Reagent control in flotation is more an art than a science. Operators vary the amount of reagents used according to the metallurgy obtained. The amount of collector may be increased, for example, if tailings losses are high and decreased if concentrate grades are low. Often control decisions are based on only one assay, such as the tailings analysis by microscopic or quick assaying techniques.
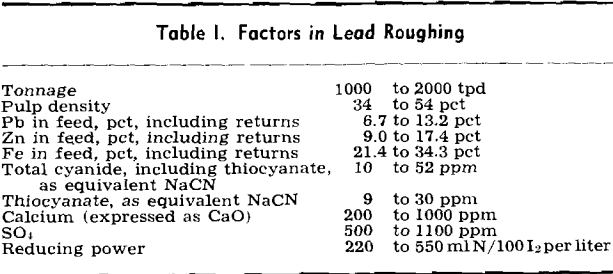
The Sullivan concentrator of The Consolidated Mining & Smelting Co. of Canada Ltd. at Kimberley, B. C., treats 11,000 tpd of complex lead-zinc ore, a scale of operations which justifies an extensive research program on automatic control.
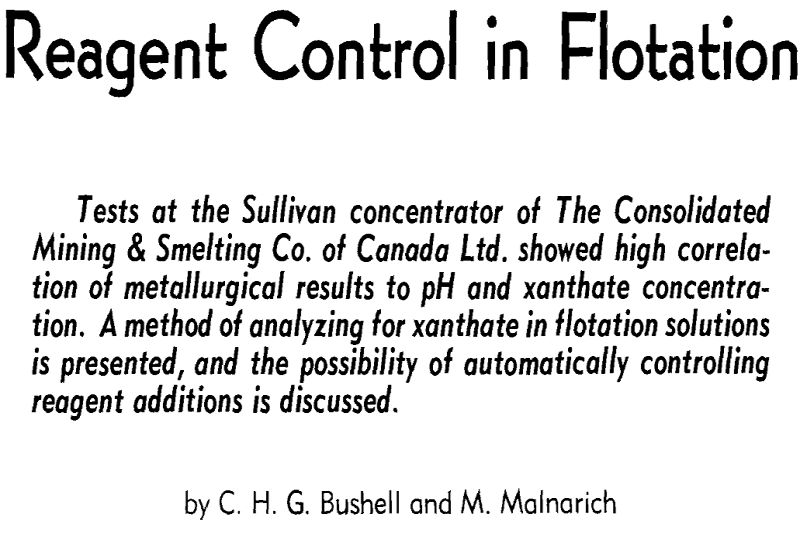
Thus the effects of all flotation reagents are direct functions of their residual concentrations in solution. The residual concentrations are related to rates of addition of reagents, but are not perfectly correlated with them because of varying composition of ore, and varying extents of oxidation. (Oxidized compounds are acidic and so affect pH, and may precipitate heavy metal collector salts.) For precise control, residual concentrations must be measured continuously and used to control reagent additions.
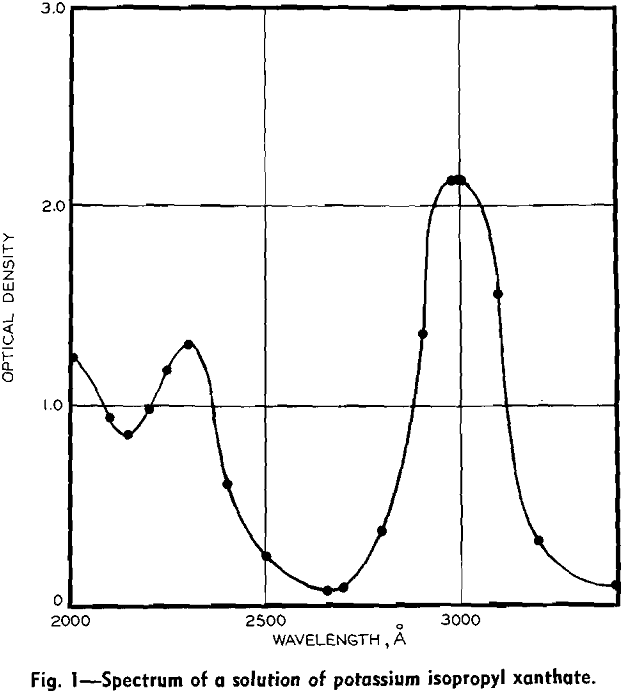
In preliminary tests at the Sullivan concentrator, xanthate concentrations were found to be in a range suitable for spectrophotometric determination, being between the extremes of 1 and 100 ppm. Variations in analysis from time to time were appreciable, confirming the desirability of more precise control.
Rates of addition of xanthate, lime, and cyanide to the lead rougher and of xanthate, lime, and copper sulfate to the zinc rougher were all varied. Filtered solutions were analyzed for xanthate, pH, total cyanide (including thiocyanate), thiocyanate, calcium, copper sulfate, and reducing power, the last analysis being an empirical test based on iodine titration. Metallurgical balances were calculated from assays of feed and products.
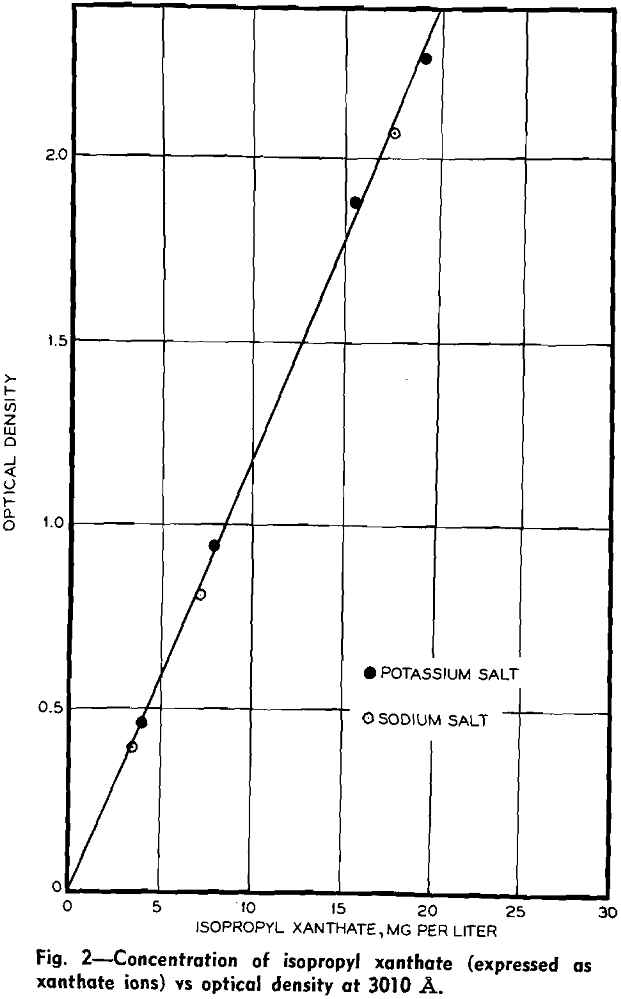
Results were analyzed statistically by both graphical and algebraic methods. The outstanding finding was very high correlation between metallurgy and both pH and xanthate concentration. The amount of copper sulfate added to the zinc roughers has significant effect on zinc metallurgy.
The high correlation between metallurgy and pH and xanthate concentration is easily shown by plotting metallurgical results vs xanthate concentration (m) multiplied by hydrogen ion concentration [H+].
With the methods of calculation used, percentage recoveries with intensive floats were most accurate for lead, next most accurate for zinc, and least accurate for iron. This accounts, in part at least, for the different degrees of scatter. Variation in ore complexity, that is, in number of combined particles, is probably also a factor.
The dependence of metallurgy on the m [H+] product deserves attention.
[X-] [H+] = constant…………………………………………………………………….[1]
Here [X-] = concentration of xanthate ions and [H+] = concentration of hydrogen ions. At mill pH’s xanthic acid is almost completely dissociated, so that m = [X-].
[H+] [X-]/[HX] = Kα………………………………………………………………….[2]
Here Kα is the dissociation constant.
The spectrophotometer measures the total xanthate concentration
[HX] + [X-] = m………………………………………………………………………….[3]
Hence,
[H+] {m – [HX]}/[HX] = Kα
[HX] = m[H+]/Kα + [H+]……………………………………………………[4]
[HX] = 1/Kα · m [H+]………………………………………………………..[5]
Flotation recovery is a function of m[H+] when b is small enough to make the term b[H+] negligible. Of course, more complex adsorption equations may actually be required. The important point is that high correlation between metallurgy and m[H+] does not in itself constitute proof as to whether adsorption of undissociated xanthic acid or competitive adsorption between xanthate ions and hydroxyl ions is the correct mechanism.