Table of Contents
Most copper is produced from chalcopyrite concentrates by smelting. While smelting is both simple and efficient, it converts the sulfur in the feed concentrate to sulfur dioxide. To meet the ambient standards for this pollutant, as established by the Clean Air Act, the Federal Environmental Protection Agency (EPA) has established a goal for smelters to recover at least 90 percent of the sulfur contained in their feed concentrates. Controls on new plants will be even stricter and, in some locations with poor meteorological conditions, new plants may not be allowed.
The only commercially proven technology for control of sulfur dioxide emissions is based on the production of sulfuric acid in contact acid plants. This is practical only for relatively concentrated gases, such as those produced in the converting and roasting operations but not for the relatively dilute gases produced by reverberatory furnaces. These dilute gases must be treated by a more expensive process, such as lime scrubbing or by one of the currently unproven processes. Furthermore, most smelters are not located near a market capable of absorbing the additional amount of acid that would be produced; thus, the acid would have to be neutralized for disposal. As a result, there is considerable interest in developing alternate processes that, unlike smelting, do not produce sulfur dioxide.
Such a process, the ferric chloride leaching of chalcopyrite concentrate, has been developed by the Bureau of Mines Reno Metallurgy Research Center. The process is hydrometallurgical and, since no roasting or smelting of the concentrate takes place, no sulfur dioxide is produced. Copper is leached from the concentrate with a ferric chloride solution leaving elemental sulfur in the leach residue. The sulfur can be recovered by leaching with ammonium sulfide.
The ferric chloride leaching process was originally described by the Bureau of Mines in 1971. As the result of additional research, improvements in method:, for recovering copper and sulfur and for regenerating the ferric chloride leach solution have been incorporated in the process and have been reported by the Bureau of Mines in 1975. Results of these investigations were used in designing a plant for the economic evaluation presented in this report.
Process Description
A plant is designed to produce 165 tons of copper per day from a chalcopyrite concentrate having the chemical analysis shown in table 1. The concentrate is assumed to be dry. The material balance and the thermal requirements for the process are shown in the appendix.

Chalcopyrite concentrate, obtained from an ore concentrator, is dry-ground and then leached with ferric chloride solution. The insoluble residue is separated from solution and treated to remove sulfur. Copper is precipitated from the pregnant solution by electrolysis, and the remaining liquor is treated with oxygen to remove excess iron as ferric oxide and regenerate the original ferric chloride leach solution. The sequence of operations is shown in figure 1.
Leaching
Chalcopyrite concentrate is delivered to the plant by truck and is dumped into a hopper from which the concentrate is conveyed to storage silos. The concentrate is withdrawn from storage as needed and fed to a ball mill where it is dry-ground from approximately 100 mesh to minus 325 mesh. The ground concentrate is fed to leaching tanks operating at about 220° F where it is leached with approximately 6 tons of leach solution per ton of concentrate. The leach solution contains approximately 36 percent ferric chloride and about 1 percent hydrochloric acid. During leaching about 99 percent of the copper, 84 percent of the iron, 90 percent of the calcium, and 96 percent of the silver are dissolved. The representative reaction for dissolving chalcopyrite is
CuFeS2 + 3FeCl3 → CuCl + 4FeCl2 + 2S.
None of the gold and molybdenum are dissolved. A small quantity of sulfur dissolves and reacts to form calcium sulfate.
The slurry from leaching is cooled and pumped to a setller, along with filtrate from a washing filter, where the undissolved material is separated from solution. Underflow from the settler is fed to a rotary vacuum drum filter where the residue is washed. The washings and filtrate are recycled to the settler, and the filter cake, containing about 75 percent solids, is conveyed to the sulfur recovery section. Overflow from the settler is pumped to the copper precipitation section.
Copper Precipitation
Solution from the settler in the leaching section contains about 5-½ percent copper, most of which can be recovered by electrolysis in a diaphragm cell.
The solution is pumped to a diaphragm cell operating at about 105° F where the silver and about 75 percent of the copper in solution are deposited on the cathode. The major reactions in the cell are assumed to be
CuCl + FeCl2 → Cu + FeCl3
and CuCl2 + FeCl2 → CuCl + FeCl3.
The cathode is periodically rapped to dislodge the copper deposited on it, which then settles to the bottom of the cell.
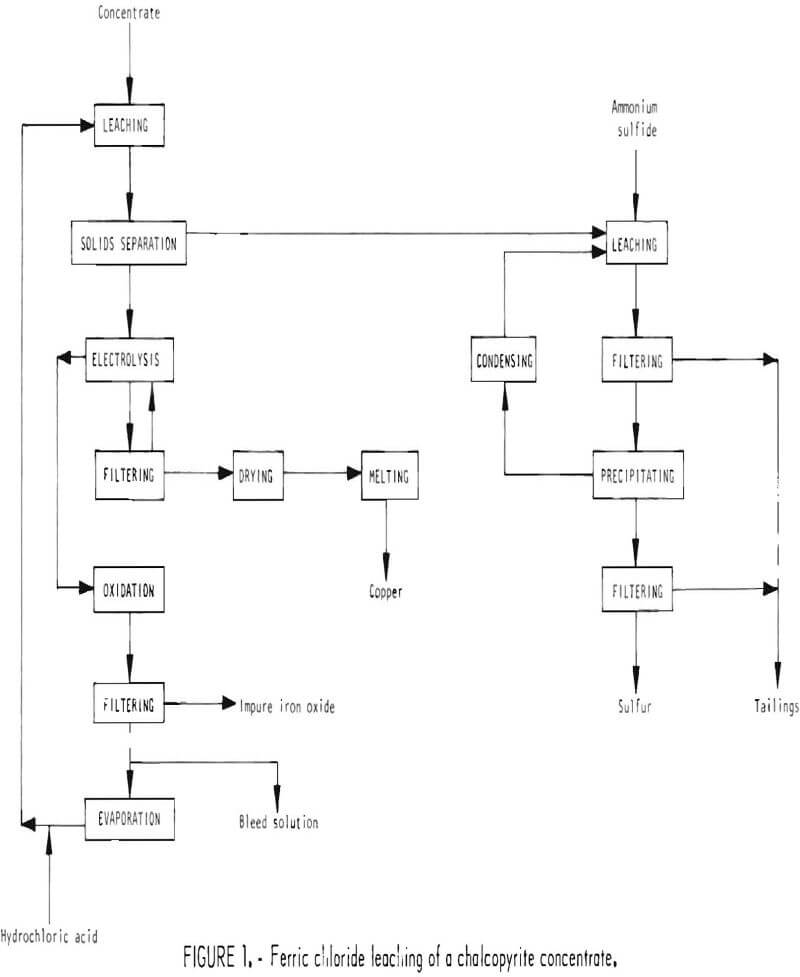
The copper slurry from the cell is pumped to a rotary vacuum filter where the copper and other solids are separated and washed. Filtrate and washings from the filter are returned to the cell as feed to the anode compartment. The filter cake contains about 25 percent moisture, which is removed in a rotary dryer. The dried cake is then melted in a furnace to remove impurities as vapor. Any slag which may form is tapped into ladles for disposal, and the vapor is scrubbed to remove condensables. The copper is tapped into ladles and cast into anodes. Further refining would be required for most uses and for recovering silver.
Stripped solution is removed from the anode compartment of the cell for processing in the ferric chloride regeneration section.
Ferric Chloride Regeneration
Direct oxidation of the spent solution from the copper precipitation section regenerates the leach solution and removes excess iron not needed for the leaching operation.
The stripped solution is pumped to turbo-oxidizers operating at approximately 175° F. The oxidation reaction is assumed to be
12FeCl2 + 3O2 + xH2O → 8FeCl3 + 2Fe2O3·xH2O.
Ferric oxide is separated from the solution and washed on a rotary vacuum filter. The ferric oxide is then mixed with water and pumped to a tailings pond for disposal. If the ferric oxide were to be sold, further purification would be necessary.
A small portion of the solution from the filter must be bled and discarded to limit any impurity buildup in the leach circuit. The remaining solution is pumped to an evaporator to remove excess water. Hydrochloric acid is added to the concentrated solution to regenerate the leach solution.
Sulfur Recovery
Residue from leaching contains free sulfur, which can easily be recovered. This residue is leached with an ammonium sulfide solution to dissolve the free sulfur. The dissolution reaction is assumed to be
xS + (NH4)2S → (NH4)2Sx+1
The undissolved solids are removed from the solution and washed on a rotary vacuum filter. The residue from the filter, which does not contain enough gold and silver for economical recovery, is mixed with water and pumped to a tailings pond.
Dissolved sulfur from filtration is precipitated by heating the solution to decompose the ammonium polysulfide. The decomposition reaction is assumed to be
(NH4)2SX + 1 → 2NH3 + H2S + xS.
Steam is injected into the solution to heat it to 203° F and to provide excess water in the offgas. Slurry from precipitation in filtered to recover a wet sulfur product, which is either shipped or stored in open piles for future shipment. If the sulfur is to be stored for an extended period of time, excessive dust losses could be prevented by moistening the surface of the storage pile. The filtrate is mixed with the sulfur leach residue and is pumped to the waste pond.
The offgases from precipitation are condensed to regenerate 90 percent of the original ammonium sulfide solution for recycle to the sulfur leaching operation.
Economics
The cost estimate presented in this report is based on data from the Reno Metallurgy Research Center. Cost data used are from published sources brought up to date by the use of inflation indexes. Freight costs for the raw materials are not included since the plant location is not considered.
Capital Costs
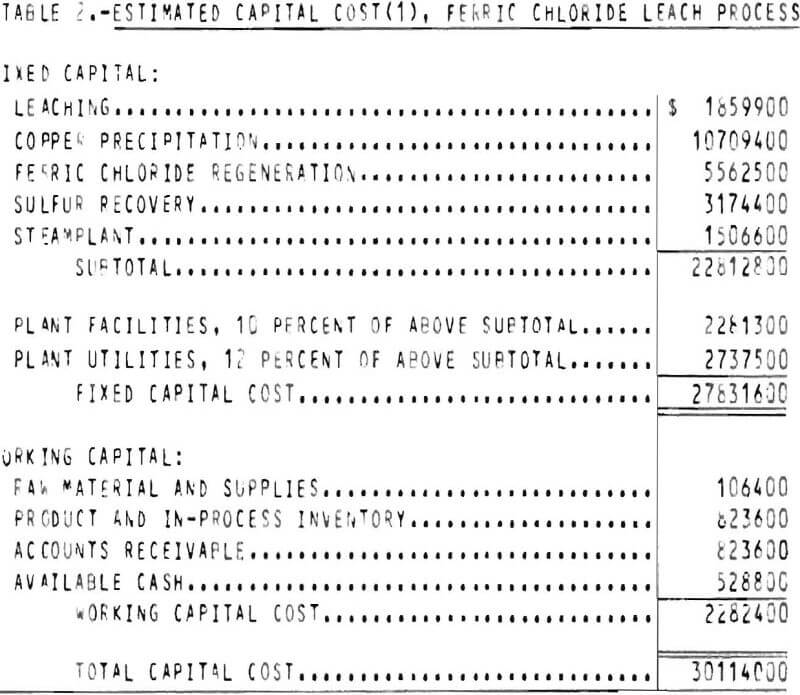
The capital cost estimate is of the general type called a “study estimate” by Weaver and Bauman. This type of estimate, prepared from a flowsheet and a minimum of equipment data, can be expected to be within 30 percent of the actual cost. The fixed capital cost on an early 1975 basis (Marshall and Swift index 437) for processing 599 tons per day of chalcopyrite concentrate is $27,831,600. The plant, which is divided into four sections, is designed to operate 3 shifts, 7 days per week, 330 days per year with the exception of the concentrate unloading facilities which operates only 1 shift per day. The total capital cost for the process is shown in table 2.
Cost-capacity data of the type presented by Bauman, Mills, and Peters and Timmerhaus are used as the principal sources of equipment costs. The costs of the major items of equipment and their accessories are tabulated for each section in the appendix. Factors for piping, instruments, and the like are assigned to each section using as a basis the effect of fluids, solids, or a combination of fluids and solids may have on each item such as piping, instruments, etc. A factor of 10 percent, referred to as miscellaneous, is added to each section to cover minor equipment and construction costs that are not shown with the equipment listed.
For each section, the field indirect costs which cover field supervision, inspection, temporary construction, equipment rental, and payroll overhead is estimated at 10 percent of the direct cost. Engineering and administration and overhead are each estimated at 5 percent of the construction cost. A contingency of 10 percent and a contractor’s fee of 5 percent are included in the section costs.
The cost of plant facilities and plant utilities are estimated as 10 and 12 percent, respectively, of the total process section costs which include a steamplant cost. The costs for plant facilities and utilities include the same field indirect costs, engineering, administration and overhead, contingency, and contractor’s fee as are included in the section costs included under plant facilities are the cost of laboratories, shops, roads, and fences. The cost of water, power, and steam distribution systems are included under plant utilities.
Working capital is defined as the funds in addition to fixed capital, land investment, and startup costs that must be provided to operate the plant. Working capital, also shown in table 2, is estimated from the following items: (1) Raw material and supplies inventory (cost of raw material and operating supplies for 1 month); (2) product and in process inventory (total operating cost for 1 month); (3) accounts receivable (total operating cost for 1 month); and (4) available cash (direct expenses for 1 month).
Land investment and startup costs are not included in this estimate.
Operating Costs
The estimated operating costs are based on the average of 330 days of operation per year over the life of the plant. This allows 35 days downtime per year for inspection, maintenance, and unscheduled interruptions. The operating costs are divided into direct, indirect, and fixed costs.
Direct costs include raw materials, utilities, direct labor, plant maintenance, payroll overhead, and operating supplies. The raw material costs do not include transportation costs because the plant location is not considered. No purchase cost has been assigned to the chalcopyrite concentrate; thus, only the operating expenses are determined. Electricity, water, natural gas, and coal are purchased utilities. Raw material and utility requirements per pound of copper are shown in the appendix.
The direct labor cost is estimated on the basis of manning the plant with 4.2 men for each position that operates 24 hours per day, 7 days per week. The direct labor assignments are shown by sections in table A-2. The costs of labor supervision is estimated as 15 percent of the labor costs.
Plant maintenance is separately estimated for each piece of equipment and for the buildings, electrical system, piping, plant utility distribution systems, and plant facilities.
Payroll overhead, estimated as 35 percent uf direct labor and maintenance labor, includes vacation, sick leave, social security, and fringe benefits. The cost of operating supplies is estimated as 20 percent of the cost of plant maintenance.
Indirect costs are estimated as 40 percent of the direct labor and maintenance costs. The indirect costs include the expenses of control laboratories, accounting, plant protection and safety, plant administration, marketing, and company overhead. Research and overall company administrative costs outside the plant are not included.
Fixed costs include the cost of taxes (excluding income taxes), insurance, and depreciation. The annual cost of both taxes and insurance are estimated as 1 percent of the plant construction costs. Depreciation is based on a straight-line, 14-year period.
The estimated annual operating cost for a plant producing 165 tons per day of anode copper with no charge for the chalcopyrite concentrate is $10,402,800, or 9.9 cents per pound of copper produced, and is listed in table 3.

The operating cost does not include credit for byproduct silver, sulfur, and iron oxide. Credit for silver, and gold if it were recovered, is not included since its value is included in the feed concentrate value, which has not been considered. The impure iron oxide produced during ferric chloride regeneration has not been assigned credit since it most likely cannot be marketed. The value of byproduct sulfur will depend on local market conditions and has not been included.
The operating cost of a conventional smelter equipped with a citrate process plant for removing sulfur from the stack gases has been estimated as 13.6 cents per pound of copper cast as anodes. This figure has been calculated by updating a previous Bureau of Mines estimate. This operating cost does not include electrorefining, a charge for the feed concentrate, or credit for recovered byproducts. Comparing this operating cost to the operating cost listed in table 3 indicates that the ferric chloride leach process should be economically competitive with the smelting process. Additionally, the fixed capital cost for the ferric chloride leach process is lower, 25.6 cents per annual pound as compared with 31.4 cents per annual pound for the smelting process.
Profitability
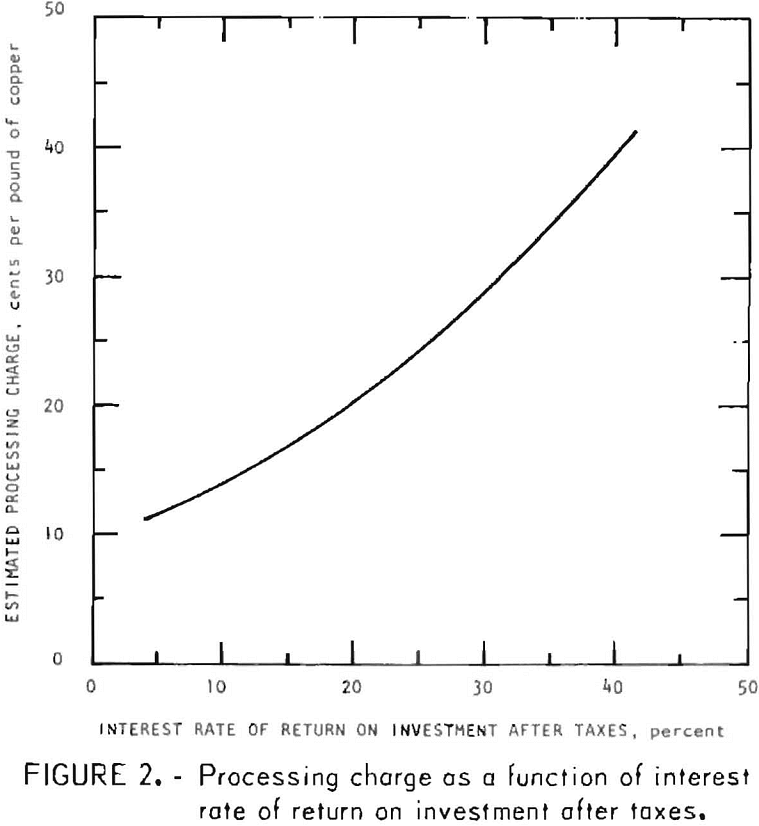
For this evaluation, processing charge is defined as the operating cost plus profit that producing anode copper from chalcopyrite by this process would require. This does not include a charge for the chalcopyrite concentrate or credit for recovered byproducts. Using the interest rate of return method to calculate the estimated processing charge, figure 2 shows the variation of the estimated processing charge, based on a 50-percent income tax, with interest rate of return on investment after taxes. If a 10-percent interest rate of return after taxes is considered the minimum acceptable rate of return, the estimated processing charge, as seen from figure 2, would be 13.9 cents per pound of copper.
Technical Evaluation
The process, as evaluated, uses a cleaner concentrate containing approximately 28 percent copper. Research indicates that a lower grade concentrate, such as a rougher concentrate, could be used. This would make the process more flexible than smelting processes which are usually designed to process a specific grade of concentrate. Additionally, the use of a rougher concentrate would reduce the cost of flotation as well as save some metal values lost during the production of cleaner concentrates.
If the value of the sulfur recovered from the leach residue is low, its recovery would be uneconomical. Under this condition the sulfur could be discarded with the leach residue. The cost to recover the sulfur is about 1 cent per pound of sulfur; therefore, its value should be at least $20 per ton before it is recovered.
When processing the concentrate used in the experimental work, the residue from the sulfur recovery section contains 0.1 ounce of gold per ton. This quantity is considered too low to justify its recovery. If more gold were present, it could be recovered in a standard cyanidation operation.