Table of Contents
- Vacuum distillation tests on a magnesium alloy containing 20 percent cadmium indicated that it would not be practicable to separate the two metals by selective vaporization of cadmium.
- A partial separation into products containing +90 percent cadmium and +90 percent magnesium, respectively, was effected by fractional distillation on a series of baffle plates between the retort and condenser.
- The best prospects for separating these metals are selective condensation of metal from the mixed vapors, careful regulation of the temperature, and proper baffling of the condenser.
- Selectivity of condensation can be markedly improved by a special condenser designed to produce a retrograde temperature distribution rather than a uniform decrease in temperature.
The Federal Bureau of Mines was requested to develop a method for recovering magnesium and cadmium from incendiary-bomb alloys held in large quantities by the General Services Administration (GSA). Preliminary studies indicated that vacuum distillation would be the most practicable method for separating the alloy constituents into products of highest purity. An investigation was started in which the objective was to determine the feasibility of applying low-pressure distillation as a method of recovery and to determine the operational conditions for optimum separation.
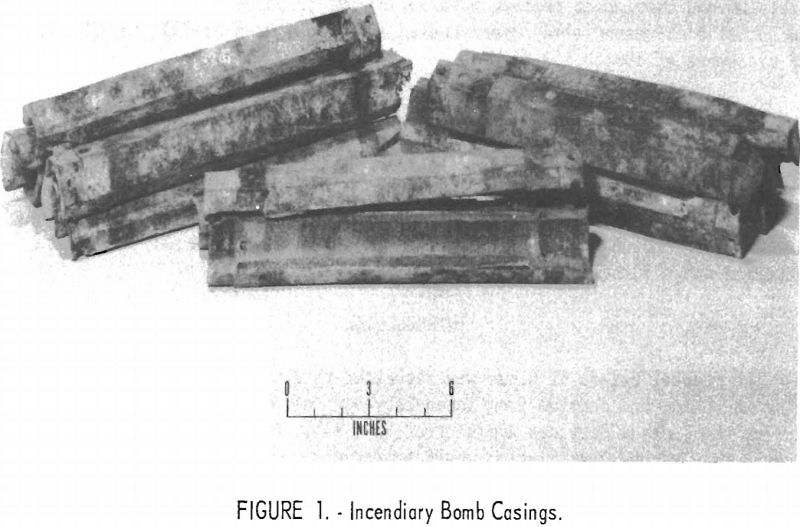
A small supply of incendiary-bomb casings was obtained through the GSA. They were cast, hollow hexagonal rods (approximately 12 inches in length with an overall diameter of 2 inches) that had been deactivated previously by removing the explosive charge and splitting the rods lengthwise into two pieces.
Figure 1 shows several broken casings ready for processing. They were appreciably corroded, as indicated by the dull-gray, powdery appearance of the surface. Analyses, consistent with composition specifications for the alloy, were made of several pieces selected at random as follows:

Composition of Condensate as Determined by Molal Composition of Charge and Vapor Pressures of Charge Constituents
It is theoretically possible to separate the constituents of an alloy by selective volatilization, if their respective partial vapor pressures differ enough. The degree of selectivity can be estimated by comparing the relative vapor pressures. The vapor pressure of cadmium for example, is about 250 times greater at 500° C than the vapor pressure of magnesium at the same temperature The ratio of vapor pressures, Cd : Mg, ranges from 250 : 1 at 500° C. to 45 : 1 at 800° C. These ratios are theoretically favorable for separating the metals by simple distillation, particularly at lower temperatures. However, the relative proportions of metals in the vapor are determined by the relative molal proportions in the alloy as well as the relative vapor pressures of the pure metals.
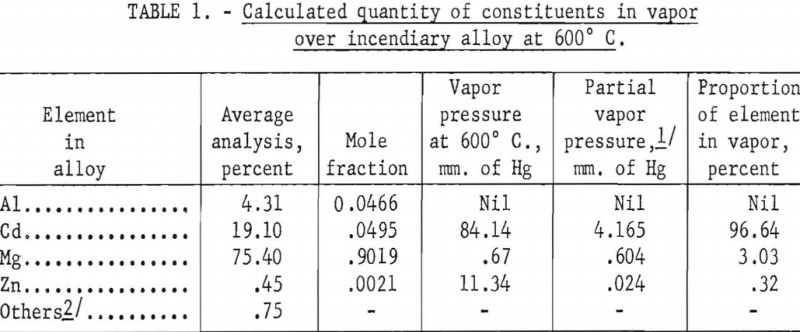
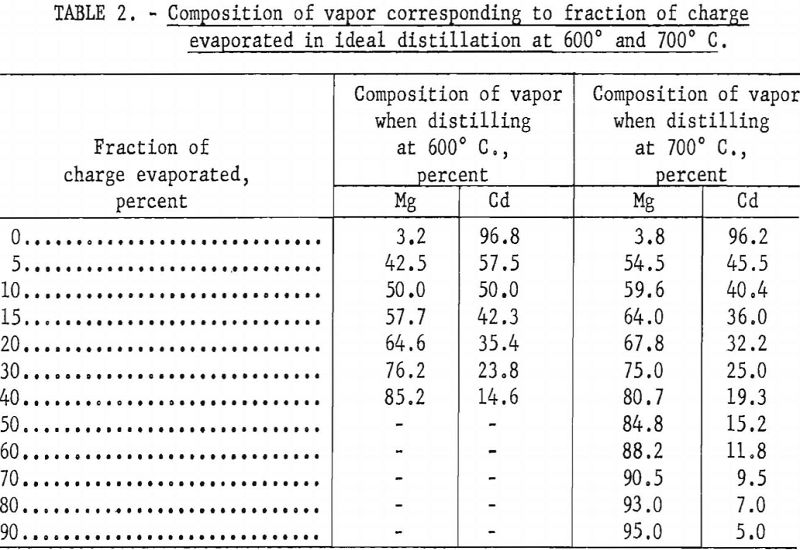
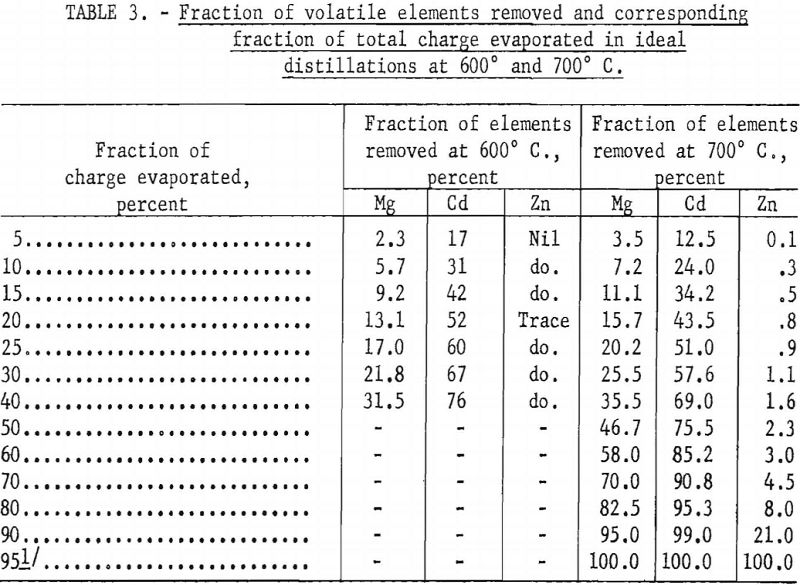
Table 1 shows the initial calculated composition of the vapor phase when a charge of incendiary alloy is distilled at 600° C., assuming that the partial pressures obey Raoult’s law. Initially, the vapor is nearly all cadmium, with only a small percent of magnesium and a fraction of 1 percent of zinc. Tables 2 and 3, calculated by the Rayleigh equation, show how the composition of the vapor changes as distillation progresses. For distillation at 600° C. the vapor contains equal proportions of magnesium and cadmium after about 10 percent of the alloy or 30 percent of the cadmium is evaporated. For distillation at 700° C. the proportion of magnesium in the vapor exceeds 50 percent after only 5 percent of the charge or 12 percent of the cadmium is evaporated. This indicates that, unless there is a strong positive deviation from Raoult’s law, cadmium cannot be effectively separated from magnesium by simple distillation. However, since information was not available on the activities of cadmium and magnesium in their binary alloys, a few distillation tests were made to determine whether these inferences could be confirmed.
Extra precautions were taken in all experimental work to avoid igniting either the charge alloy or condensates because of the high toxicity of cadmium vapors.
Simple Distillation Experiments
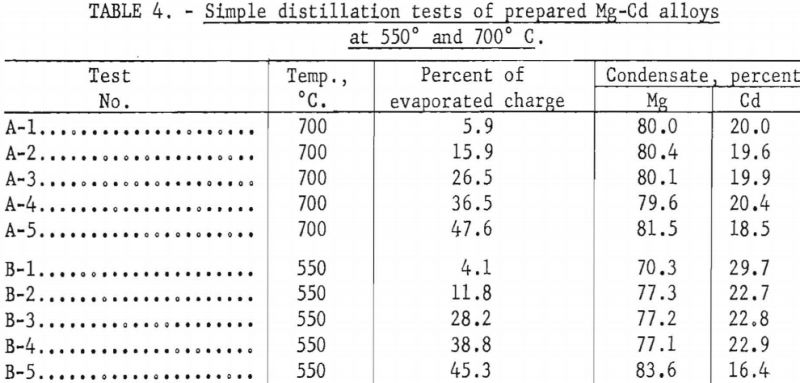
Two series of simple distillation tests on a binary alloy of 80 Mg -20 Cd were run at 700° and 550° C., respectively. The distillation apparatus shown in figure 2 was comprised of a crucible, A; cylindrical condenser, D E F; and water jacket, G. It was enclosed in a vacuum-tight, fused-silica vessel, V. A vacuum adapter head, U, fitted with steel flanges and rubber sealing gaskets, was secured to the top of the silica tube.
The condenser was fitted with a 24-gage, black-iron insert sleeve that could be removed at the conclusion of a test and unrolled to facilitate stripping and sampling the solid condensate. The crucible A and charge B were heated by electrical induction furnace coil W that surrounded the lower end of the silica tube. The temperature was measured by thermocouple T, which was vacuum tight and sealed into the lower end of the silica tube so that the junction entered a shallow depression in the bottom of the crucible. Metal vapors C traveled as indicated from heated charge B to cooled condenser D E F.
In these experiments the entire condensate was removed and crushed to about 35-mesh, so that a representative sample could be split from the total.
Table 4 shows the analyses of five successive condensates for each series. Even the first condensate has the same analysis as the charge for distillation at 700° C. At 550° C. the first condensate contained about 30 percent cadmium as compared with 20 percent in the alloy, but the vapor composition of subsequent condensates approaches that of the residual metal.
It is interesting to note that, if the partial pressures follow Raoult’s law, vapor having a composition of 80 Mg – 20 Cd would have to come from an alloy containing 99 percent magnesium and only 1 percent cadmium. This indicates that the composition of the surface is enriched in magnesium, probably because the cadmium diffuses relatively slowly from the interior of the melt to replace the cadmium that has been evaporated. Cadmium, having an atomic weight much greater than magnesium, will not diffuse to the surface as rapidly as it is initially evaporated. Furthermore, as the surface is no longer enriched in cadmium, the liquidus temperature rises so that at any temperature below the melting point of magnesium the surface may be covered with a film of solid magnesium-rich alloy, which also inhibits the evaporation of cadmium.
Segregation of Constituents in Condensates
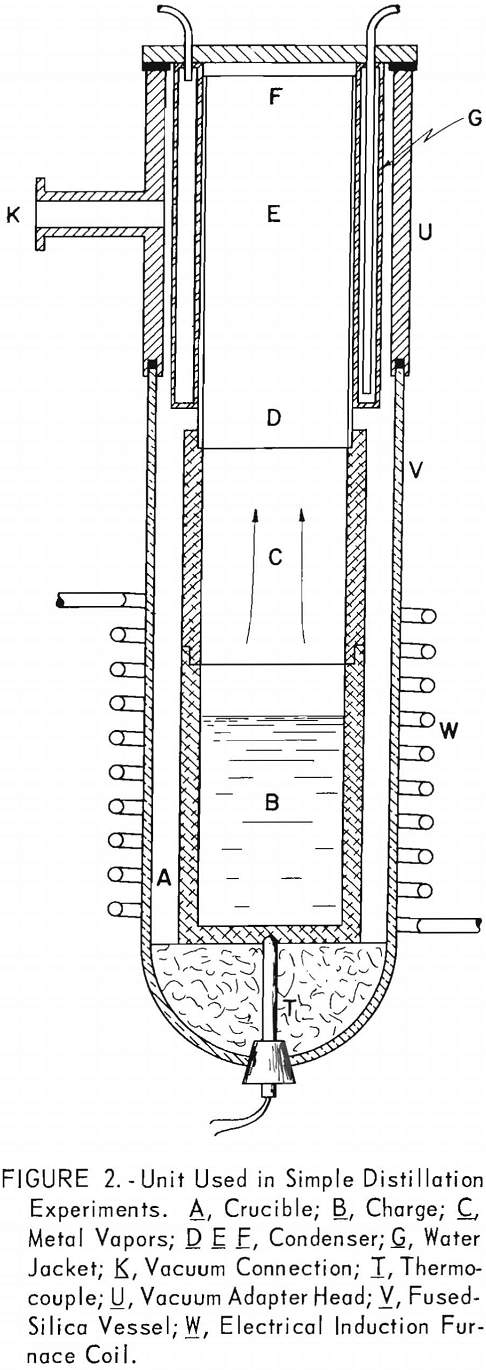
Although it was evident that it would not be practicable to volatilize cadmium selectively from magnesium, there remained the possibility of effecting the separation during condensation. Therefore, another series of experiments was run to study segregation of the two metals in the condensates. The condensates were separated into sections that were analyzed individually. Table 5 gives results of five successive experiments.
The condensate of test 1, which was not separated, was 70 percent richer in cadmium than the charge. In test 2 the condensate was separated into two parts; the top section was enriched 170 percent in cadmium and the bottom section 15 percent in magnesium. In tests 3, 4, and 5 the condensate was divided into top, center, and bottom sections; test 3 was run at 700° C. and tests 4 and 5 at 600° C. The results of those tests were similar to the results of test 2, except that the separation was more effective for the condensates distilled at the lower temperature. The top and bottom sections for distillation at 600° C. contained more than 90 percent of the cadmium and magnesium, respectively. The center sections, which make up about one fourth of the total weight of the condensate, contained 30-50 percent cadmium.
Fractional Distillation Experiments
Further tests were designed to investigate fractional distillation methods. Actual bomb casings were used in these and all subsequent distillation tests so that every influencing factor would be present during the tests.
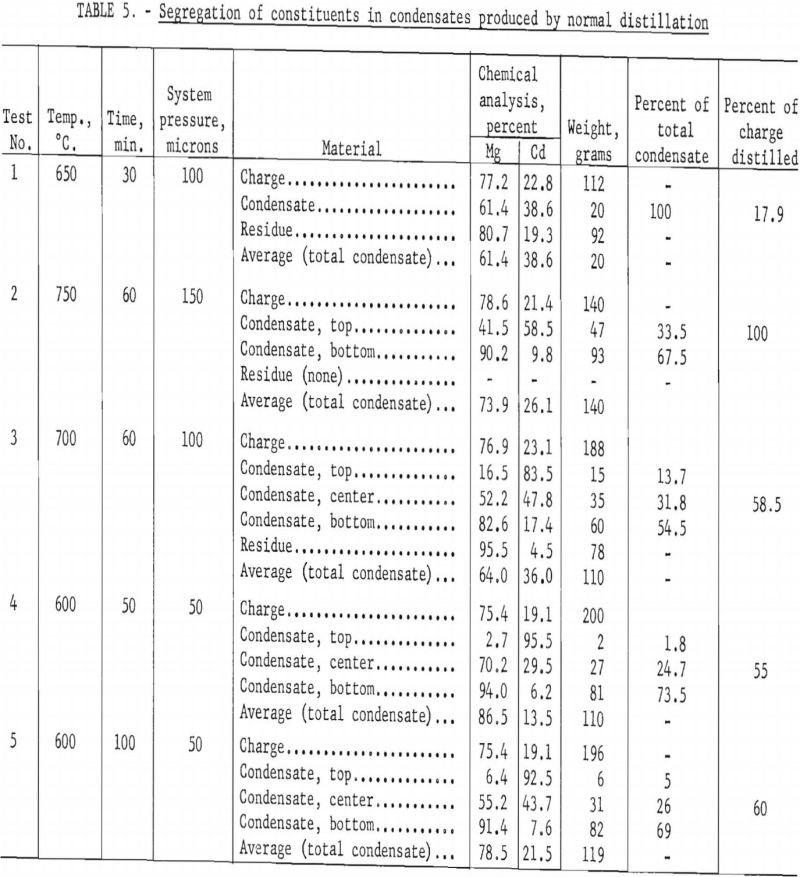
A simple fractionating column was provided by placing six carbon-plate baffles in staggered positions between the crucible and the condenser, as shown in figure 3. It was intended that the lower plates would become heated by the rising vapors to a point where only the magnesium vapor would condense. It was also intended that any cadmium vapor would diffuse from the hot lower plates to the cooler higher plates. The temperature of the plates could not be controlled except indirectly by regulating the rate of evaporation or, to a limited extent, by shifting the induction coil.
The plates were removed at the end of each test, and the adhering condensate was weighed and analyzed. Plates were numbered consecutively 1 through 6, beginning with the plate nearest the crucible. Table 6 gives results of the tests.
In test 6, 95 percent of the charge was distilled in 60 minutes at 700° C., but there was general mixing of constituents on all plates. The magnesium-rich condensate on plate 3 represents 37 percent of the total condensate and contained about 3 per-cent cadmium. The cadmium-rich condensate, in the condenser proper, represents only about 5 percent of the total condensate and contained 8 percent magnesium. The residue analysis showed that essentially all the cadmium had been distilled, This would be expected, as 95 percent of the charge had distilled.
The temperature for tests 7 and 8 was reduced to 600° C., but all other conditions remained unchanged. Only 30 to 40 percent of the charge was distilled in 60 minutes at this lower temperature, indicating the rate of distillation had dropped about half by lowering the temperature 100° C.
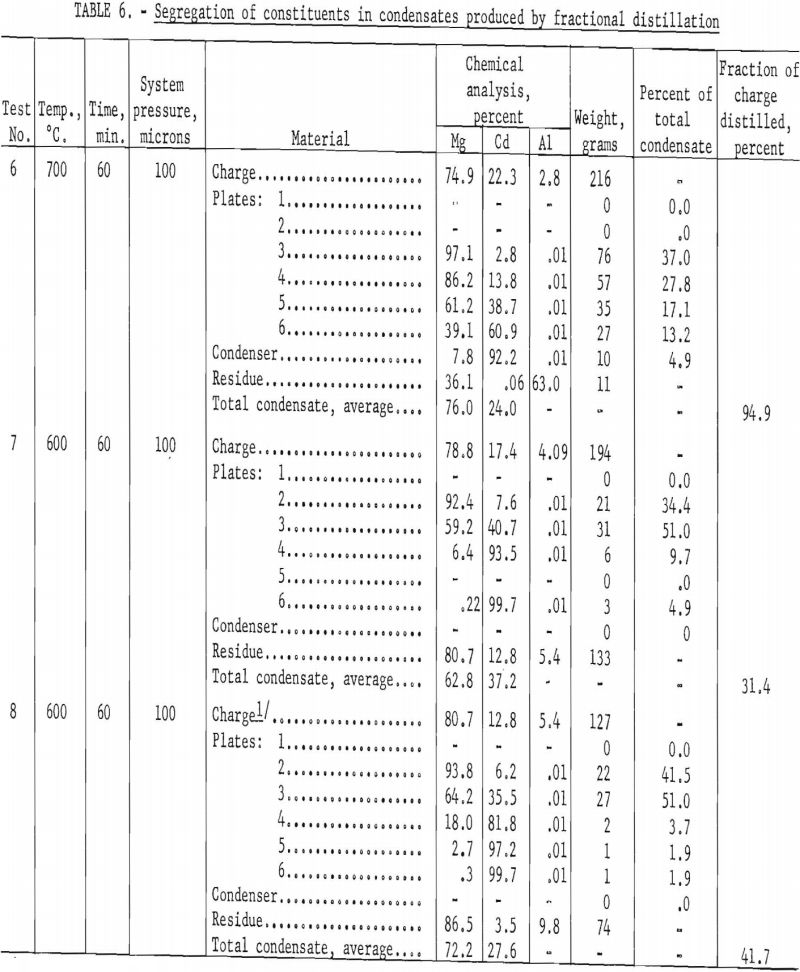
In both tests at 600° C. the best products were condensed on plates 2 and 6. In test 7 the 99.7-percent cadmium condensate on plate 6 contains only 0.22 percent magnesium and represents only 5 percent of the total condensate. The condensate for the same plate in test 8 is essentially the same, except that the deposit represents only 2 percent of the total condensate. The magnesium-rich condensates on plate 2 in tests 7 and 8 contain about 35 to 40 percent of the total condensate. In all instances 45 to 50 percent of the condensate would have to be recycled with new charge alloy, and the balance would have to be redistilled separately to effect good refining. Results by fractional distillation show improvement in separation over results by simple distillation, but much better selectivity would be required for a commercial application.
Experiments With Vapor Baffle
Previous results show conclusively that any practicable degree of refining incendiary alloy by distillation methods must be done by selective condensation rather than selective volatilization.
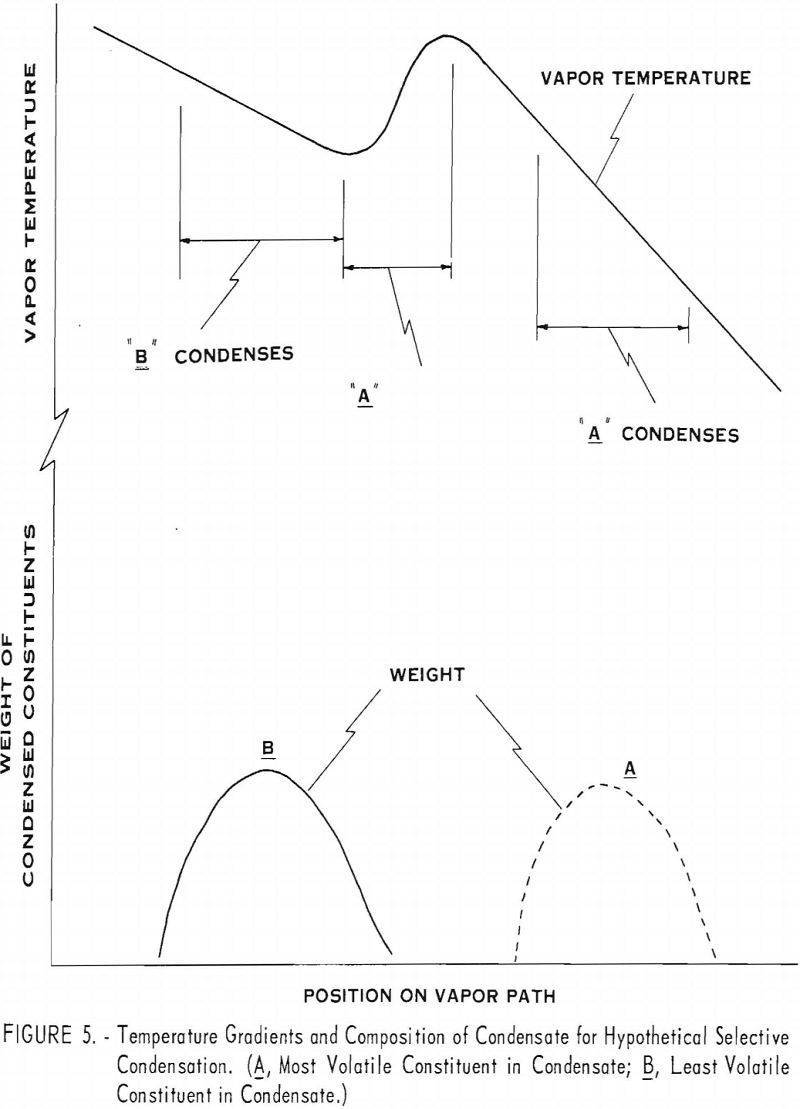
Condensing vapors of mixed metal such as cadmium and magnesium is illustrated graphically in figure 4. The curve at the top of the figure (vapor temperature) represents the temperature of the vapors with respect to location in the system. The lower curves represent the distribution of constituents A and B in the condensate when A is the more volatile constituent.
Although there is a degree of selectivity in condensing the constituents, the zones in which A and B condense overlap. The composition of the condensate at any point depends on the temperature, the relative concentrations of constituents in the vapor, and the relative rates of diffusion for the two kinds of vapor molecules. The separation is not sharp because as the B molecules condense, they leave a preponderance of A molecules near the condenser wall. The large number of A molecules near the condenser wall increases the probability that some of them will condense prematurely with the B molecules. Under these circumstances, some B molecules diffuse much farther than they would otherwise, and many of them condense belatedly with the A molecules.
A greater degree of selectivity would be expected under conditions shown in figure 5. The vapor temperature in this hypothetical distillation does not decrease uniformly along the condenser as in figure 4 but rises where the two constituents otherwise tend to condense together. Reheating the vapor in this region causes the molecules to diffuse more rapidly and allows more effective remixing of the vapor as the B molecules condense. The zones where A and B have the greatest tendency to condense are thereby effectively separated, and the selectivity of the condensation is improved accordingly.
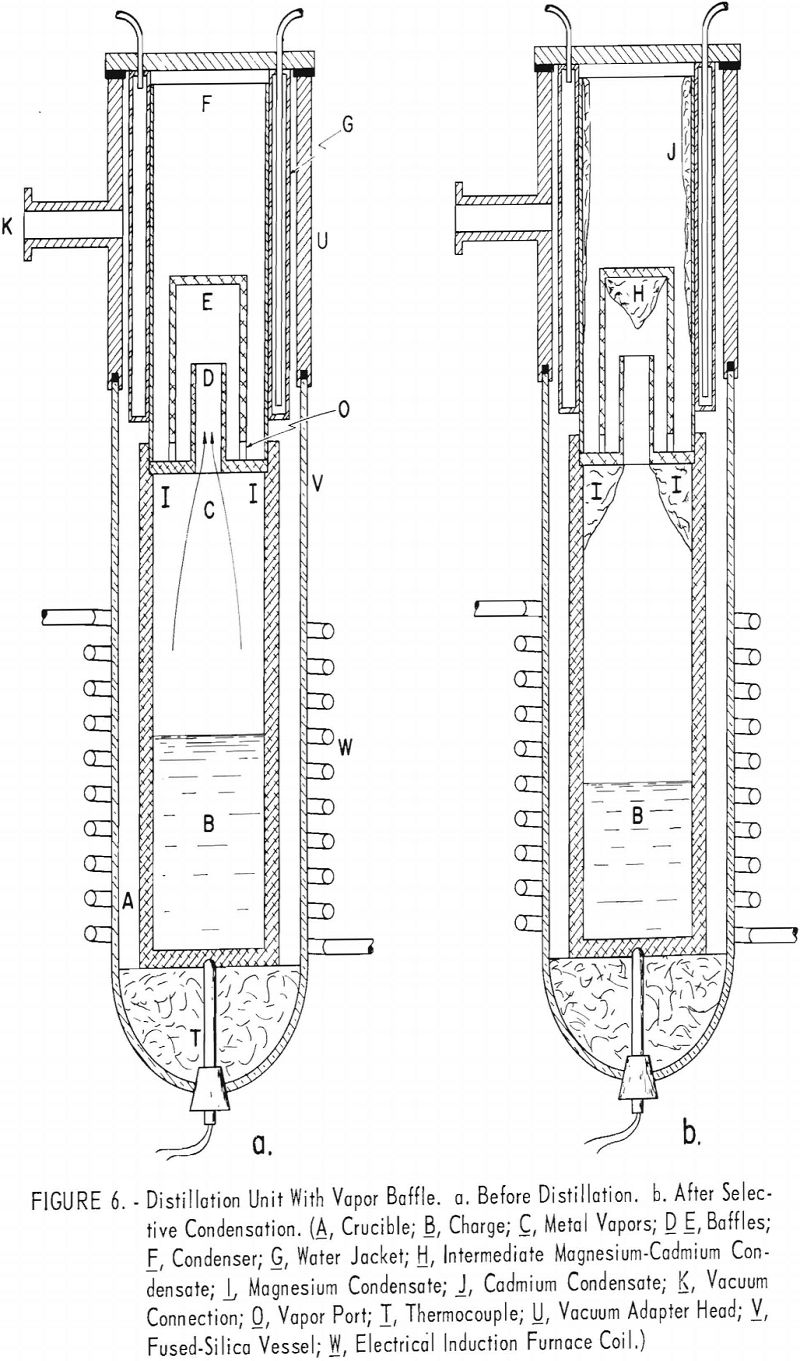
Description of Vapor Baffle
The vapor baffle illustrated in figure 6(a) was designed to produce the temperature distribution shown in figure 5. Its function is described as follows: The vapor cools slightly as it passes through tube D. Heat is absorbed by the walls of the tube and transferred to the vapor passing downward along the outside of the tube. This produces the retrograde temperature distribution shown in figure 5.

Figure 6(b) shows the relative locations of the cadmium, corresponding to constituent A, and magnesium, corresponding to constituent B, as follows: The deposit I represents magnesium condensed before the vapor passes through the baffle. The deposit H represents an intermediate-grade of Mg-Cd condensate condensed at intermediate temperatures, and the condensate J represents refined cadmium condensed beyond the baffle.
The effects of the baffle are shown by the results of tests 9 and 10 (see table 7). H, J, and I, refer to condensates at respective positions shown in figure 6. About two-thirds of the condensate was collected at position I and contained about 99 percent magnesium. About 15 percent of the condensate was deposited at position J as 99 percent cadmium. An intermediate grade of magnesium deposited at H, amounting to a little less than a fifth of the condensate, contained 60 to 80 percent magnesium. The I and J deposits were of satisfactory purity, but the H deposits, being relatively high in cadmium, would have to be recycled.

Distillation into a Temperature-Controlled Condenser
Although practicable separation may be possible if a vapor baffle is used, the net rate of diffusion of vapor to the condenser is decreased by any constriction in the vapor path. Therefore, an additional test was made to determine whether condensation could be made sufficiently selective without a baffle by properly controlling the condenser temperature.
This test was made in a large, gas-fired vacuum retort shown in figure 7. The retort was supported in a horizontal position on three firebrick columns. A 10-inch combustion space was provided between the retort and the firebrick furnace shell. Two gas burners were positioned along the length of the retort so that the flames spiraled around the retort and the gases were exhausted at the back end of the furnace. The retort and the condenser were fitted with water-cooled flanges and a rubber sealing gasket. The inside dimensions of the retort were 11 inches in diameter by 72 inches long. The approximate maximum capacity was 1,000 pounds. The condensing chamber was 11 inches in diameter by 48 inches in length. Only the back part of the retort was used for distillation in this experiment. The front end was used as a condenser in which the temperature was controlled independently.
An attempt was made to condense magnesium selectively in the front hot zone of the retort by holding the temperature about 80° lower than the retort temperature so that magnesium vapor would condense but cadmium vapor would diffuse toward the cold end of the condenser. The charge was placed in the back half of the retort and heated by the backburner. The retort temperature was controlled manually by adjusting the gas burner in response to a pyrometer actuated by a thermocouple placed in the vapor stream near the front end of the charge. For this test the condensing section included about one-third of the length of the retort section in addition to the regular condenser section, giving the condensing section a total length of 7 feet from end to end. A
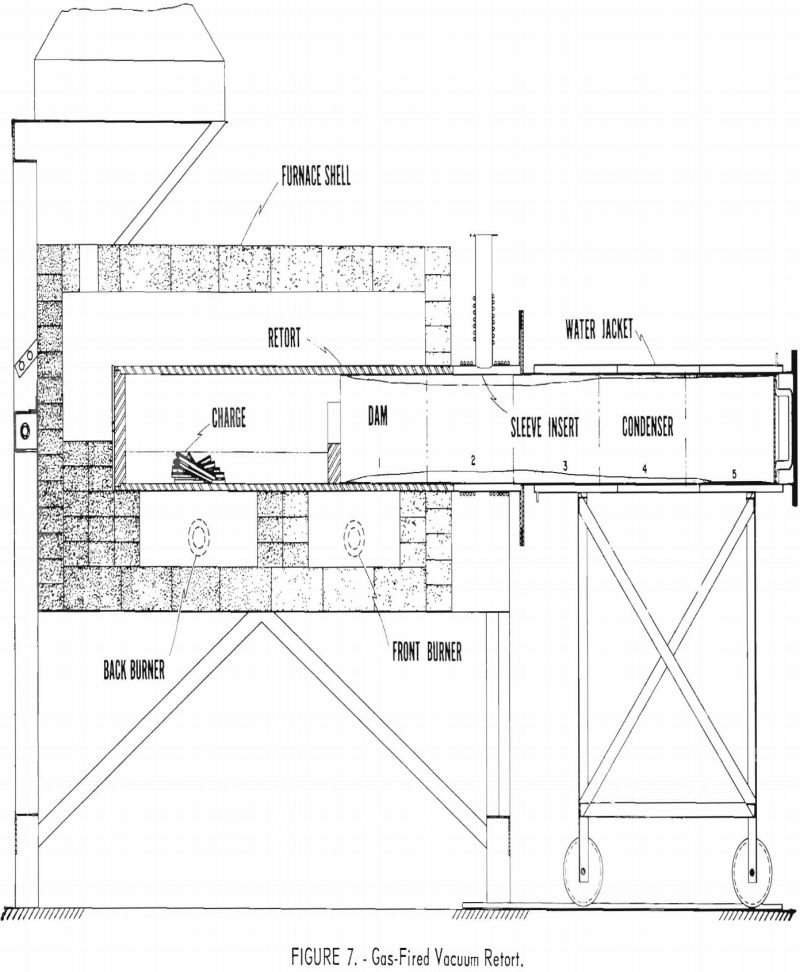
28-gage, black-iron insert sleeve was used inside the condenser to collect the condensate and facilitate stripping.
Table 8 shows that 80 percent of the 7,258-gram charge was distilled in 5 hours at 550° C. The residue was composed of partly coalesced pieces of metal that showed some bright metallic areas and a greater area of dark adhering film presumed to be a mixture of oxides, carbides, and aluminum metal. The general appearance of the residue indicated considerable sublimation. The condensate had a bright metallic luster and occupied a zone about 72 inches long beginning approximately 2 inches from the charge zone. The condensate was cut into five 14-inch sections, as shown in figure 7. A representative sample was taken from each section for chemical analyses.
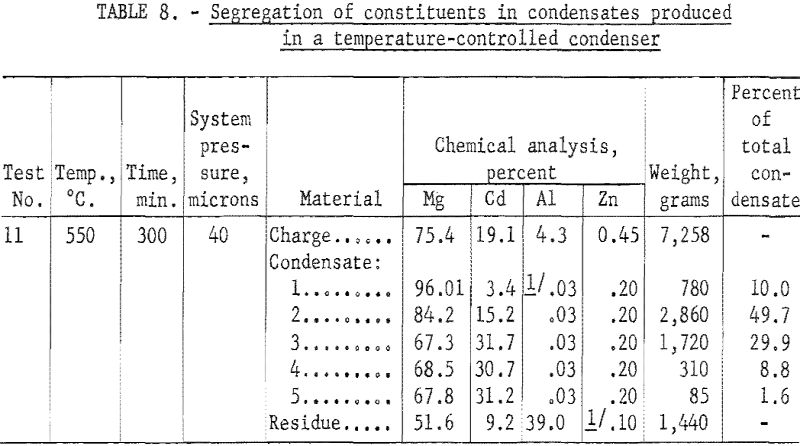
Table 8 shows that selectivity of condensation was not sharp even at the high-temperature zone. There was some mixing of constituents in all sections. Only 10 percent of the condensate was deposited in the hot zone as magnesium of 96 percent average purity. Although the purity was high, the small amount of condensate deposited in this zone shows that much of the magnesium vapor diffused beyond the hot zone before it could condense.
The vapor baffle system produced better separations even though its use may have reduced the rate of evaporation by constricting the vapor channel. The charge for this test was distilled at a relatively high rate without difficulty, thus showing that horizontal distillation retorts are completely satisfactory.
