospho-molybdate the following composition:
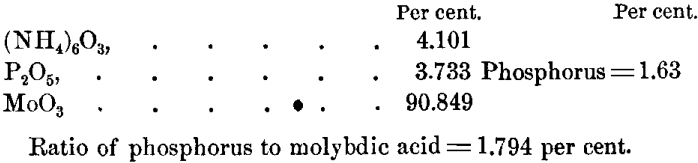
In view of the difficulties attending the analysis of this salt and the well-known law of chemical reactions that the ratio between combining atoms is simple, it seems rational to assume that the composition of the salt corresponds to the formula (NH4)6 O3,24MoO3, P2O5, with a percentage composition of:
Reduction of Molybdic Acid.—In reducing MoO3 preparatory to its oxidation with K2Mn2O8, I use a modified form of Jones’s reductor, consisting of a tube about 5/8-inch internal diameter, filled with powdered zinc to about 6 inches.
The reduction seems to be more perfect than with the addition of zinc to a sulphuric-acid solution, as used in Emmerton’s original method. With a K2Mn2O8 solution (1 c.c= 0.006703 iron), I obtained the following determinations of K2Mn2O8, corresponding to 0.5 grammes of phospho-molybdate, viz.: 77.14, 77.15, 77.10, 77.15, 77.20; average, 77.15 K2Mn2O8. Assuming the salt to contain 1.63 per cent, of phosphorus, and the ratio of phosphorus to molybdic acid to be 1.794, molybdic acid= iron x 87.847.
A number of determinations were made with varying quantities of molybdic acid, and all ranged from 87 to 88. For the purpose of comparing these results with Emmerton’s, the following determinations were made, as in Emmerton’s original paper, using 20 grammes of zinc for reduction. The results from 0.5 grammes of phospho-molybdate were: K2Mn2O8, 74.65, 74.65, 74.60; average, 74.63. This gives molybdic acid = iron x 90.814 as against the theoretical value of 90.76. This would make for the solution of K2Mn2O8, 1 c.c. = 0.006436 iron = .0001 P., instead of 1 c.c. = .006141 iron = .0001 P.
If the MoO3 were reduced to Mo2O3, we should have MoO3 = iron x 85.714. Hence the reduction seems to be at an intermediate point between Mo2O3 and Mo12O19, the formula quoted by Emmerton from Werncke for the sulphuric acid and zinc reduction.
This is an interesting point, and I should be glad if other members of the Institute felt disposed to make comparative tests.
Speed of Reduction.—Incidental to the reduction with K2Mn2O8, I noted the effect of the speed of reduction and the rate of oxidation of the reduced solution when exposed to the air.
Varying the speed of reduction of a 200 c.c. solution acidified with 10 c.c. H2SO4 from twelve minutes to two minutes, I found the results to be coincident. With greater speed the reduction was less complete, so that in one case, where the time was thirty seconds, nearly 10 per cent, escaped reduction.
The reduced solution, after standing exposed to the air for thirty minutes, showed no oxidation.
Temperature.—To determine whether all the phosphorus was precipitated at 25° C., whether the ratio of phosphorus to molybdic acid remained constant regardless of the temperature of precipitation, and whether the presence of Fe2(NO3)6 had any effect on the composition of the phospho-molybdate, 0.100 P was added to a steel solution and precipitated at 25° C. and 85° C., the proper checks being made on the steel at those temperatures. The results were:

The difference is within the limits of experimental error, and shows that all the phosphorus is precipitated at 25° C. with five minutes’ shaking, and that the composition of the phospho-molybdate is constant.
