Table of Contents
The discovery of low-grade gold-silver deposits in the United States has resulted in construction of several cyanide-leach carbon-in-pulp (CIP) milling facilities. Gold and/or silver ores typically contain other metals such as mercury and a variety of base metals. Of all the metals most likely present, mercury presents the most serious problem.
Typically, 10 to 30 pct of the mercury and 75 to 95 pct of the gold and silver are solubilized during cyanidation. Mercury is adsorbed on carbon with gold and silver, then stripped along with the precious metals with caustic cyanide solution, and electrowon on steel wool cathodes. Mercury must be either recovered or precipitated because of the health hazard during cathode smelting and carbon regeneration.
Metal sulfides, such as calcium, sodium, silver, zinc, and iron sulfides, have been used to precipitate mercury from gold cyanide solutions. Silver sulfide would tie up considerable silver, and iron sulfide forms ferrocyanide, resulting in cyanide loss. Calcium and sodium sulfides are effective in precipitating mercury and are not harmful to the gold recovery process. Redissolutlon of mercury occurs when sodium sulfide is used; when calcium sulfide (CaS) is used, the dissolution of the precipitate is minimized. Mercury precipitation with CaS is shown by the chemical equation
Hg(CN)4²- + CaS → HgS + Ca²+ + 4CN-
Silver is similarly affected with CaS addition. Although gold is unaffected, some of the silver precipitates, with the mercury.
The objective of this investigation was to study (1) silver loss during CaS precipitation of mercury in gold-silver cyanide-leach slurries and in solutions prepared from mercury-containing gold- silver ores and (2) methods to prevent silver loss.
Material Equipment and Procedure
Ore samples used in this investigation were obtained from the Carlin Gold Mining Co., NV, Cortez Gold Mines, NV, Getty Minerals Co., UT, and 5-M, Inc., UT.
Mineralogical examinations were conducted on all four ores. The Carlin ore consisted of calcite (CaCO3), with smaller amounts of gypsum (CaSO4·2H2O), feldspar minerals, clay minerals, quartz (SiO2), and hematite (Fe2O3). The Cortez ore consisted of limestone, crystalline calcitic dolomite [Ca,Mg(CO3)2], gypsum, barite (BaSO4), and clay minerals. This sample also contained a few percent pyrite (FeS2) and traces of zinc and antimony. The Getty ore consisted of limestone, with some quartz and clay minerals. Minor amounts of limonite (Fe2O3·nH2O), pyrite, realgar (AsS), and orpiment (As2S3) were also present. The 5-M ore consisted of quartz, calcite, muscovite [H2KAl3(SiO4)3], biotite [H2K(Mg,Fe)3(Al,Fe)(SiO4)3], hematite, malachite [CuCO3·Cu(OH)2], cerussite (PbCO3), and a small amount of mercury and silver. The mineralogy of the silver and mercury could not be determined in any of the ores.
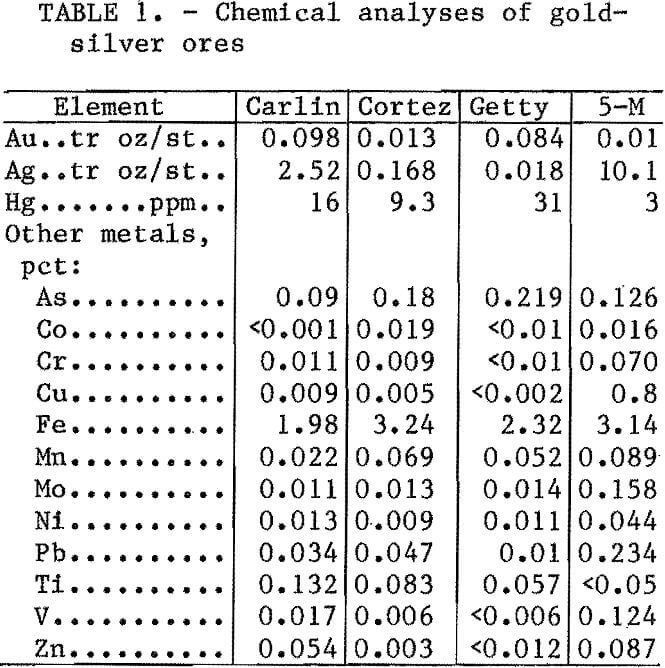
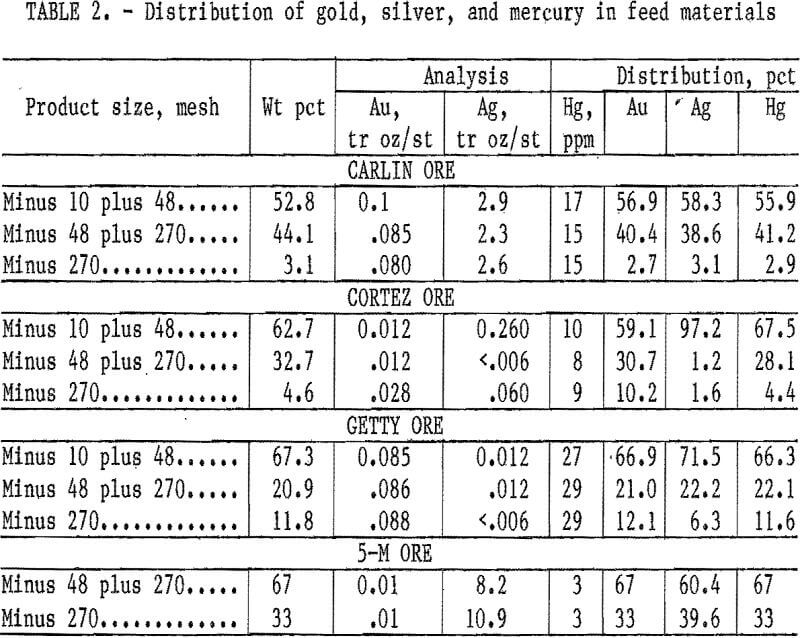
Chemical analyses of the four ore samples are listed in table 1. Analyses show that in addition to gold, silver, and mercury, other metals, including arsenic, cobalt, copper, iron, manganese, molybdenum, nickel, lead, vanadium, etc., are also found in these ores. Chemical analyses of minus 10- plus 48-, minus 48- plus 270-, and minus 270-mesh-size screen fractions of the four ore samples showed mercury evenly disseminated throughout these size fractions (table 2).
The NaCN (sodium cyanide), lime, CuCN (cuprous cyanide), and CaS used in this study were all reagent grade.
Leaching tests were performed by adding 50 to 1,000 g of dried, 90-pct minus
200-mesh ore to between 200 and 3,000 mL of pH 11 leach solution at ambient tem-perature. Lime was used to adjust pH. The slurries were leached in closed plastic bottles placed on laboratory rolls for periods of up to 24 h. The leached slurries were filtered and the resulting residues washed and dried at ambient temperature. The residue and solutions were analyzed by inductively coupled plasma (ICP) and/or atomic absorption (AA) techniques.
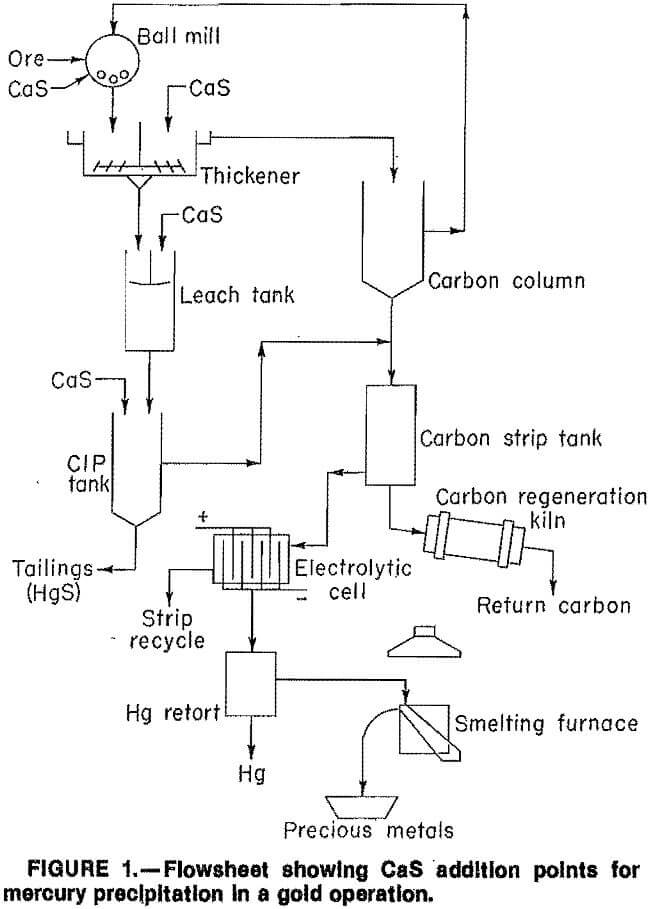
In mercury precipitation tests in a ball mill, CaS was added to the slurry in the mill along with cyanide and lime. The slurry mixture was ground for 45 min, washed from the ball mill with water, and leached for periods of up to 24 h at 25 pct solids. Additional mercury precipitation tests were conducted by adding CaS directly to the pregnant cyanide leach slurry, followed by three-stage CIP adsorption to recover precious metals. In other tests, CaS was added directly to the leach solution and the precipitate was recovered by filtration. CaS additions are reported in pounds per short ton of ore, whereas NaCN is reported in pounds per short ton of solution.
Results and Discussion
Mercury Extraction During Cyanide Leaching
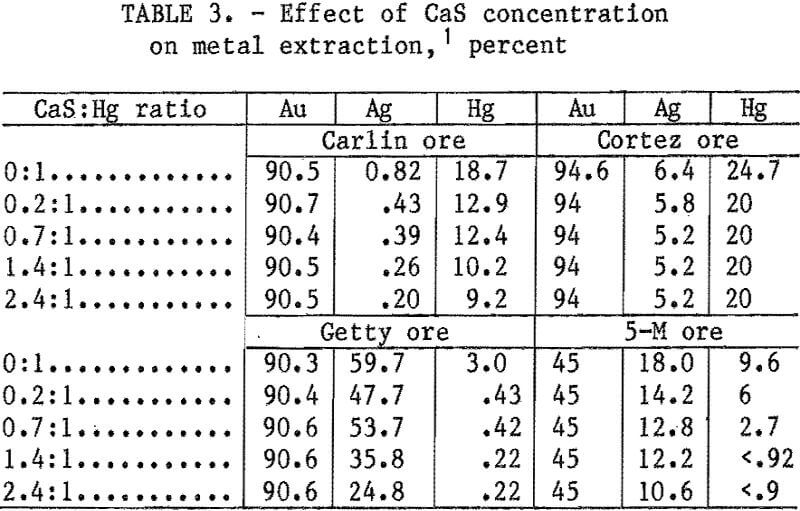
NaCN leaching tests were conducted with all four ores to compare mercury and precious metal extraction with and without CaS addition. All ores were leached at 25 pct solids with 1 lb/st NaCN at pH 11 and at ambient temperature for 24 h. CaS was added at the beginning of the leach; the CaS:Hg ratio was varied from 0:1 to 2.4:1. Results of these tests are listed in table 3. Increasing the CaS:Hg ratio from 0:1 to 2.4:1 decreased mercury and silver extraction but had little effect on gold for all ores.
Addition of CaS at Other Points in the Process
Because CaS addition precipitated silver as well as mercury, further testing was necessary. Previous research indicated CaS could be added to (1) the grinding circuit and (2) the pregnant cyanide leach slurry prior to the CIP circuit to precipitate mercury (fig. 1).
Mercury Extraction in a Laboratory Ball Mill
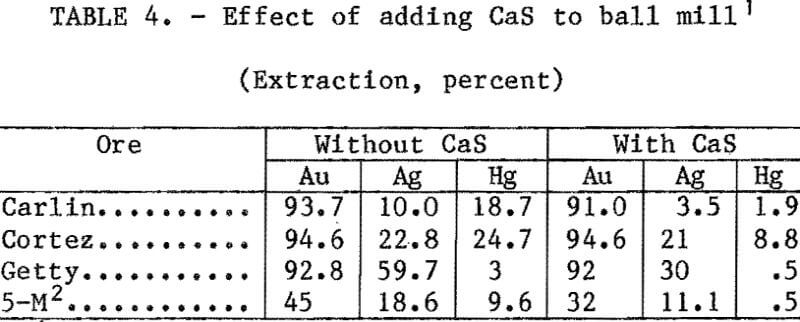
Tests were conducted with all four ores to determine the effect of CaS addition on mercury, silver, and gold during grinding. A 50-pct-solids slurry containing 1 lb/st NaCN, enough lime to give a pH of 11, and 0.1 lb/st CaS was added to a laboratory ball mill and ground for 45 min. The slurry was washed from the ball mill and leached for 24 h at 25 pct solids. This same procedure was repeated without CaS. Results of these tests are listed in table 4. With all ores, mercury extraction was greatly reduced with CaS addition; however, silver extraction was also reduced by 8 to 65 pct.
Mercury Precipitation Prior to Carbon Adsorption
Previous research demonstrated that precipitated mercury sulfide redissolved with time. To decrease this redissolution, CaS was added just prior to carbon adsorption. Tests were conducted by adding CaS to a pregnant NaCN leach slurry prior to contacting with activated carbon. All ores were leached for 24 h at ambient temperature with 1 lb/st NaCN and enough lime to give a pH of 11 at 25 pct solids. Following the 24-h leach, varying amounts of CaS were added and the slurry was mixed by rolling for 1 h. The slurry was then contacted with 6 g of minus 6- plus 16-mesh carbon for 1 h and and screened. To simulate a three-stage CIP system, the slurry was contacted a
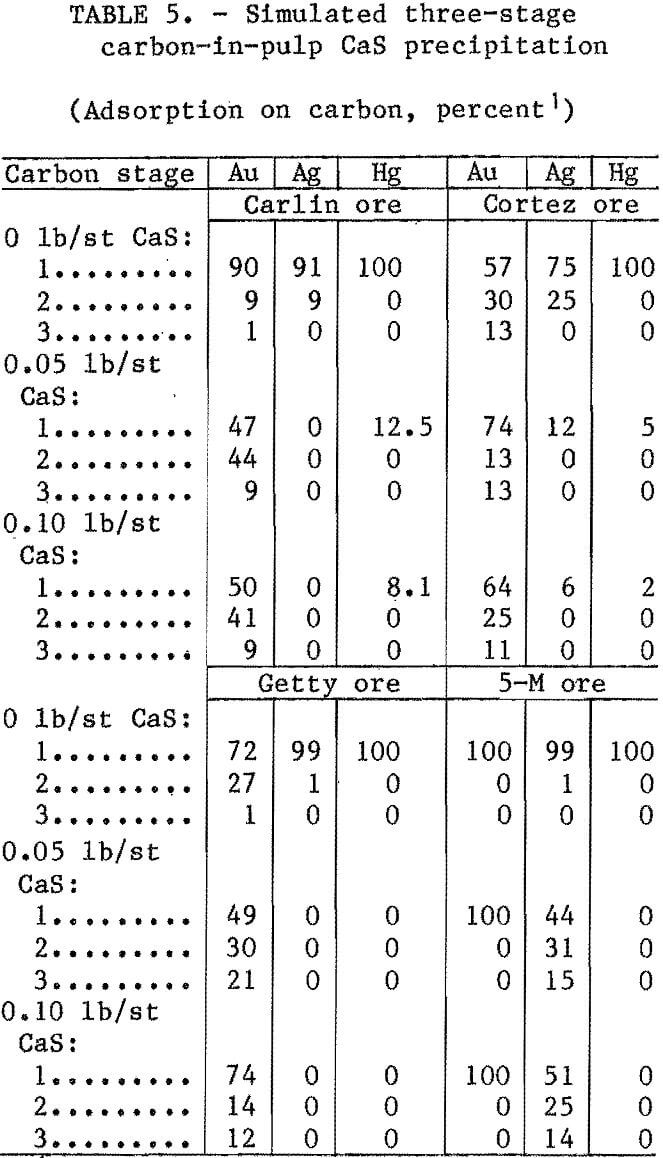
second and third time with an equal amount of fresh carbon. At the end of each test, the slurry and loaded carbon were analyzed. Test results are listed in table 5.
Adsorption of gold, silver, and mercury was 100 pct without CaS addition. With the addition of CaS, adsorption of mercury was zero for Getty and 5-M ores; however, for the Carlin and Cortez ores, small amounts of mercury were adsorbed during the first carbon contact stage. The addition of CaS prevented adsorption of silver from the Carlin and Getty ore slurries, significantly reduced adsorption from the Cortez ore slurry, but had little effect on adsorption from 5-M ore slurry. Over 90 pct of the 5-M silver adsorbed on the carbon. Although all the solubilized gold adsorbed on the carbon, with and without CaS addition, the rate of adsorption was considerably slower with CaS addition for all ores except the 5-M ore,
Copper Addition to Eliminate Silver Loss during Mercury Precipitation
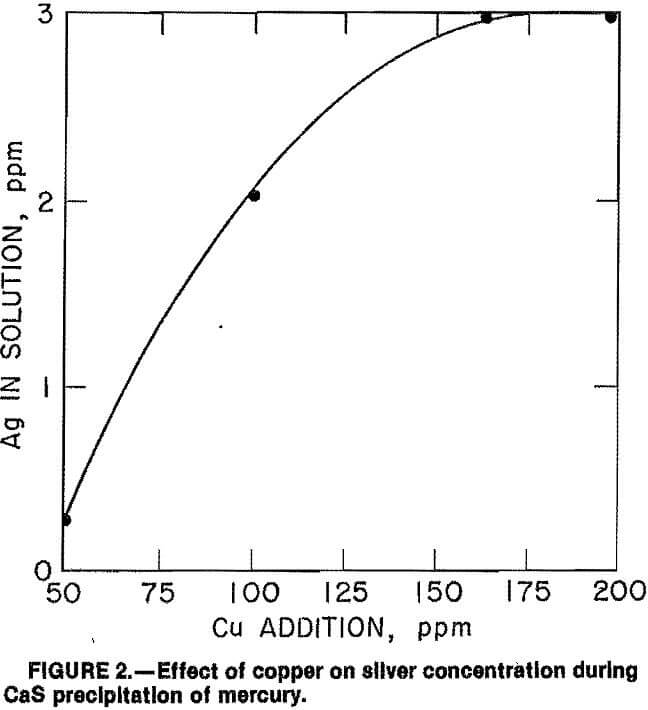
Additional tests were initiated to determine why silver was not precipitated from the 5-M slurry during mercury precipitation. Analysis of the 5-M pregnant leach solution showed that 170 ppm Cu was dissolved in it. To determine the effect of copper during mercury precipitation, varying amounts of copper (added as CuCN) were introduced to a pregnant leach solution containing 1,5 ppm Au, 3 ppm Ag, and 0.76 ppm Hg prior to the addition of 0.1 lb/st CaS. The
results, given in figure 2, show that silver content in solution increased rapidly as copper addition increased from 50 to 160 ppm. With the addition of 160 ppm Cu, silver loss was eliminated while mercury precipitation was unaffected.
The tests described in table 5 were repeated, but with 235 ppm Cu added to the ores prior to the CIP step. Following the 24-h leach, 0.1 lb/st CaS was added to the slurry, which was rolled for 1 h and then contacted with carbon. Results listed in table 6 show that silver recovery was( greatly increased with copper addition. Adsorption of silver from Carlin and Cortez ores was increased from 0 to 80 pct and 6 to 90 pct, respectively. With the Getty ore, copper addition appeared to have no effect on silver recovery. However, with only 0.018 tr oz/st Ag in the Getty ore, these
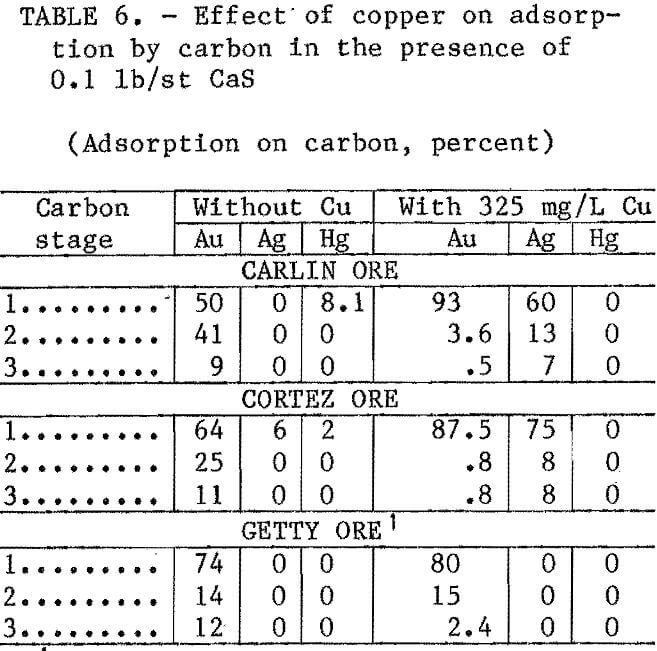
suits may be suspect. Adsorption of gold was reduced by about 10 pct with Cortez ore and about 3 pct with Carlin and Getty ores. Adsorption of mercury was zero for all ores when CuCN was present.
Sequential tests (i.e., mercury precipitation, carbon adsorption and stripping, and electrowinning) were conducted with a copper-containing heap leach solution, to determine the effect of copper on silver loss during both mercury precipitation and gold-silver adsorption on carbon. Test results are illustrated in figure 3. After CaS was added to the heap leach solution containing 1.4 ppm Au, 2.92 ppm Ag, 0.76 ppm Hg, and 270 ppm Cu, mixed for 2 h and filtered, nearly 98 pct of the mercury and 0.6 to 0.7 pct of the silver and copper were precipitated. The filtrate was pumped through a carbon column at 0.13 BV/min to recover the gold and silver. Nearly all the gold and silver, plus 0.7 pct of the copper, and nearly 69 pct of the 0.016 ppm Hg remaining in solution after precipitation were adsorbed. Stripping the carbon at 90° C, using a solution containing 0.1 pct NaCN plus 1 pct NaOH at a pumping rate of 0.13 BV/min, produced an eluate containing 5 ppm Au, 19 ppm Ag, 16 ppm Cu, and 0.28 ppm Hg. Subsequent gold and silver recovery by electrolysis was >98 pct at 3 V and 60° C in 30 min. Although the mercury content in the electrolyte was only 0.28 ppm, mercury was recovered with the precious metals. Increased copper in the
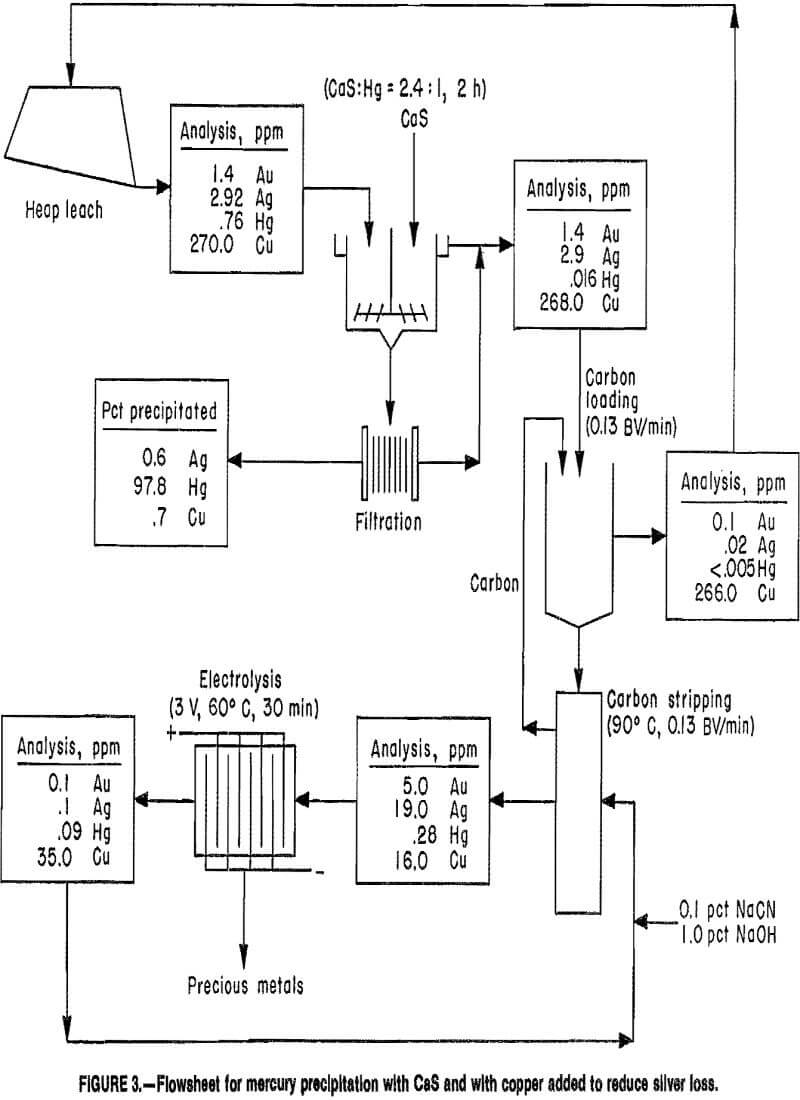
spent electrolyte was probably due to contact of the strong cyanide solution with the copper anode connector.
Results indicate that copper addition is an effective approach to eliminating silver loss during mercury precipitation with CaS. Less than 1 pct of the copper and silver were lost during mercury precipitation, and less than 1 pct of the copper was adsorbed on the activated carbon. These results also indicate that even low quantities of mercury are electrowon from the pregnant electrolyte. To eliminate the need for retorting the gold-silver cathodes prior to smelting, the mercury must be reduced to levels even lower than the 0.28 ppm achieved in this test.
Summary and Conclusions
Mercury extraction from Carlin, Cortez, Getty, and 5-M ores ranged from 3 to nearly 25 pct when using 1 lb/st NaCN without CaS. Carbon adsorption of gold, silver, and mercury from leach pulps or solutions is usually 90 to 100 pct; however, the addition of 0.1 lb/st CaS to the slurry reduced mercury and silver adsorption to between 0 and 8 pct and 0 and 6 pct, respectively, with all ores except the high-silver-copper 5-M ore. With this ore, 0 pct of the mercury and 90 pct of the silver adsorbed on the carbon. Further analysis showed that the 5-M leach solution contained 170 ppm Cu. Addition of copper to the Carlin and Cortez ore slurries prior to mercury precipitation and carbon adsorption resulted in adsorption of none of the mercury and 80 to 90 pct of the silver. Further testing with a heap leach solution containing gold, silver, mercury, and copper showed that 98 pct of the mercury was precipitated, with about 0.7 pct of the silver and copper coprecipitating. Nearly all the gold and silver and only 0.7 pct of the copper adsorbed on the activated carbon.
These results show that mercury can be precipitated from gold-silver cyanide leach slurries or solutions without severe losses of silver if copper is present.
