Table of Contents
For several years, the Bureau of Mines has been actively involved in developing techniques for recovering and recycling metals and alloys from industrial scrap, including waste materials generated by the electronics industry. Among the latter materials is tin-lead solder that has become contaminated with gold and other impurities to a point where it no longer has the physical properties requisite for use in fabricating circuit boards or other electronic components. These solders are nominally 60-40 tin-lead mixtures containing 50 to 175 oz. gold per ton, and other major impurities such as Ag, Sb, Cu, and Fe.
Recognizing the need for processes to recover gold and reclaim the solder in relatively pure form, studies were conducted at the Reno Metallurgy Research Center to evaluate a molten salt electrolytic recovery technique. The present studies were conducted to determine the applicability of a simple phase-separation method.
The objective of the phase-separation study was to develop a procedure similar to the Parkes process for softening lead. In the Parkes process, zinc metal is added to molten silver-bearing lead metal. The zinc combines with the silver to form the high-melting, insoluble compound Ag3Zn3 which rises to the surface and is skimmed off as dross.
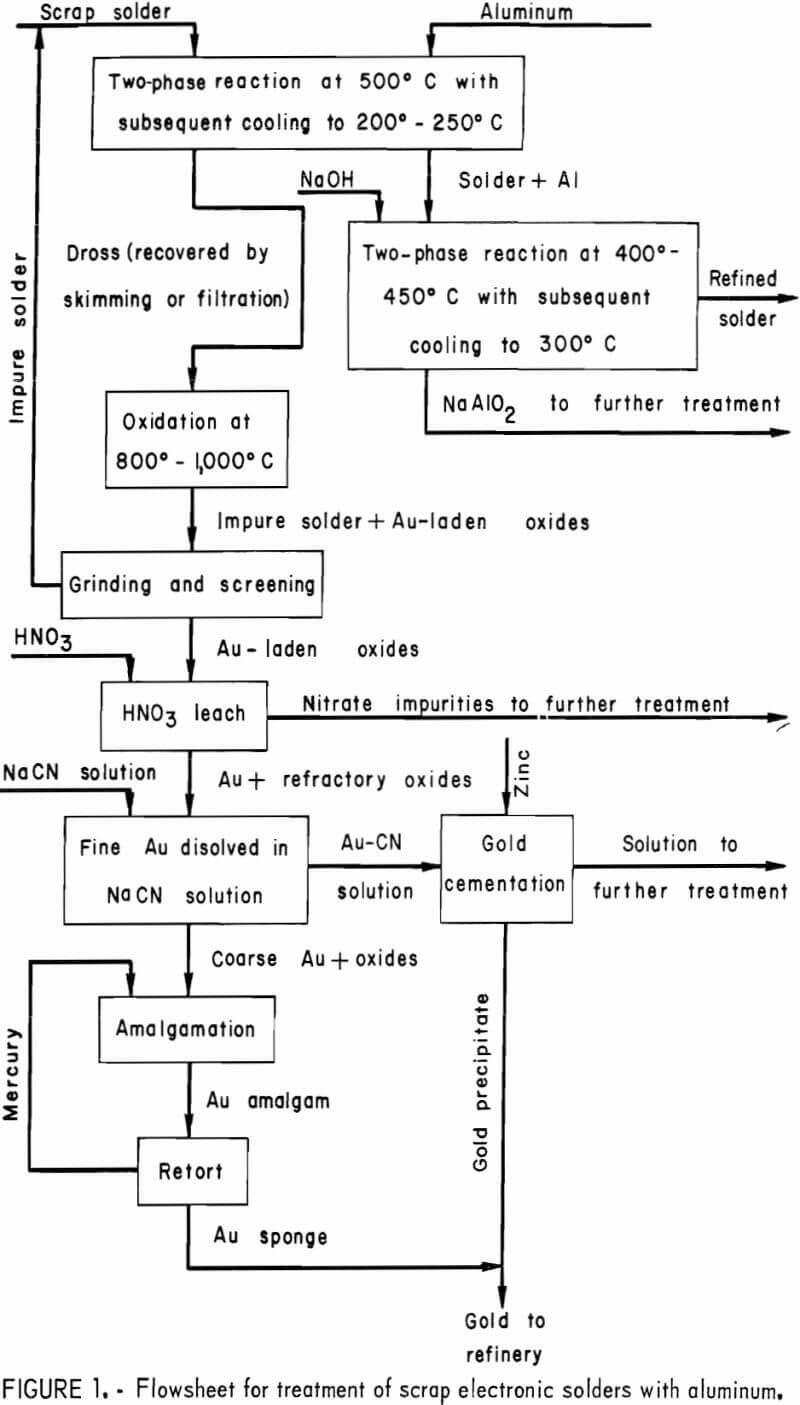
Application of the separable phase principle to contaminated solders involved the addition of aluminum or zinc metal to molten solder. This results in formation of high-melting, low-density intermetallic compounds such as Al2Au which float to the surface along with any undissolved additive metal, such as aluminum, yielding a dross easily removed by skimming or molten metal filtration. Included in the investigation was an evaluation of techniques for recovering gold from the resulting dross.
Materials Equipment And Procedure For Drossing
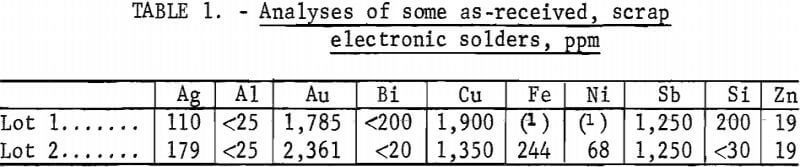
Electronic solders used in this study were nominal 60-40 (tin-lead) solders that had become contaminated during the wave soldering of printed circuit boards to a degree that they would no longer form acceptable bonds. Although there is no such thing as a typical scrap electronic solder, the solders used in this study were fairly representative and contained 50 to 60 oz gold per ton of solder together with appreciable quantities of copper and silver and lesser amounts of Fe, Ni, Bi, Si, and Sb. Analyses of two as-received solders are noted in table 1.
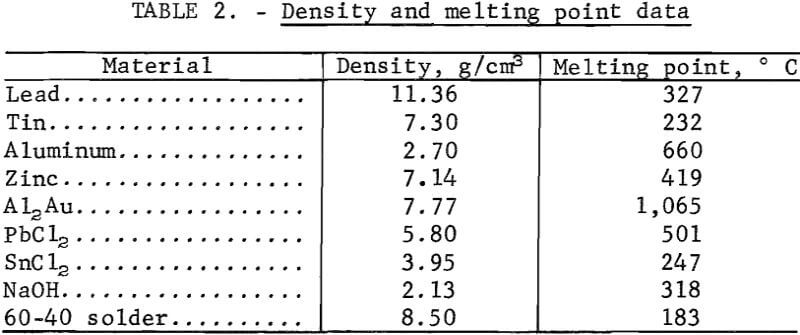
The recovery of gold from electronic solders by phase separation requires a density difference between the solder and the drossing agent. Scrap electronic solders had specific gravities from 8.45 to 8.85 g/cm³, while the specific gravities of aluminum and zinc are 2.70 and 7.14 g/cm³, respectively. Other specific gravities of interest, together with melting point data, are given in table 2. Quantities of scrap solder ranging from 200 to 4,000 g were melted in open clay and clay-graphite crucibles in a conventional pot furnace. Bath temperatures were held at 550° C for aluminum-treated solders, and at 350° C for zinc-treated solders. Wires of the additive metal were wrapped around a carbon stirring rod and immersed in the molten solder bath. This technique provided for greater surface contact between the two phases. Agitation of the bath during cooling helped phase disengagement. The bath temperature was then lowered to 200° to 250° C, and the dross was removed from the solder by ordinary skimming methods or by filtering in equipment similar to that reported by Ruppert and Sullivan.
Skimming was the simpler of the two methods, requiring only a rake or perforated ladle to remove the dross from the solder. Filtration required more elaborate equipment but provided better separation of the dross from the molten solder. Briefly, the filtering unit consisted of a cylindrical upper chamber into which the treated solder and dross were poured, and a cylindrical graphite-lined lower chamber that received the filtrate. The filtering medium consisted of a double thickness of glass cloth supported on a perforated stainless steel plate held between the upper and lower chambers. The lower chamber was connected to a mechanical pump which sustained a vacuum of 25 inches of mercury. The filtration apparatus was contained within a vertical tube furnace which was maintained at 200° to 225° C.
Solder samples for analysis were taken from the molten bath by dipping out a 6- to 12-g portion with a graphite cup or by pouring a 1/8- by ¾-inch slab into a copper mold. Dipped samples were used to determine gold and silver by fire assay, and poured samples were used to determine other metals by X-ray fluorescence, atomic absorption, and spectrographic analysis. Analyses were carried out on the as-received solder, solder after dross removal, and solder after final purification. Dross products and subsequent gold products were analyzed by fire assay.
Results and Discussion
Initial experiments on aluminum drossing focused on the effect of temperature on the two-phase operation. A bath temperature of 550° C was decided upon because at lower temperatures solder could not break through the Al2O3 film on solid aluminum and means of injecting molten aluminum would have to be devised. Bath temperatures much higher than 550° C would require bath protection to prevent excessive oxidation of the solder.
Oxide coating on the aluminum inhibited its reaction with the molten solder; however, once this film was broken the solder reacted rapidly with the aluminum metal. Small amounts of PbO or SnO that formed when the solder was heated were reduced by molten aluminum or zinc.
The effect on gold recovery of adding different ratios of aluminum-to-gold and using a different number of contacts of aluminum-to-goId was studied. As noted in table 3, experiment A was conducted by adding aluminum in three successive increments to the solder. The first contact removed only 57 pct of the gold, while the second contact brought the recovery figure to 92 pct.
After a third contact, gold recovery was essentially complete. In the first step of experiment B, more aluminum was used than had been used in all three steps of experiment A, but only 74 pct of the gold reported to the dross phase. Increasing the molar ratio Al:Au to 9.0, however, removed essentially all of the gold from the solder. The third experiment utilized a very high molar ratio Al:Au in a single contact. Gold recovery was 98.2 pct.
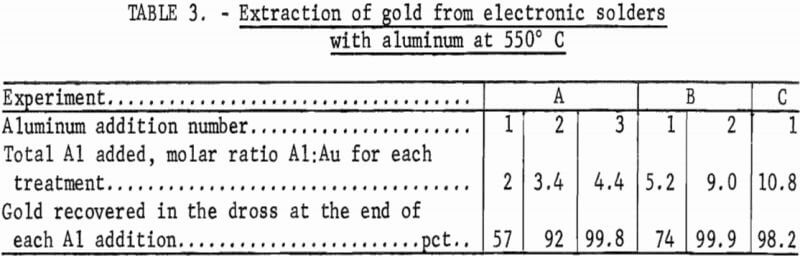
It is evident that multiple contacts are more effective for removing gold from electronic solders, but that a very high percentage of the gold can be removed from the solder in one step if an excess of aluminum is used.
In addition to gold, the aluminum also removed significant amounts of Sb, Cu, Fe, and Ni from the solder. Aluminum was not effective for silver removal.
Zinc drossing was conducted at 350° C as opposed to the 550° C necessary for aluminum drossing. Zinc was as effective as aluminum for removing gold from solder and also removed Ag, Cu, Fe, and Ni, but not Sb.
The use of zinc, however, imposed distinct disadvantages. Approximately eight times as much zinc on a molar basis as aluminum was needed to completely remove gold from the solder. Zinc dross did not become as viscous as aluminum dross as the bath temperature was lowered, thus complicating phase separation. In addition, solder dissolved larger quantities of zinc than aluminum, making it more difficult to repurify the solder to an acceptable electronic grade.
In general, aluminum drossing is preferred over zinc drossing except for solders containing substantial quantities of silver.
Separation of Aluminum Dross From Treated Solders
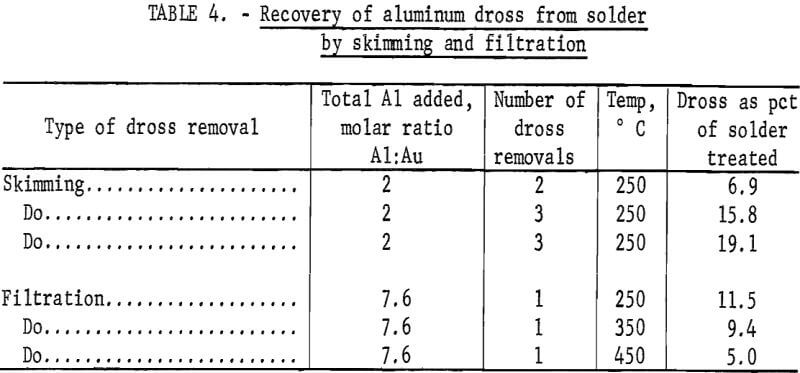
The gold-laden aluminum dross was removed from the solders by skimming or by filtration. Skimming required multiple stirring-skimming operations for complete removal of the dross, whereas filtration removed all of the dross from the solder in one pass.
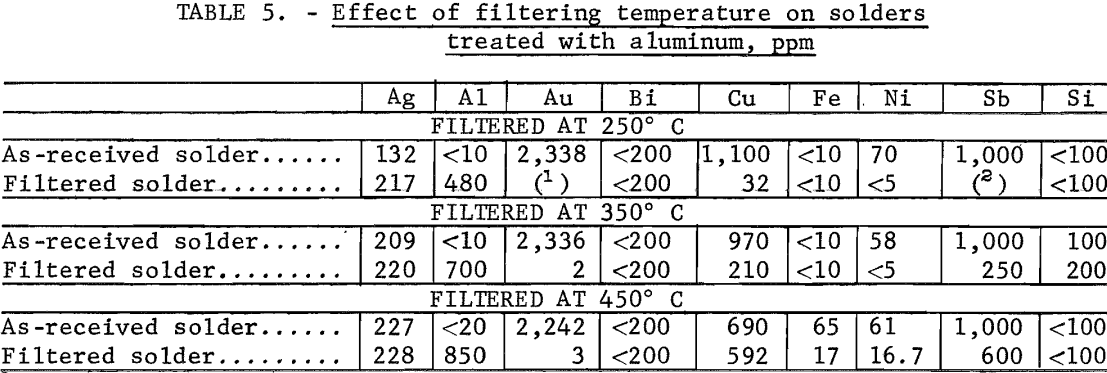
Table 4 presents data for skimming and filtration operations. The dross had such a low viscosity at 350° and 450° C that it could not be effectively removed by skimming. Skimming removes considerably more material than does filtering, and the total amount removed increased with the number of skimmings and with the amount of aluminum added. Filtration temperature was also an important factor because higher temperature operation, 450° C, resulted in separation of a smaller amount of dross.
The effect of filtration temperature on the removal of metal contaminants was also checked. Gold removal was not affected by filtration temperature, but increasing the filtration temperature decreased the extent of Sb, Cu, and Ni removal (table 5).
Recovery of Gold from Aluminum Dross
Dross recovered by filtration contains essentially all of the gold originally present in the solder and will typically represent about 10 pct of the weight of the solder. This dross can be further processed to concentrate the gold since about 85 pct of the dross is entrained solder.
Heating the dross in the range of 800° to 1,000° C will oxidize aluminum and aluminum-gold compounds together with some of the lead and tin. Most of the metallic solder in the dross, amounting to 15 to 40 wt-pct of the dross, remains in the metallic state and can be separated from the oxidized material by grinding and screening. The metallic portion remained on a 200-mesh screen whereas the oxidized material passed through. The solder recovered from the dross contains about the same amount of gold as the original solder and can be recycled with a fresh batch of scrap.
The gold in the oxide phase is in the free metallic state and can be reclaimed by aqua regia digestion or by a combined cyanidation-amalgamation procedure. If the aqua regia route is chosen, the oxides should first be treated to remove base metal oxides so that they do not report to the aqua regia with the gold. This is best accomplished by leaching the oxides successively with a 50-pct NaOH solution and concentrated HCl. The NaOH solution removes most of the Al2O3 , while HCl dissolves the remaining soluble impurities . After filtering and washing, the solutions are discarded. The remaining solids are treated in two steps with aqua regia followed by filtration and washing. Approximately 98 pct of the gold dissolves in the aqua regia. Gold can be recovered from the aqua regia solution by conventional cementation procedures. In this form, the gold can be handled easily by a gold smelter for final purification.
The second technique utilizes combined cyanidation-amalgamation. Neither cyanidation nor amalgamation alone is sufficiently effective because of the variation in size distribution of the gold particles. The larger particles are removed by amalgamation, while the smaller particles are dissolved by the cyanide solution. To use the combined cyanidation-amalgamation procedure, the oxides must first be treated with 5-N HNO3. This is necessary to remove from the gold a tarnished surface film which hinders reaction of the gold either with the cyanide solution or with mercury. Nitric acid also dissolves unoxidized solder and some base metal oxides.
Cyanidation was accomplished by making a 60-pct solids slurry with a 0.3-N-NaCN solution, adjusted to a pH range of 9 to 11. The slurry was mixed from 12 to 72 hours under oxidizing conditions, after which the unreacted material was filtered and washed. Gold was recovered from the cyanide solution by cementation with zinc as in the standard Chiddey method.
The residue from the cyanide operation contained the larger gold particles which amounted to 25 to 30 pct of the gold present in the oxides. The amalgamation technique involved contacting 200 g of residue in a water slurry with 20 g of mercury for 1 hour. It is essential that the mixture be constantly stirred or kept in motion by some means such as by rotating a jar containing the mixture. After 1 hour the gold amalgam was separated from the residue by conventional panning. Gold can be recovered from the amalgam either by dissolving the mercury with acid or by distilling off the mercury and leaving a gold sponge. In either case, gold recovered from amalgamation can be combined with the gold recovered from the cyanide operation and forwarded to a gold smelter for final purification.
The combined cyanidation-amalgamation is generally preferred over the aqua regia route because of the highly corrosive nature of aqua regia, which complicates the selection of construction materials.
Purification of Treated Solders
Solders that have been contacted with molten aluminum or zinc dissolve some of either metal and are unsuited for recycling as electronic solder. Drossed solders were found to contain about 800 ppm aluminum or 15,000 ppm zinc versus the 50 ppm specified for each substance in electronic solders. The purification process, therefore, requires the removal of the aluminum and zinc from the solder.
Aluminum can be removed from solder by reacting it with solid NaOH to form sodium aluminate. The reaction occurs rapidly at 400° to 450° C. After cooling to 300° C, the sodium hydroxide-sodium aluminate phase solidifies and can be removed by skimming. This procedure lowers the aluminum content of the solder to <25 ppm.
Sodium hydroxide can also be used to remove zinc from solder, but the reaction is slow and it is difficult to reach the 50-ppm level. A more effective approach is to utilize a displacement reaction in which zinc displaces tin or lead from a chloride salt. Tin chloride is preferred over lead chloride because of its lower melting point (246° versus 501° C). Bath temperatures of 300° C suffice for the SnCl2 treatment. Fuming of the SnCl2 is mild at this temperature and the SnCl2 could be filtered or washed out of the gas stream. This operation is similar to the aluminum removal procedure and involves a reaction between molten SnCl2 and molten solder, followed by cooling and congealing of the SnCl2-ZnCl2 salt and its removal by skimming. This technique lowers the zinc level in the solder to <10 ppm. A flowsheet for treatment of scrap electronic solders with aluminum is given in figure 1.
Conclusions
Gold can be efficiently removed from scrap electronic solders by drossing with aluminum and recovering the gold from the roasted dross by a combined cyanidation-amalgamation procedure. The solder can be upgraded to electronic quality by treatment with NaOH to remove dissolved aluminum.
Alternate technological schemes include using zinc in place of aluminum for drossing and using aqua regia to recover gold from roasted and filtered aluminum or zinc dross. If zinc drossing is utilized, electronic-grade solder can be produced by treating the solder with SnCl2 to remove dissolved zinc.
