Table of Contents
Rhenium is industrially recovered all over the world as a by-product of molybdenite processing. There are no separate deposits of rhenium minerals. Molybdenite concentrates, produced as co-products of porphyry copper deposits, contain 0.01 to 0.2% rhenium occuring as a substitutional impurity in the molybdenite lattice.
Process Synthesis
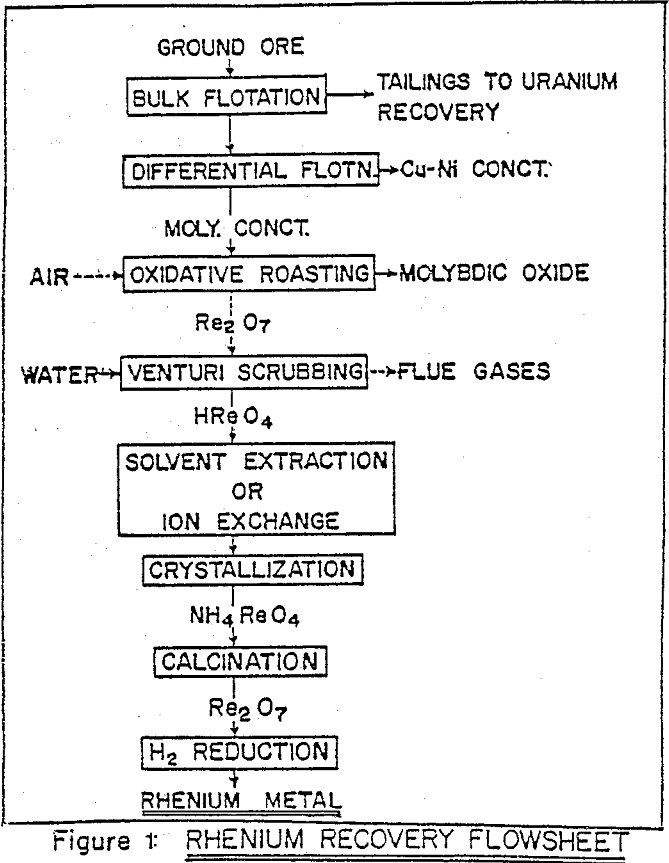
The solvent extraction process is integrated with other processing steps to obtain the ultimate product. The industrial processes for recovery of metal values from low-grade complex sulphide ores usually involve a bulk flotation stage where the sulphide minerals are collectively separated from silicious gangue.
Due to volatile nature of rhenium heptoxide, in contrast with low volatility of other metal oxides, there is high separation factor during oxidative roasting of molybdenite. One of the principal operating variable which influences subsequent rhenium processing is the flow rate of air in the roaster. In conventional multiple hearth roaster large excess of air is needed for rapid convective transfer of reaction heat, and this dilutes rhenium content of the gases and hence of the liquor, for a scrubber operating at constant optimal liquid/gas ratio.
Experimental Work
The experimental work done in the Ore Dressing Section includes the generation of equilibrium separation data under varying process conditions, and the use of this data in process design for treatment of 250 liters of highly impure scrub liquors.
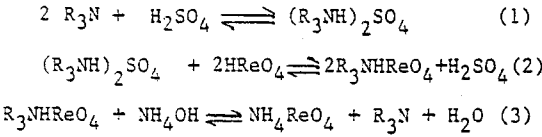
Recovery of rhenium by solvent extraction can be considered to be a two step process involving concentration of a low grade highly contaminated dilute feed to a relatively pure concentrated feed, and final purification and refining of medium grade concentrated feed to the desired level for crystallization.
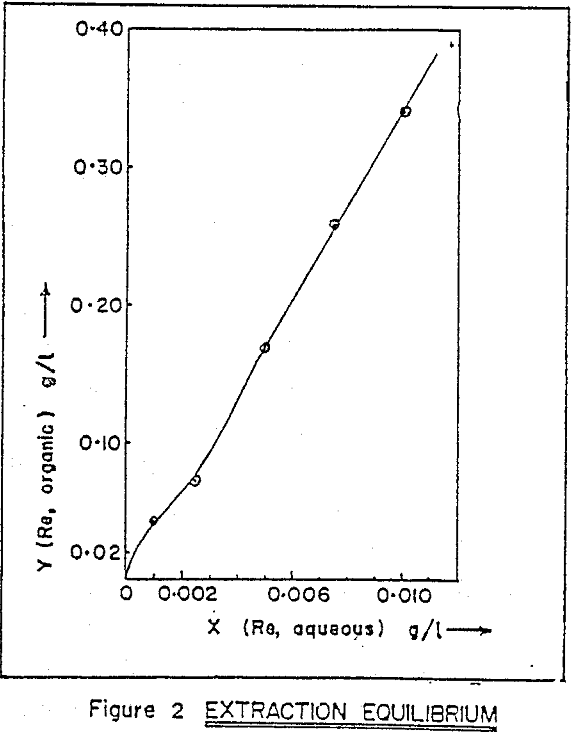
Alamine 336, a long chain tertiary amine. Kerosene is used as diluent, and 2% octanol is added for phase stability. Extraction equilibrium curve. Experimental results indicate that distribution ratio of rhenium increases sharply with increasing amine concentration. Effect of operating phase ratio on the distribution ratio of rhenium shows that as aqueous to organic ratio (A/O) increases from 1 to 15 , distribution ratio (DRe) decreases from 48 to 23, for extraction with 1% amine at a pH of 1.5.
The strip solution obtained after first cycle of concentration and purification contains 0.10-0.60 g/l of rhenium and 0.5-1.5 g/l of molybdenum and needs to be further purified to over 99%. The process alternatives are
- Amine re-extraction.
- Amine-D2EHPA extraction.
- Amine-Ammonium salt extraction.
Pilot Plant Extraction Study
During preliminary testing of prototype multiple hearth furnace for oxidative roasting of molybdenite, over 250 liters of rhenium liquor was obtained by scrubbing the flue-gases in a packed bed. While the concentration of rhenium was as low as 0.005 g/l, that of impurities like iron exceeded 10 g/l. This extremely dilute and highly impure solution was taken up for pilot plant runs of solvent extraction process.
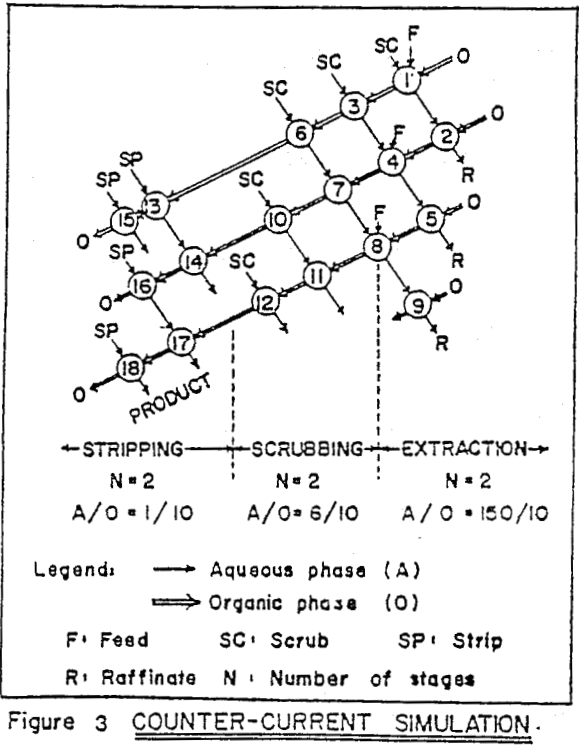
Mixer-settlers were used as the contactors. For operation at high phase ratios a recycle type of design was adopted, while at lower phase ratios a centrifugal type of unit was used. Aqueous feed was controlled at 1 lpm by a peristaltic sigma-pump. Other stream flows were adjusted by constant head gravity feed tanks. The mixer-settlers were made of flexiglass, and reagent tanks were of high density polyethylene (HDPE).