Table of Contents
A major overall objective of the Bureau’s research program on rare-earth elements is to make ultrapure individual rare-earth metals and compounds and to determine some of their properties—properties that are not yet known or not well defined. Recognition and definition of characteristics of a rare-earth element will create new interest in it and eventually lead to new uses.
Ultimately, research and development must provide the basic knowledge for obtaining marketable products from ores of rare-earth metals. One of the most important ores contains minerals of bastnasite (a fluocarbonate of rare- earth metals of the cerium group. Cerium can be separated easily by chemical means, but the other rare-earth elements are so nearly alike chemically that chemical separation is tedious; and a different technique, such as in ion-exchange separation, is necessary. Removal of cerium chemically before application of the ion-exchange step is advantageous because: (1) Cerium comprises over half of the rare-earth-element content of bastnasite; and (2) chemical processing is faster and more economical.
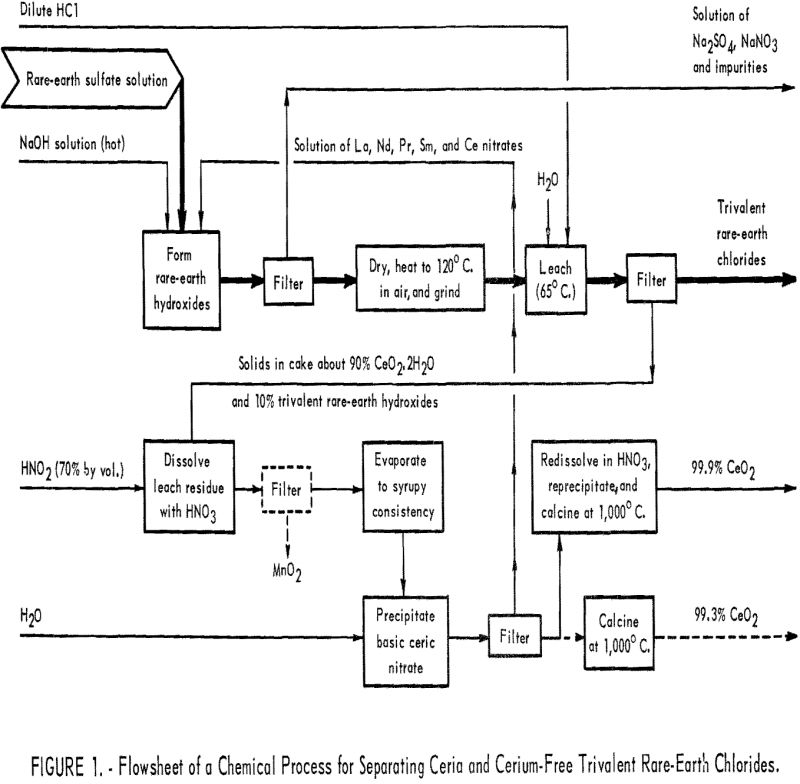
The starting sulfate solution was prepared by treating bastnasite concentrate with sulfuric acid, and it contained the equivalent of 50-60 grams of rare-earth oxides per liter. The distribution of rare-earth elements in the oxides is given in table 1.
The main objectives of the research were to develop a chemical method for preparing a rare-earth chloride solution, which is necessary for ion-exchange separations, from the sulfate solution obtained from bastnasite concentrates. Another objective was to prepare high-purity cerium dioxide for reduction to metal.
Description of Process
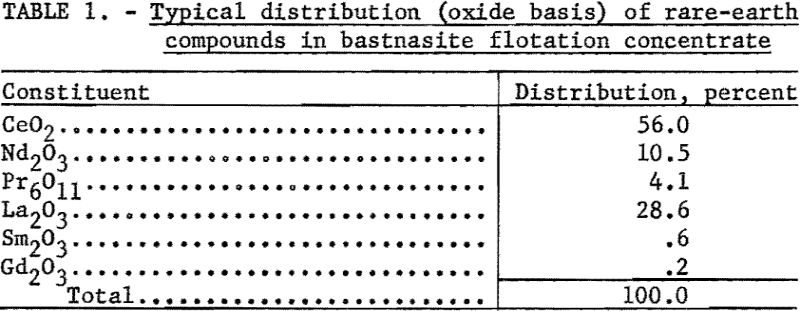
Essential steps of the method are as follows: (1) Conversion of rare-earth sulfates to hydroxides; (2) filtering, drying, and heating the hydroxides to oxidize the cerium; (3) leaching the trivalent rare-earth hydroxides away from the hydrous ceric oxide; (4) obtaining pure cerium dioxide from the acid residue; and (5) recovering trivalent rare-earth elements found in the leach residue. Although experimental work has not gone far enough to define conditions precisely, a procedure has been established that gives a solution of rare-earth chlorides and pure cerium dioxide. More detail for each step will be found in the next section. A flowsheet of the process is outlined in figure 1.
Experimental Procedure and Results
Typical data on operating conditions and yields are included, along with description of experimental work on each step in the process. These data are intended to provide a skeletal picture of the requirements of the process with respect to chemicals and equipment and the results that can be expected.
Cerium Separation
A method of oxidizing cerium in a mixture of rare-earth hydroxides and separating the cerium component from the hydroxides of accompanying rare-earth elements is described in patents by Powell, Blumfeld, and the Societe de Produits Chimiques des Terres Rares. Information on this subject also has been presented by Pilkington and Wylie. The method depends on the high solubility of the trivalent rare-earth hydroxides in dilute hydrochloric (or nitric) acid and the relative insolubility of the tetravalent cerium component at selected pH.
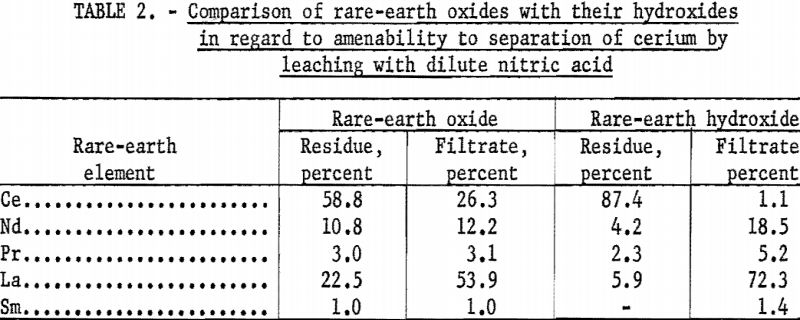
The first experiments in this research tested the separation of cerium from the rare-earth elements by leaching dried hydroxides and ignited oxides with dilute acid. The ignited oxides were included because an early concept supposed that ignited oxides would be prepared during the production of dried hydroxides. The ignited oxides were obtained by heating rare-earth oxalates at 900° C., and the dried hydroxides were prepared by method given under Long Oxalate Method in the appendix. The dried hydroxides and ignited oxides were leached with dilute nitric acid under the same conditions. Results in table 2 show that separation of cerium is good when dried hydroxides are used and poor when ignited oxides are used.
Preparation of Rare-Earth Hydroxides
Acid leaching of dried hydroxides required conversion of bastnasite-derived rare-earth sulfates, in solution, to hydroxides. Four different methods were tried. Technical information in three of the methods is recorded in the appendix. The best method of preparing rare-earth hydroxides consisted of treating rare-earth sulfate solution with sodium hydroxide and precipitating the mixed rare-earth hydroxides. Following are details of the procedure used, including data from a typical run.
Fifty-percent caustic solution was prepared by dissolving 430 grams of sodium hydroxide in an equal quantity of water. This was heated to boiling, and 10-¾ liters of rare-earth sulfate solution containing the equivalent of 538 grams of rare-earth oxides was added gradually to the boiling caustic solution. The sulfate solution, fed through a separatory funnel, was introduced near the bottom of the vessel containing the caustic solution.
Heating was continued during the addition of the sulfate solution, and the liquid in the reaction vessel was stirred continuously. Rate of addition was such as to keep the liquid at a slow boil, and a total of 30 minutes was required to feed in all the sulfate solution. Stirring and heating (slow boil) were continued for another 30 minutes. After a few minutes for the precipitate to settle, which allowed most of the liquid to be decanted, the rare-earth hydroxides were filtered and washed with 6 liters of water.
Analysis of the filter cake showed small quantities of iron, aluminum, and manganese usually less than one-half percent each. The amounts of these impurities varied from one run to another, probably owing to variations in compositions of the sulfate feed solutions. The washed moist hydroxides had a pH of 10 to 11.
Oxidation of Cerium to Tetravalency
The step of oxidizing cerium by air-drying of (bastnasite) rare-earth hydroxides in the filter cake was developed into a satisfactory operation. However, the influences of certain factors are important enough to be reviewed briefly. Exploration of a number of temperatures for drying the hydroxide filter cake was performed but 99.5 to 99.9 percent of the cerium was oxidized at 120° C., and this practice was adopted. At higher temperatures (such as 150° C. or higher) dissolution of trivalent hydroxides in dilute acid was reduced and made the cerium separation more difficult.
When the rare-earth hydroxides were precipitated with ammonium hydroxide, the cerium was not completely oxidized to the quadrivalent states upon drying. However, if the mixed hydroxides were given a single wash with dilute sodium hydroxide, the proportion of cerium oxidized by air was increased. Physical factors that influenced oxidation of cerium by air occasionally were thickness and denseness of cake.
Acid Leaching of Trivalent Rare-Earth Elements From Hydrous Ceric Oxide
Since data and discussions on the separation of cerium by leaching with dilute hydrochloric acid are meager in the patent literature, some details on the step will be included here. For these experiments the dried hydroxides were pulverized to 35-mesh to insure uniformity in experimental samples.
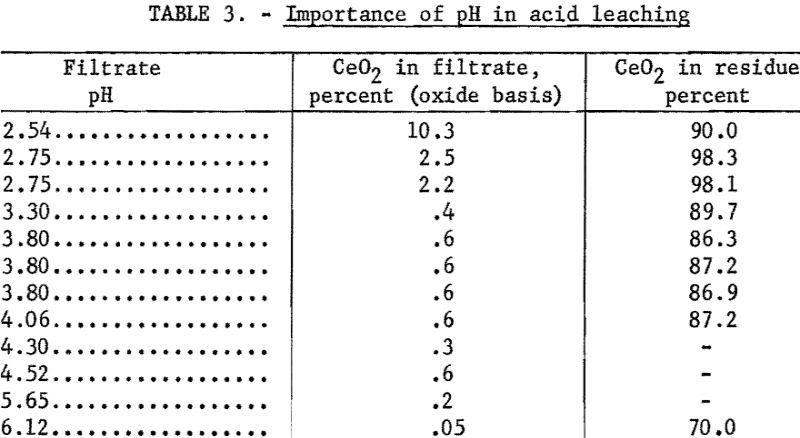
The basis of the cerium separation is that dilute acid dissolved the trivalent rare-earth hydroxides, leaving the tetravalent cerium as a hydrous oxide. The amount of cerium dissolved depended on the hydrogen-ion concentration maintained during the acid leach. Rapid addition of dilute hydrochloric acid will give a localized high acid concentration, and more cerium will go into the chloride filtrate. Similarly, improper stirring can give a localized excess of acid. The correlation between final pH and the amount of cerium in the trivalent chloride solution is shown in table 3.
A satisfactory temperature for leaching the dried hydroxides was 65° C. Experiments at other temperatures showed that this temperature at a pH of 3 gave a good rate of dissolution for the rare-earth hydroxides and almost no dissolution of tetravalent cerium.
The time of contact between chloride solution and leach residue was not critical. In one experiment the residue was left in contact with chloride solution for 16 hours; pH increased from 3.2 to 3.6, and cerium dioxide in solution increased from 0.51 to 0.64 percent.
The weight of trivalent oxides in a given amount of dried hydroxides was determined from their chemical analysis, and the weight of hydrochloric acid used for leaching was determined by calculating the weight of acid required to change the oxides to chlorides. A short-cut method for determining the approximate volume in milliliters of hydrochloric acid (sp, gr., 1.19) was to multiply the weight in grams of trivalent oxides by 1.5.
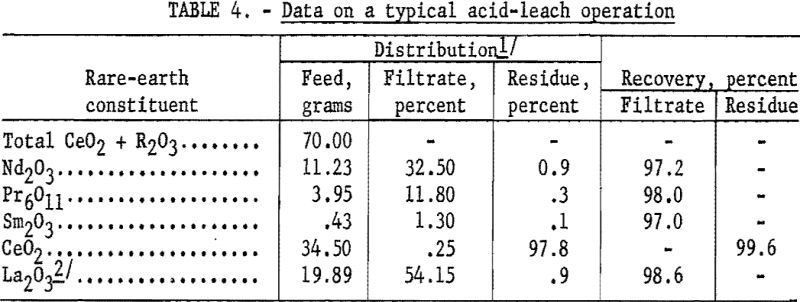
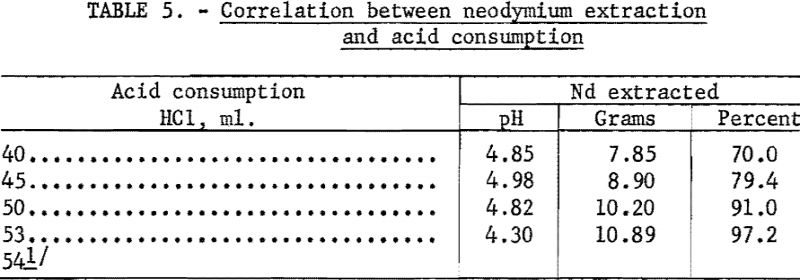
A decided improvement in controlling the quantity of rare-earth elements left in the leach residue was obtained by following the dissolution step with a spectrophotometer. The point at which addition of dilute hydrochloric acid should cease could be judged by following the increase in concentration of certain elements in the liquor with a spectrophotometer. Its use was particularly valuable in experimental work, because conditions were frequently changed, but in a plant where conditions would be more constant, a pH meter probably would suffice. The results given in table 4 show the degree of separation from cerium that was obtained. Table 5 shows how sharply the end point of acid addition can be determined by following the percentage of neodymium extracted.
Refining Acid-Leach Residue to Pure Cerium Dioxide
Filter-cake solids from experimental acid-leaching operations ranged from 75 to 98 percent hydrous cerium dioxide; the remainder was hydroxides of other rare earths and small amounts of impurities (about 2 percent of total). A purity of better than 99 percent CeO2 was desired for studies on reduction of the oxide to metal, so the acid-leach residue was purified further by precipitation as basic nitrate.
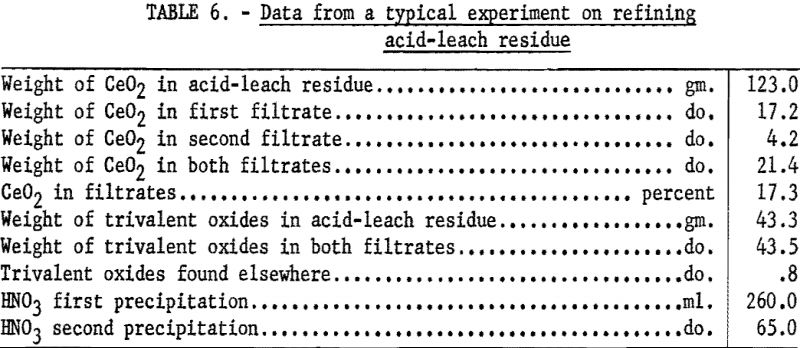
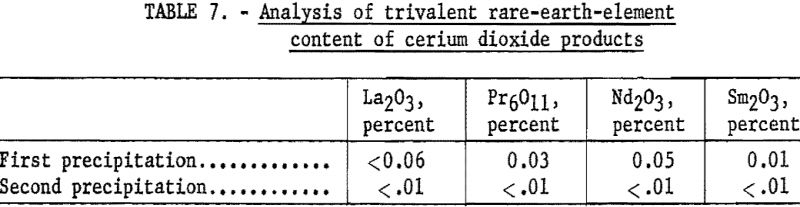
In a typical run 200 grams of the residue was dissolved in 260 ml. of nitric acid (sp. gr. 1.42). The resultant solution was filtered through a glass filter to remove any manganese dioxide. The filtrate was evaporated to a paste and added to 3-½ liters of hot water. The precipitate of basic eerie nitrate was coagulated by boiling a few minutes, allowed to settle, and removed by filtration. Ignition of the basic nitrate at 950° C. yielded cerium dioxide that was 99.3 percent pure. To obtain a product of higher purity, this material was redissolved in nitric acid and reprecipitated. After filtration, the resulting basic ceric nitrate was ignited as before, yielding 99.9-percent cerium dioxide (see tables 6 and 7). The trivalent rare-earth elements in the filtrates were recovered by treatment with sodium hydroxide and addition of precipitate to the main body of rare-earth hydroxides before drying at 120° C.
In the experimental test run, there was considerable variation with respect to the amount of manganese dioxide in acid-leach residues. Some contained none, others over 1.0 percent. The manganese dioxide was removed by filtering the nitric acid solution before evaporation to low volume. The reason for the variation in amount of manganese dioxide is not apparent; but, since some sulfate solutions gave no MnO2, it is believed that further study would make it possible to leave the MnO2 behind.
In precipitating basic nitrate, some residues gave up to 95-percent recovery of the cerium while others yielded only 75-percent recovery. Some of the residues were found to contain oxalate and others to contain a large proportion of trivalent cerium. Residues that gave low recoveries of cerium (for example, 75 percent) could be raised to 95 percent by ignition at 400° C.
The recovery of trivalent rare-earth elements was high because any missed in the hydrochloric acid leach were recovered from the first basic nitrate filtrate. Less than 1 percent of the trivalent rare-earth elements should be lost in the second basic ceric nitrate filtrate. The largest loss of cerium was in its purification by precipitation as basic nitrate. The losses were 5 to 25 percent of ceric oxide in the leach residue.
