Table of Contents
Al2O3 used as feed for electrolytic cells in the production of aluminum metal is obtained in the United States from bauxite by the Bayer process. The United States is dependent on imports for its bauxite supply, and despite adequate world reserves rising prices and competition by other industrial nations for bauxite suggest a need to develop technology for production of Al2O3 from domestic sources.
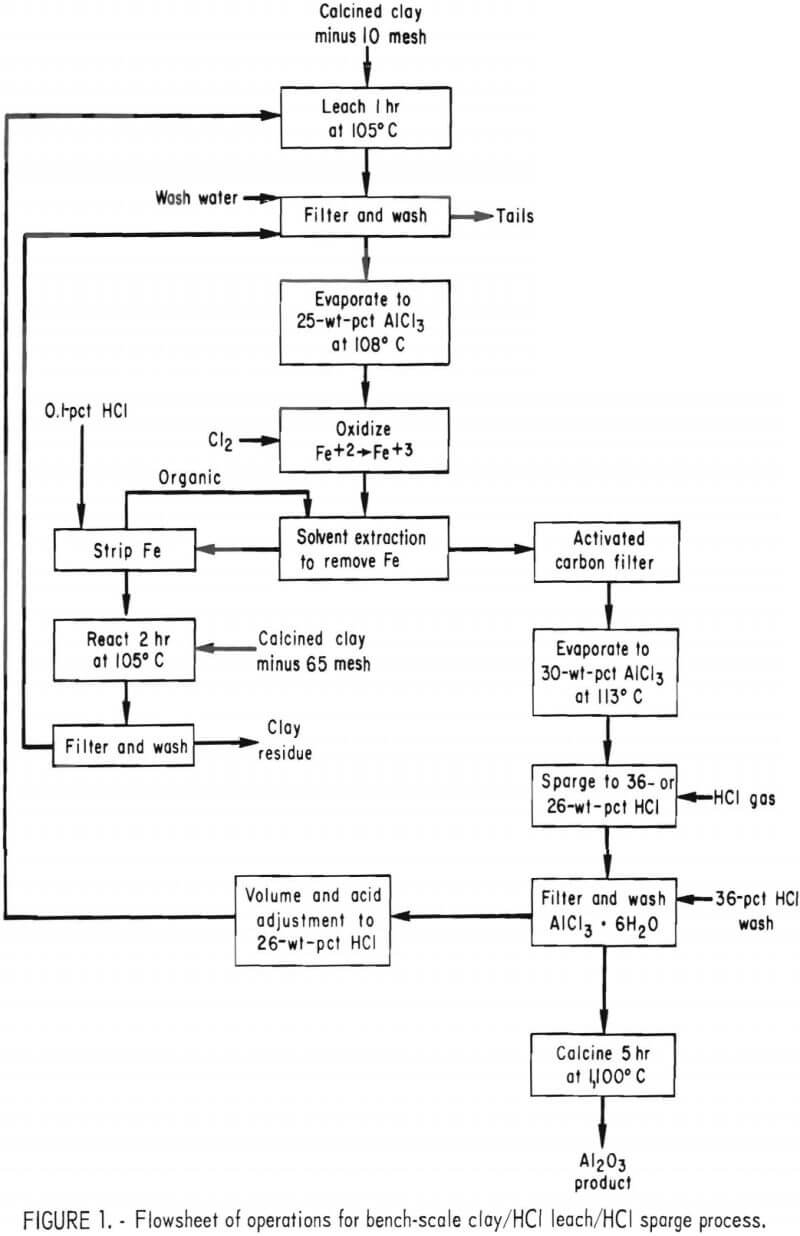
The United States has large resources of kaolin clay that cannot be treated by the Bayer process because of its high SiO2 content. However, a great deal of research has been conducted to devise processes for making high purity Al2O3 from domestic resources of clay and anorthosite. The most promising of several acid processes investigated appears to be HCl leaching of the clay followed by crystallization of AlCl3·6H20 by sparging with HCl gas. To obtain Al2O3 by this process, calcined clay is leached with 26-pct-HCl, a solid-liquid separation is made, and the liquor is treated by solvent extraction to remove iron. The purified leach liquor is evaporated to increase its AlCl3 concentration and AlCl3·6H2O is crystallized by the injection of HCl gas, since AlCl3·6H2O is practically insoluble in strong HCl. The crystals are calcined to Al2O3, and HCl is recovered from the offgases. The mother liquor is heated to recover some HCl as gas; it is then adjusted to the proper acid concentration and returned to leaching.
In 1973, the Bureau began a miniplant test program at its Boulder City Metallurgy Engineering Laboratory (now the Boulder City Engineering Laboratory) in Boulder City, Nev., to evaluate methods for recovering Al2O3 from clay and other domestic raw materials. The objective of this program is to develop engineering and cost data needed for the design and operation of larger scale demonstration plants. The clay/HCl leach/HCl sparge process has been intensively studied in the Boulder City miniplant.
Although the miniplant operation made it possible to study various unit operations of this process, such as leaching and solvent extraction, one parameter that has not been adequately defined is the purity of the product Al2O3. Data on the purity of Al2O3 that could be expected to be produced by a continuous process can only be obtained from a knowledge of the concentration of impurity elements that would be expected in the feed stream being crystallized. To obtain this information, the process liquor must be cycled repeatedly until the impurity level approaches steady state. The effect of impurity level on Al2O3 quality can then be determined by crystallization tests. There are stringent limits on the impurities that can be tolerated in Al2O3 that is to be used in the production of aluminum metal; therefore, it is important to determine if Al2O3 produced by any given process will fall within the specified limits for impurities.
The Bureau designed a bench-scale test program to supply data on the purity of Al2O3 produced by the clay/HCl leach/HCl sparge process, since it would require a major undertaking to recycle plant liquors in the miniplant a sufficient number of times to obtain this data. The bench-scale operations approximated those of the proposed clay/HCl leach/HCl sparge process as closely as practicable in order to simulate conditions of impurity buildup in actual practice. Detailed information on the proposed clay/HCl leach/HCl sparge process is available in references (1) and (3).
Materials, Equipment, and Procedures
Operations that were used to obtain data on the purity of Al2O3 produced by the clay/HCl leach/HCl sparge process are shown in figure 1 and are described in detail below.
Leaching
Kaolin clay was obtained from the Thiele Kaolin Co., Sandersville, Ga. It was calcined and sized to minus 10 mesh before use. An analysis of the calcined clay is given in table 1.
The clay was leached with 105 pct of the stoichiometrically required 26-wt-pct HCl in a 4-1 glass reaction vessel fitted with a condenser. For the first cycle, reagent grade 36-pct HCl was diluted to 26 pct. For subsequent cycles, the combined mother liquor and acid wash liquors were adjusted to the required acid concentration and volume for leaching.
To start the first cycle, 800 grams of clay was used. For each subsequent cycle, the amount of clay was decreased by a factor that represented the loss of liquor that resulted from sampling. Thus, the system had virtually no bleed stream; the only exiting materials were the AlCl3·6H2O crystals and the small amount of liquor not washed out of the clay residue (which was less than 2 pct of the soluble components).
Because of the vigorous nature of the clay-HCl reaction, the charge materials were not initially heated together. The clay was mixed with one-third of the leach acid and heated until the reaction started. The remainder of the cold leach acid was added in increments to cool the hot pulp and control frothing. When all the acid had been added, the pulp was held at 105° C for 1 hour to complete leaching.
Filtering and Washing
Tails were separated from pregnant liquor and washed in a centrifuge with a polyethylene-cloth filter basket. The first wash was dilute AlCl3 solution generated from the solvent extraction step. Three washes of dilute HCl (0.1 pct) were used to remove soluble aluminum from the tails (the dilute acid prevented hydrolysis of AlCl3). Total wash volume was
approximately 3 1. This wash volume is proportionally greater than would be used in a miniplant, but it insured that 98 pct of the solubilized aluminum was washed out of the tails. The pregnant solution and washes, which were cloudy after centrifugation, were polished by filtering through paper on a Buchner funnel, The washed tails were dried, weighed, and sampled to determine aluminum extraction from the clay.
Evaporation to 25 Wt-Pct AlCl3
Because of the large volume of wash solution used, it was necessary to concentrate the combined filtrate. This was done by heating the AlCl3 solution in large flasks while directing a flow of air across the liquid surface. Prior experimentation had shown that when the temperature reached 108° C, the concentration of the solution increased to 25 wt-pct AlCl3. Therefore, evaporation was stopped when the solution reached this temperature. The solution was then cooled to below 80° C. At this point, its volume was between 1.5 and 2.5 1.
Oxidation
Iron was not extracted in the solvent extraction step unless it was in the ferric state. Although most of the iron in the leach liquor was in the ferric form, there was also a small amount of ferrous iron present. Oxidation was accomplished by injecting chlorine gas into the liquor while it was agitated vigorously. Oxidation was controlled by measuring the electromotive force of the solution with a platinum electrode. Prior experimentation had shown that at 650 mv, less than 5 ppm Fe+2 was present.
Solvent Extraction
Iron was extracted from the oxidized liquor with an organic phase containing 15 vol-pct Alamine 336 (a tertiary amine), 10 vol-pct decyl alcohol, and 75 vol-pct kerosene. Although solvent extraction in a continuous-mode operation is usually carried out in a multi-stage contact device such as a mixer-settler, solvent extraction for this study was performed in separatory funnels. The feed liquor was extracted with three 200-ml portions of organic phase. This gave an overall volumetric phase ratio of roughly 4:1 aqueous-to-organic. Extraction was checked by titrating 1.0 ml of the raffinate for iron content.
The combined organic phase was stripped by contacting it with nine 50-ml portions of 0.1N HCl. This gave a phase ratio of 4:3 organic-to-aqueous. The iron content of the strip liquor was determined by titrating a 1.0-ml sample. The stripped organic phase was stored until needed for the next cycle.
Care was taken during extraction to make clean separations between organic and aqueous phases, in order to minimize organic phase entrainment in the purified leach liquor. However, there were always a few organic slicks on the surface of the leach liquor. Most of these could be removed by passing the liquor through a clean, dry separatory funnel where the organic phase tended to cling to the walls. The liquor was then poured through a column of activated carbon to remove all traces of organic material.
Reaction of Iron Strip Solution with Clay
In order to recover the chloride content of the strip solution and transform the iron into an easily disposable form, the iron-loaded strip solution was reacted with an excess of calcined clay. According to the reaction,
2FeCl3 + Al2O3 → Fe2O3 + 2AlCl3,
Fe2O3 is precipitated on the clay and an equivalent amount of aluminum dissolves in the solution as a chloride. The strip solution and 20 grams of minus 65-mesh clay were heated to 105° C for 2 hours in a glass vessel; the pulp was filtered on a Buchner funnel and washed; and the combined filtrate, a dilute AlCl3 solution, was used for the first wash of the leach tails. A 1.0-ml aliquot was titrated to check iron removal.
Evaporation to 30 Wt-Pct AlCl3
The purified leach liquor was evaporated a second time to increase its concentration of AlCl3 before sparging crystallization. Evaporation was continued until the boiling point reached 113° C, which prior experimentation had shown to correspond to 30-wt-pct AlCl3. When the liquor reached this concentration it was cooled to room temperature, weighed, and a 50-ml sample was taken for analysis. Because this liquor is the sparger feed from which AlCl3·6H2O can be crystallized by HCl injection, its composition plays a major role in determining the purity of the crystals. The sparger feed sample was analyzed for the following components by atomic absorption spectrophotometry: Fe2O3, Na2O, K2O, CaO, MgO, MnO, CuO, NiO, PbO, and Cr2O3. AlCl3, S04=, and P2O5 were analyzed by wet chemical methods, and F was analyzed with a specific ion electrode.
Sparging With HCl Gas
Although AlCl3 is very soluble in solutions containing negligible amounts of free HCl, solubility decreases as HCl concentration increases. This is the basis for crystallizing AlCl3·6H2O from the leach liquor by the injection of HCl gas, which is rapidly absorbed by the liquor. Current proposed process flowsheets call for introducing the HCl gas in two stages. In the first stage, the gas would be injected until the mother liquor is 15-wt-pct HCl; in the second stage, the gas would be injected until the liquor is 26 wt-pct-HCl. According to equilibrium solubility data, 26-wt-pct HCl should crystallize approximately 85 pct of the contained AlCl3.
AlCl3·6H2O crystallization is a step that cannot be easily duplicated on a bench scale. Because one cycle must be completed before the next begins, crystallization must be done in batches rather than in a continuous manner, and this may affect crystal growth. Work conducted at the Boulder City Engineering Laboratory has shown that crystallization is the step for which bench-scale results diverge most widely from miniplant results.
Prior test work had established that sparging to 36 pct HCl in the laboratory apparatus resulted in about 90 pct crystallization of AlCl3 and that sparging to 26 pct HCl resulted in about 70 pct crystallization. Since it was not possible to obtain the desired combination of 85 pct crystallization with 26-pct HCl in the mother liquor, two separate runs were planned, In one run of 20 cycles, the feed was sparged to 36 pct HCl. In the second run of 10 cycles, the feed was sparged to 26 pct HCl. This made it possible to compare results and determine the effect of acid strength on the cocrystallization of impurities.
Sparging was done in a 4-1 glass vessel surrounded by a. water jacket that made limited temperature control possible. The temperature averaged 50° C; it was lower at the start of the HCl injection process and was higher when the process was complete. HCl gas was introduced through a glass tube at the bottom of the vessel while the feed was mildly agitated. The entire apparatus was situated on a balance so that the amount of HCl that was absorbed could be estimated; however, this provided only an approximate measurement. When weighing indicated that approximately the correct amount of HCl had been added, the exact acidity of the mother liquor was determined by titrating a 1.0-ml sample. When the desired acidity was reached, HCl injection was stopped, but gentle agitation was continued for 1 hour so that the liquor would more nearly reach equilibrium. The total crystallization time was about 3 hours.
Filtering and Washing AlCl3·6H2O
On a larger scale, the crystal slurry would be centrifuged and the crystals would be washed with concentrated HCl by displacement. No suitably sized corrosion-resistant centrifuge was available for this study, so the crystals were separated from the mother liquor by filtering on a glass-fiber filter pad on a Buchner funnel. A preliminary test was done to determine how many displacement washes were needed to remove most of the mother liquor; the objective was to wash the crystals as clean as possible, rather than to minimize wash volume.
The method devised for washing was as follows; Vacuum on the Buchner funnel was broken before wash acid was poured on the crystal filter cake, and the acid level was raised just enough to cover the filter cake. Vacuum was then restored to pull the acid wash through the cake and was turned off before the next wash was added. Three displacement washes done in this mode removed 99 pct of the mother liquor from the crystal surfaces. A total of 1 to 1.5 liters of 36 pct HCl was used to wash each batch of crystals (batches ranged from 800 to 1400 grams AlCl3·6H2O).
After the last wash, the crystals were dried on the filter with vacuum for several hours; they were then transferred to glass pans and dried in an oven at 95° C for 12 to 24 hours. The dried crystals were weighed and a portion was taken for calcining.
Calcination
Two hundred and fifty grams of each crystal batch were calcined to provide about 50 grams of Al2O3 for analysis. The crystals were slowly heated to 600° C in a tube furnace to expel most of the HCl. (HCl was not recovered here or in the acid-adjustment step for recycling; instead, fresh HCl was used for makeup.) The temperature was then raised to 1,100° C and held for 5 hours.
Al2O3 samples were analyzed by X-ray fluorescence for the following: Fe2O3, Na2O, K2O, CaO, SO4, NiO, TiO2, V2O5, CuO, MnO, AnO, Cl, and P2O5. Although X-ray fluorescence is not an accurate method for determining low levels of P2O5, it was adequate for the levels that were encountered. Cr2O3 and SiO2 were analyzed spectrographically, and MgO was analyzed by dissolution of the sample and atomic absorption analysis.
Acid Adjustment
The combined mother liquor and HCl washes were adjusted to give the correct amount of HCl for leaching calcined clay in the next cycle. The amounts of clay and acid needed for each succeeding leach cycle were calculated based on the decrease in weight of sparger feed due to sampling (bleed was zero). Besides the major sample of 50 ml, 4 ml of feed was removed for titrations (this amount could increase if repeated titrations were required in the sparging or acid-adjustment steps), and a 2-ml loss on glassware was assumed. The volume decrease was small for each cycle, but it was also cumulative; by the 20th cycle, only 438 grams of clay was leached, compared to 800 grams for the first cycle.
Because of the relatively large amount of 36-pct HCl used for crystal washing, it was necessary to reduce the volume and acid content of the combined filtrate by boiling it in an open flask. Acidity was measured by titrating samples as evaporation proceeded. It would be difficult and time consuming to stop evaporation at a precise acid concentration. Therefore, excess HCl was boiled off, and the solution was brought back to the correct strength and volume by adding reagent grade 36-pct HCl and/or distilled H2O. This solution, after cooling, was then used to leach additional clay in the next cycle.
During the 10-cycle 26-pct-HCl sparge run, a difficulty was encountered in the acid-adjustment step. Since only about 75 pct of the AlCl3 was crystallized in each sparging cycle, the mother liquor contained significant quantities of AlCl3. When the 26-pct-HCl mother liquor and 36-pct-HCl washes were combined, a second crop of AlCl3·6H2O was crystallized during some cycles. This second crystal crop was dried, weighed, and put back in the solution that was being evaporated to prepare sparger feed for the next cycle.
Results and Discussion
20-cycle 36-pct-HCl Sparge Run
Table 2 gives the mass balance for aluminum extracted and recovered in the 20-cycle 36-pct-HCl test. An average of 95 pct of the contained aluminum was extracted from the clay. The weight of AlCl3·6H2O extracted from the clay has been corrected by the decrease factor so that it reflects the amount of AlCl3·6H20 that was in the sparger feed after sampling. For each cycle, the amount of AlCl3·6H2O in the sparger feed differed from the amount extracted from the clay. In the first cycle, less AlCl3·6H2O appeared in the sparger feed that was extracted because of hangup of solution in such places as the activated carbon column. In following cycles (except No. 15) more AlCl3·6H2O appeared in the sparger feed than was extracted from the clay because there was a carryover of AlCl3·6H2O that was not crystallized by sparging. According to the equilibrium solubility curve, 99 pct of the AlCl3·6H2O should crystallize from 36-pct HCl. Evidently, equilibrium was not reached under these test conditions since the recovery of crystals varied between 89 pct and 99 pct. Of the 23.08 kg of AlCl3·6H2O extracted from the clay, 22.34 kg, or 97 pct, was accounted for in the recovered crystals and in the AlCl3 left in the mother liquor.
Table 3 gives the analyses of sparger feeds for the 20-cycle tests. The values shown are corrected to 30.0 wt-pct AlCl3 in order to provide a basis for comparison of the analysis data, although the feeds entering the sparger varied from 26 pct AlCl3 to 30 pct AlCl3. The weight-percent value for Fe2O3 remained constant (except for an anomaly at cycle 16); this was expected, since titrations showed that the solvent extraction system functioned well. The concentration of other impurities increased with each cycle, but not at a constant rate and not at the same rate for all impurities. This can be seen more clearly in figure 2, which shows weight-percent plotted against cycle number for the major impurities. At 20 cycles, the concentration of MgO continued to increase at the greatest rate, but the concentration of Na2O had almost reached a steady state. From these values it is possible to calculate the steady-state impurity level in the sparger feed for a given size bleed-stream. Cycle 10, for example, corresponds to a 10-pct bleedstream; cycle 20 corresponds to a 5-pct bleedstream.
Since the amount of impurities leached at steady state must be removed in the bleedstream, the concentration of impurities in steady-state liquor can be calculated from the concentration of impurities in one-pass leach liquor. The equation used for this calculation is
Csteady – state = Co/fraction to bleed
where C = concentration of impurities and C0 = the concentration of impurities in one-pass leach liquor. Less of each impurity is leached from the clay during each succeeding cycle as impurity concentration in the recirculating leach liquor build up. Therefore, more accurate impurity data are obtained by actually leaching for 20 cycles and measuring the impurity level than by dividing the concentration of one-pass leach liquor by .05.
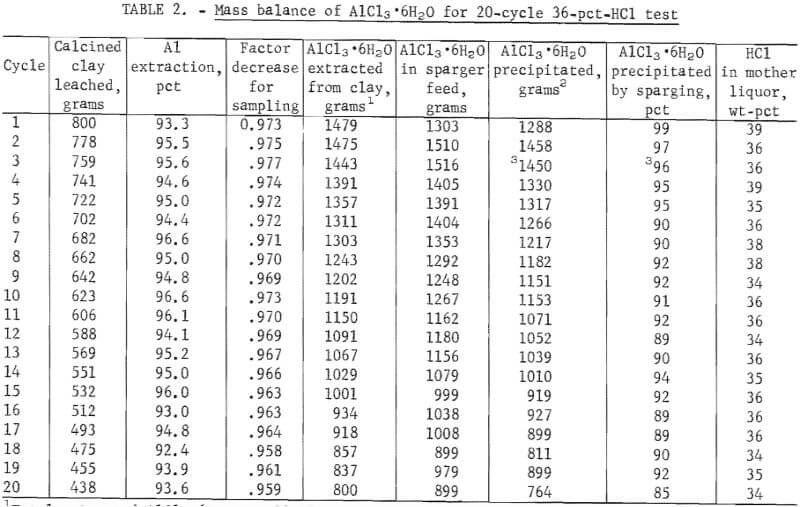
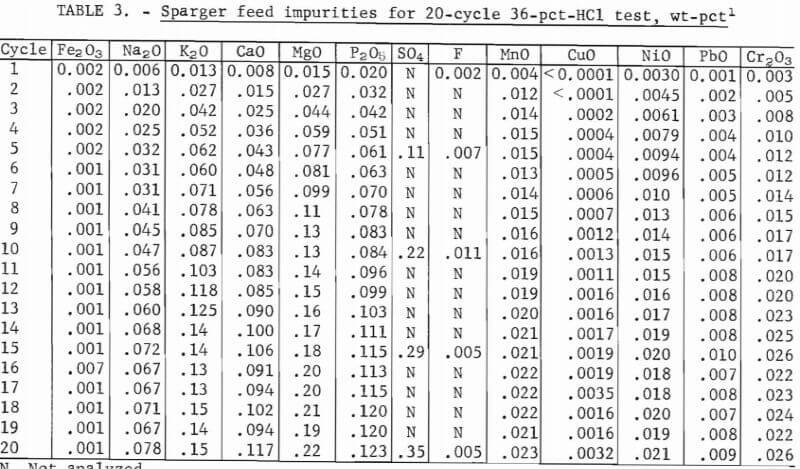
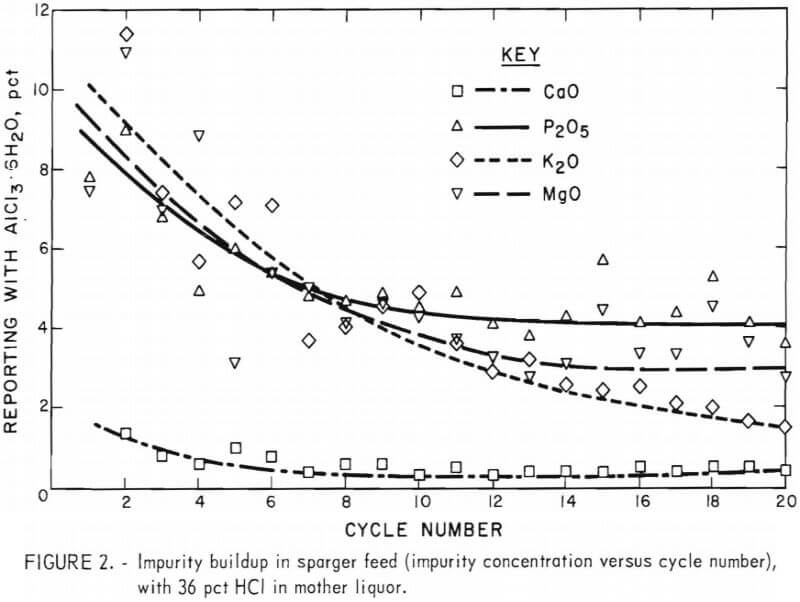
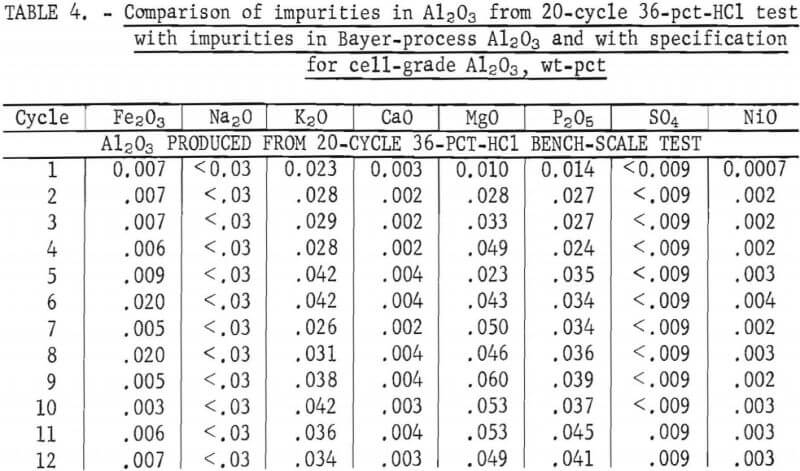
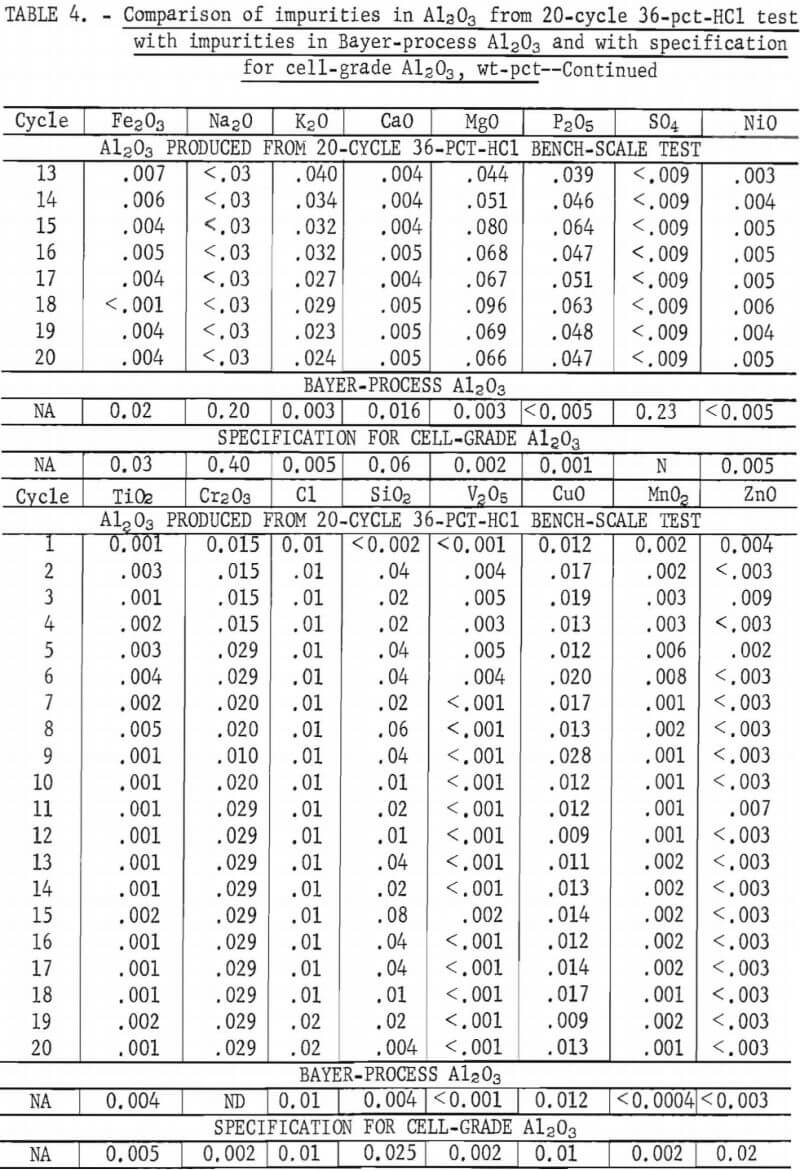
Table 4 shows the analyses of Al2O3 produced from each test cycle. An analysis of Bayer-process Al2O3 analyzed by the same techniques is also shown for comparison, along with specifications for cell-grade Al2O3 (as defined by several major aluminum companies).
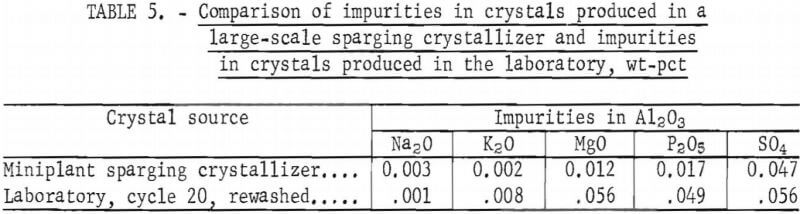
Subsequent work performed in the Boulder City miniplant’s gas-sparging crystallizer established that crystals produced in a large-scale continuous unit with recirculating mother liquor differ from crystals produced batchwise in the laboratory. The miniplant crystal product was produced from a synthetic cycle-20 sparger feed solution. In table 5, the impurity levels of cyrstals produced in the miniplant are compared with those of crystals produced in the laboratory. Some of the cycle-20 laboratory crystals were repulp washed three times (after the displacement washes) with 36-pct HCl to insure maximum crystal purity. It is these rewashed laboratory crystals that are compared in table 5 with the miniplant crystals. This comparison shows that the levels of impurities in laboratory crystals were from three to five times higher than the impurity levels of crystals produced in the large-scale sparger (except for Na2O). This difference in impurity levels indicates that the Al2O3 products prepared in this study cannot be used to predict product purity for the clay/HCl leach/HCl sparge process. However, removal of the AlCl3 from the leach liquor by sparging made it possible to simulate the large-scale process, to follow impurity buildup in the liquor, and to determine liquor composition for larger scale sparging tests.
When AlCl3·6H2O crystals from some of the cycles were placed in dispersion oil and examined under a microscope, a number of fluid inclusions could be seen (fig. 3). It was hypothesized that fluid inclusions of sparger  feed in the crystals might be the major cause of contamination. If fluid inclusions were the major contamination source, the impurities should appear in the Al2O3 in the same ratio that they are found in the feed liquor. Figure 4 shows the percentage of each impurity that reported to the crystals at each cycle, calculated from the weight and impurity levels of the sparger feed and from the Al2O3 impurity levels.
feed in the crystals might be the major cause of contamination. If fluid inclusions were the major contamination source, the impurities should appear in the Al2O3 in the same ratio that they are found in the feed liquor. Figure 4 shows the percentage of each impurity that reported to the crystals at each cycle, calculated from the weight and impurity levels of the sparger feed and from the Al2O3 impurity levels.
Three different patterns were evident from the impurity data presented in figure 4. First, only a small amount of CaO was found in the AlCl3·6H2O crystals, even though its concentration in the sparger feed was approximately the same as the K2O and P2O5 concentrations, and a proportionate level was therefore expected in the crystals. Second, K2O showed a steady decrease in the percentage reporting to the crystal phase. (It can be seen from
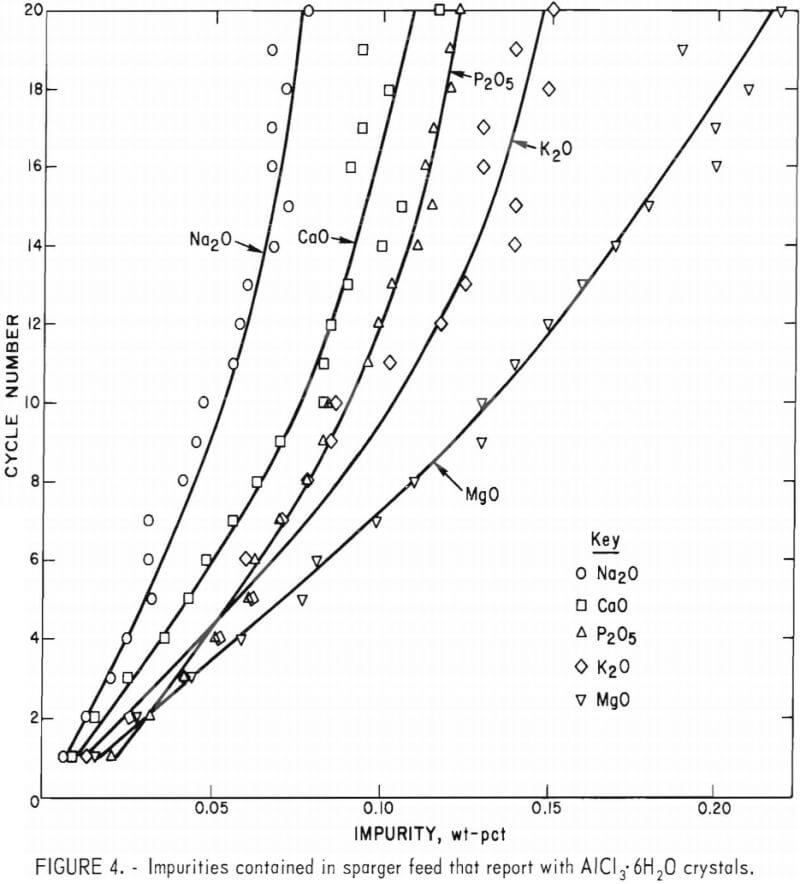
table 4 that the absolute quantity of K2O decreased slightly.) The third pattern observed was that the MgO and P2O5 curves are similar and may actually coincide. (A constant percentage of the amounts of these impurities in the sparger feed was crystallized along with the AlCl3·6H2O.) The conclusion is that although the occluded fluid may have been responsible for some level of impurity in the Al2O3 product, a significant amount of certain impurities are also present in the AlCl3·6H2O crystal lattice. It is also apparent from these curves (fig. 4) that about eight cycles are needed for the system to reach a steady state.
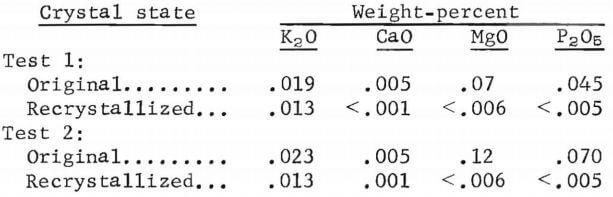
To determine the effectiveness of a second crystallization step in reducing the residual impurities in the product, a recrystallization test was performed. This test was successful in reducing the levels of CaO, MgO, and P2O5, but there was little change in the K2O level. The crystals for this test were produced from a synthetic cycle-10 liquor at 36 pct HCl. A portion of the dried crystals was calcined to Al2O3 and analyzed. The remainder of the crystals was dissolved in an equal weight of distilled water and again sparged to 36 pct HCl. The crystals, after they were dried and calcined to Al2O3, contained the following impurities:
10-cycle 26-pct HCl Sparge Run
A mass balance was prepared for aluminum entering and leaving the system for a 10-cycle 26-pct-HCl test (table 6). Of the 13.7 kg of AlCl3·6H2O that was extracted from the clay, 13.0 kg, or 95 pct, was accounted for in the crystals and in the AlCl3 remaining in the mother liquor. An average of 76 pct of the contained AlCl3 was crystallized at an HCl concentration of 26 wt-pct.
Table 7 gives the concentration of impurities in the sparger feeds, corrected to 30.0 wt-pct AlCl3. Results of the 20-cycle 36-pct HCl run are included for comparison. Impurity concentrations were lower for the 26-pct- HCl run than for the 36-pct-HCl run. This was because the larger mass of the sparger feed in the 26-pct HCl run produced a dilution effect. (The larger feed mass resulted from recirculation of 24 pct of the AlCl3 in the 26-pct-HCl run.)
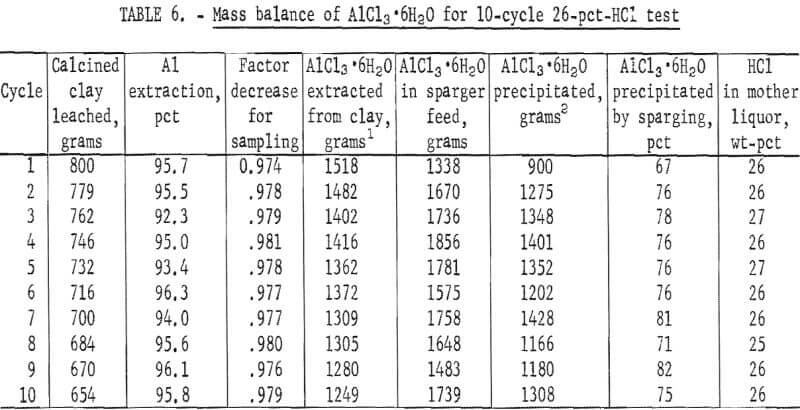
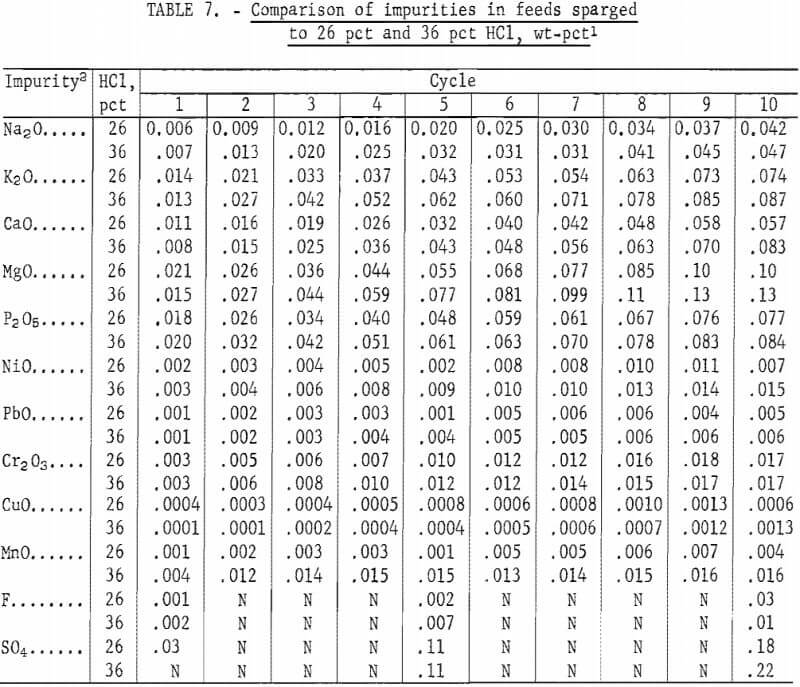
Table 8 presents the Al2O3 analyses for the 10-cycle 26-pct-HCl run. Values for the 20-cycle 36-pct-HCl run are also shown for comparison. The aluminas produced from crystals sparged from 36-pct HCl had higher impurity levels in almost every case. The most noticeable difference was in K2O; in the 36-pct-HCl run, the K2O impurity was approximately three times the K2O impurity in the 26-pct-HCl run. This is not surprising since the solubility of KCl is sensitive to HCl concentration; it rapidly decreases as HCl concentration increases.
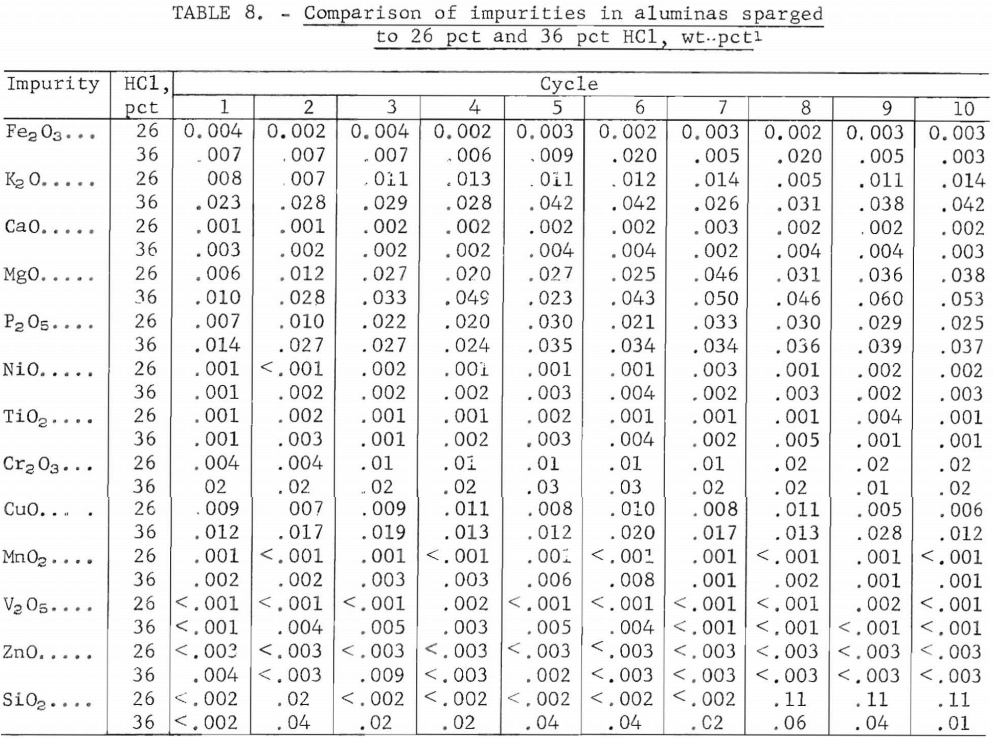
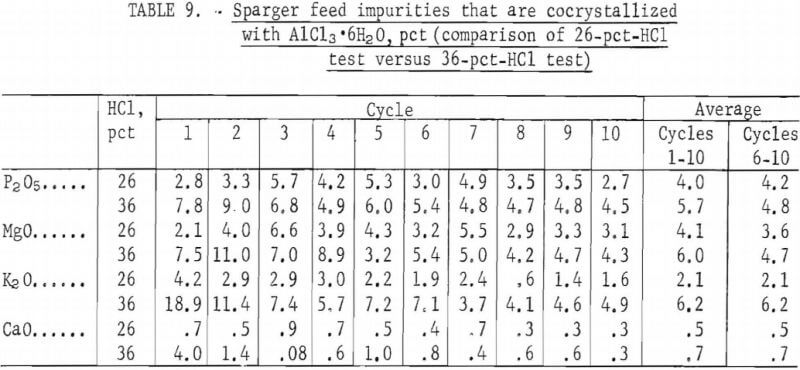
To determine if the same percentage of impurities was crystallized with AlCl3·6H2O in both series of tests, values for selected elements were compared as shown in table 9. Comparison of the first 10 cycles showed that a higher percentage of impurities was crystallized in the 36-pct-HCl system. However, if averages for only the last five cycles are compared, the differences in impurities between the two runs are smaller, except for K2O. (The first five cycles can be justifiably omitted because, as shown in figure 4, they were not representative of the crystallization patterns.) Evidently, the levels of P2O5, MgO, and CaO that were crystallized with AlCl3·6H2O are not especially sensitive to the difference between 26-pct HCl and 36-pct HCl, but K2O is sensitive to this difference.
Hydrochloric Acid Process in Summary
These laboratory tests have determined the approximate compositions of process liquors that should be produced by the clay/HCl leach/HCl sparge process, It is not possible to accurately study the crystallization step or the expected product purity on a bench scale unless more sophisticated equipment is developed to simulate operation in a large-scale crystallizer. Analysis of Al2O3 produced from AlCl3·6H2O indicated that not all of the major sparger feed impurities (P2O5, MgO, K2O, and CaO) are removed from solution by the same mechanism. K2O is an impurity that is sensitive to HCl strength. CaO is only slightly cocrystallized, despite its high concentration in the sparger feed. Recrystallization significantly reduces the levels of P2O5, MgO, and CaO in the AlCl3·6H2O crystals, but not the level of K2O.
https://www.youtube.com/watch?v=nDvTjR63cLA
The Bureau of Mines conducted bench-scale cyclic tests to determine the composition of recycled leach liquor in the Bureau’s proposed clay/HCl leach/ HCl sparge process for producing Al2O3 from clay. The data developed from these tests make it possible to synthesize leach liquors for predetermined steady-state operating conditions when conducting large-scale crystallization tests.
The results of two sparge tests were compared. One was a 20-cycle test with a 36-pct-HCl concentration in the mother liquor; the other was a 10-cycle test with a 26-pct-HCl concentration in the mother liquor. The effect of impurity concentrations in the leach liquor on the purity of aluminum chloride (AlCl3·6H2O) crystals was determined, but there was poor correlation between this data and results that have been obtained from miniplant-scale work.
