Table of Contents
The precipitate method described herein is advantageous because of its rapidity and relative simplicity. This method eliminates the standardization of reagents, second filtration, washing of precipitate, and dissolving of ammonium phosphomolybdate precipitate, all of which are required in the titration step of the usual alkalimetric method. The precipitate method does not approach the accuracy of refined analytical methods. However, this study indicates that, within the grade ranges determined, readings of at least ±2.6 percent at the 95-percent confidence limits can be obtained by use of an appropriate exponential calibration curve.
The maximum time required for carrying through a single complete determination is about 25 minutes. With the development of greater skill, the time can be reduced to less than 15 minutes. By using the equipment recommended, four determinations can be made simultaneously.
This study indicates that the described method may be applicable as a simple process control in the beneficiation of sedimentary phosphate rock, and possibly could be applied as a simple, reliable means for guiding phosphate mining operations and in blending phosphate ores.
This report summarizes studies by the Bureau of Mines in developing a simple method for estimating the phosphorus pentoxide (P2O5) content of finely ground sedimentary phosphate ores and beneficiated products. The method used involved leaching, filtration, and precipitation. The Bureau found that measurement of the volume of ammonium phosphomolybdate precipitate obtained from samples containing 16.8 to 37.7 percent P2O5 and the use of an appropriate calibration curve permitted estimates of percentage of P2O5 content accurate within ± 1.1 to ± 2.6 percent, respectively, at the 95-percent confidence limits.
Incidental to mineral dressing and petrographic investigations of western phosphate ores in 1959, the Bureau of Mines undertook development of a simple process-control method for rapid on-the-spot evaluation of phosphate-mineral recovery. Because of the fine-grained and obscure character of western phosphate ores, chemical, rather than microscopic or petrographic, techniques were investigated. The described method involves acid decomposition of the ore or product, filtration, addition of ammonium molybdate reagent to the filtrate, and centrifuging and measuring the volume of yellow ammonium phosphomolybdate precipitate produced.
Routine determinations of phosphorus content by similar methods have been reported by Eggertz, Goetz and Wedding, and Baulieu between the years 1860
and 1925. However, application of these methods has been largely restricted to quick determinations of the relatively small quantities of phosphorus in bronze and steel. In contrast, the method described in this report is for determination of the relatively large quantities of phosphorus in phosphate ores and concentrates.
Materials and Equipment
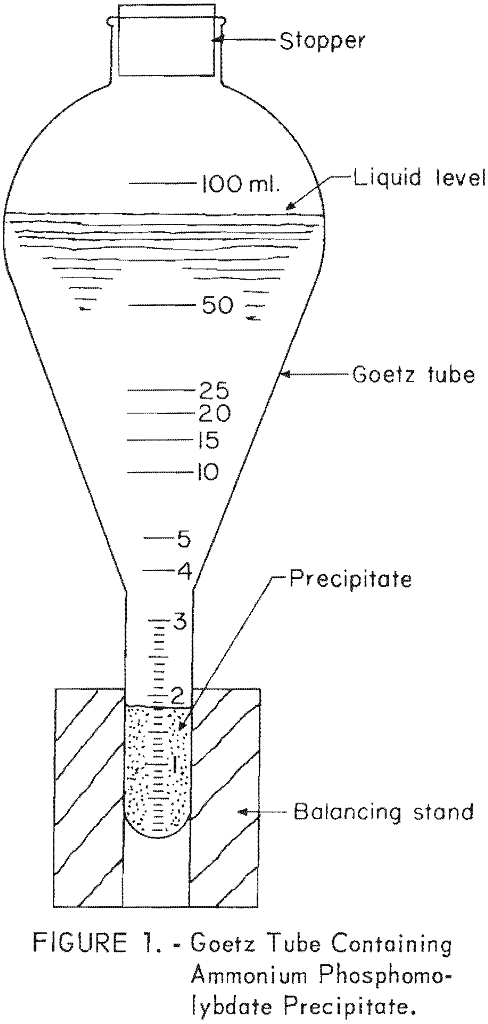
To provide a simple, accurate means of measuring precipitate volumes, 100-milliliter Goetz tubes with stems graduated from 0 to 3 milliliters by one-tenth-milliliter increments were used (figure 1). In this range the tubes have a tolerance of ± 0.05 milliliter.
The centrifuge used was an oil-testing model capable of holding four 100-milliliter Goetz tubes. Special aluminum cups were used for holding the tubes during centrifuging.
All filtrations were made through Whatman filter paper No. 30. However, on three trial runs with a roasted high-grade ore, the much faster Reeve Angel No. 711 filter paper gave comparable results even though some suspended material passed through.
The ammonium molybdate reagent was prepared as follows:
- Add 7 pounds of 70-percent nitric acid solution to 3,600 milliliters of water in a 10-liter bottle.
- In a 3-liter beaker, mix, stirring constantly, 480 milliliters of ammonium hydroxide with 1,400 milliliters of water. Then add 708 grams of 85-percent molybdenum trioxide and continue stirring until clear.
- Add about half of the molybdate solution (2) to the acid mixture (1) by swirling to prevent precipitation. Dilute the remainder of the molybdate solution (2) with 1 liter of water and continue adding to acid solution (1) with swirling.
- To the clear mixed solution, add a drop of H3PO4 and allow to settle. The clear solution is decanted for use.
Description of Method
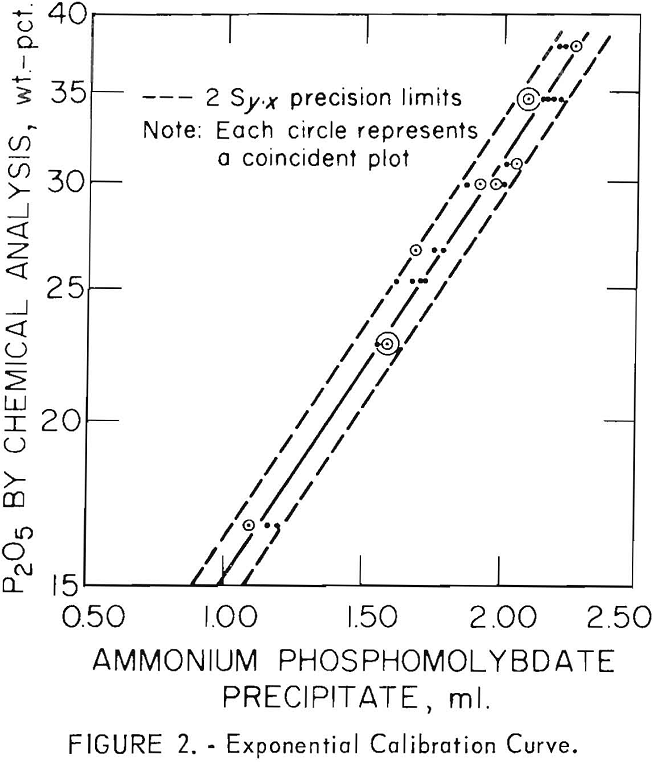
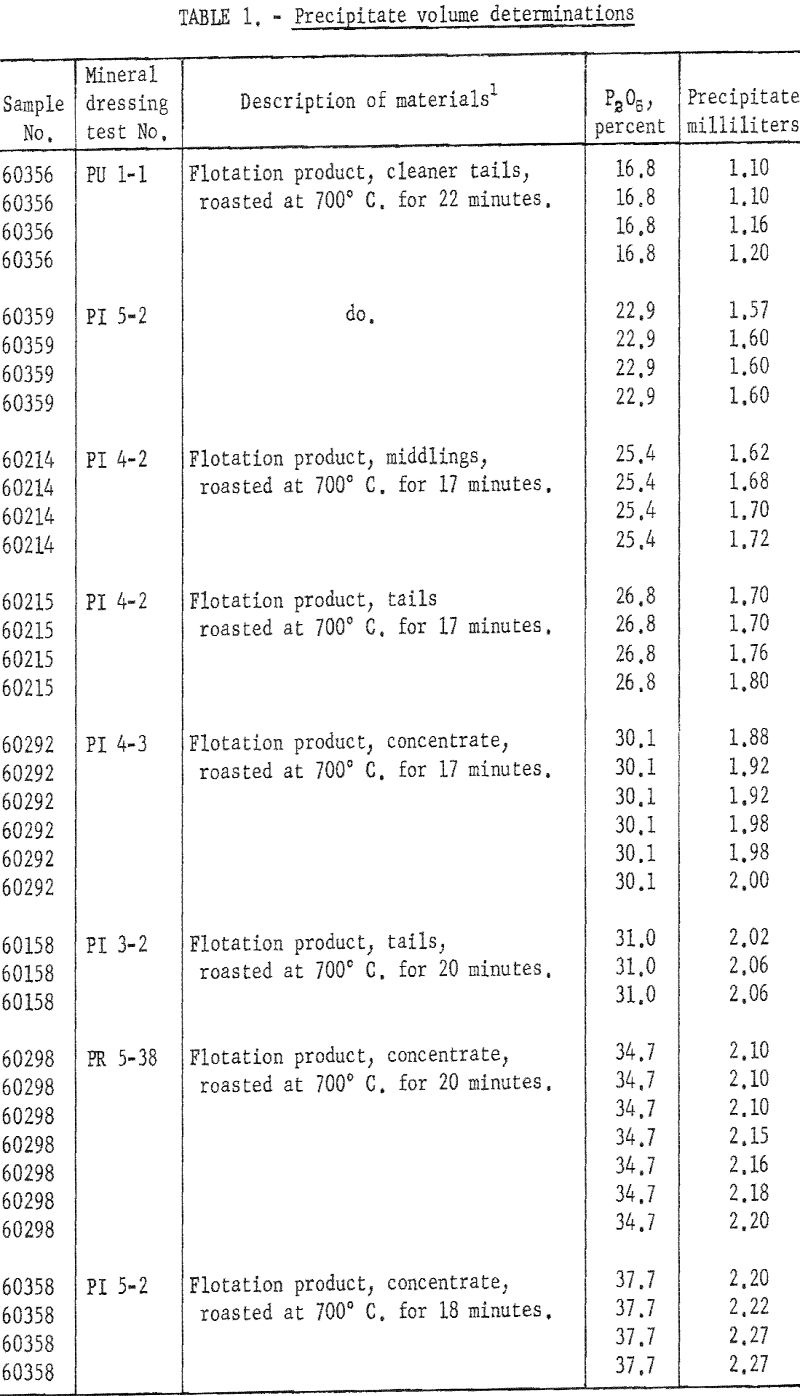
Thirty-six precipitate-volume determinations were made on eight roasted flotation products containing between 16.8 and 37.7 percent P2O5 as determined by the alkalimetric method. The results of these tests, along with sample descriptions and P2O5 contents, are given in table 1, and the plots of the determinations are shown in figure 2. From these data best fitting linear and exponential calibration curves were then calculated to show percent P2O5 versus milliliters of precipitate. All determinations were made at room temperatures ranging between 23° C. and 26° C. The samples were run in groups of four. The following procedure was used.
From phosphate ores and ore products ground through 80 mesh, weigh 0.300- gram samples and place them in 50 —milliliter beakers. Add 10 milliliters of 70-percent nitric acid, and while stirring, barely heat to fuming. Then add 10 milliliters of tap water. After alternate stirring, heating (but not to boiling), and cooling, bring the solutions to room temperature by running tap water on the outside of the beakers. Filter the cooled solutions through a fast acid-resistant filter paper into 100-milliliter Goetz tubes having stems marked off in tenths of a milliliter up to three milliliters. Apply a 10- milliliter wash solution of 14-percent nitric acid to each emptied beaker and then to the residue on the filter paper.
After collection of the entire filtrate, add 55 milliliters of ammonium molybdate reagent to the Goetz tubes and then completely mix the reagent and filtrate by repeated inversion of the tubes. To develop enough heat for precipitation to take place rapidly, hand-warm the tubes for approximately 15 seconds.
Next, precisely balance two pairs of Goetz tubes and centrifuge at 1,250 revolutions per minute for five minutes. Remove the tubes, mount them on ringstands, and level the precipitate surface by very gentle tapping with the fingers. After a 3-minute settling period, read the precipitate volumes. These values are used in setting up calibration curves and in making estimates of percent P2O5 from the curves.
In most sedimentary western phosphates, the phosphorus is almost entirely tied up in carbonate fluorapatite. The described method of acid leaching is largely limited in its effectiveness to the carbonate fluorapatites commonly present in sedimentary phosphate rock. In general other phosphate minerals will require more drastic acid treatment. For example, if much wavellite is present in the sample, a more prolonged 70-percent nitric acid leach may be necessary.
Determination of Best Fitting Curves
Plots of data for charges of 0.300 grams were made on double-logarithmic, semilogarithmic, and rectangular coordinate paper. The latter two plots appeared to yield almost straight lines. By the use of standard regression equations, best fitting linear and exponential curves were calculated. The best fitting linear and exponential equations were found to be
Percentage of P2O5=18.48x-5.29
Percentage of P2O5=0.879 (10)0.309x
respectively, where x equals milliliters of precipitate.
Accuracy
The standard error of estimate (S y.x) for the linear equation was,
Sy.x=1.13,
and for the exponential equation;
Sy.x=0.0143.
Using the formula for the coefficient of correlation,
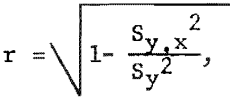
a larger correlation coefficient (r=0.990) was obtained with the exponential equation than with the linear equation (r=0.984). Therefore, the exponential equation offered the best correlation of the data.
With the linear plot, errors no greater than ± 1.1 and ± 2.3 percent P2O5 can be expected at the 68- and 95-percent confidence limits, respectively. However, with the exponential plot, errors less than ± 1.3 and ±2.6 percent P2O5 are expected with readings of 37.7 percent P2O5 at the 68- and 95-percent confidence limits. With readings of 16.8 percent P2O5, deviations in the order of ± 0.6 and ± 1.1 percent P2O5 are expected at the 68- and 95-percent confidence limits, respectively.
Possible Improvement
The time required for precipitate-volume determinations possibly can be significantly reduced by the following:
- Higher speeds and shorter periods in centrifuging.
- Reduction of the time required for acid leach by closer investigation of time and also of acid strengths.
- Use of faster filter paper to increase the rate of filtration.
Running samples in duplicate and averaging the results may improve the accuracy of the method considerably. Accuracy also might be improved by the formulation of individual calibration curves for each Goetz tube.
