Table of Contents
- Single-Contact Tests
- Equilibrium Characteristics of the Resin-Leach Liquor System
- Stripping Characteristics
- Discussion
- Conclusions
- Appendix
- Preparation of Synthetic Leach Liquor
- Procedure for Multiple-Cycle Tests
- Test results:
- Procedure 2-Recycling of 80 volume-percent of eluate
- Procedure 3—Elimination of washing step
- Procedure 4—Elimination of washing step—Recycle of 80 volume-percent of eluate
- Procedure 5-Recycle of 80 volume-percent of barren
- Procedure 6—Recycle of 60 volume-percent of barren
The leaching solutions circulated at copper dumps in the United States contain an appreciable concentration of aluminum-as much as 5 to 10 grams Al2O3 per liter in some solutions. This aluminum passes through the copper-recovery operations and recirculates to the leach dump. The aluminum concentration in the leach liquor appears to be in an equilibrium condition that is established between the dump rock and the recirculating leach solutions. The dynamic leaching rate of aluminum in the leaching operation at copper dumps has not been established. However, if the leaching rate were such that the aluminum concentration would remain at about the equilibrium concentration when an aluminum recovery system was operating, the potential U.S. production from leaching operations at copper dumps would be in excess of 500,000 tons per year of alumina. This is approximately equivalent to the production of many operating Bayer alumina plants.
Since high flow rates of up to 50,000 gallons per minute are encountered in copper-dump leaching operations, a noncomplex alumina-recovery technology would be particularly desirable. Other preferable processing criteria include the following:
- The acidity of the recirculating process liquor should be maintained at a pH of 3.0 or lower without supplemental acid.
- The aluminum-processing procedures should not significantly increase or decrease the volume of the recirculating leach liquor.
- Any ions added to the recirculating leach liquor should not be detrimental to the copper-leaching operation.
- The aluminum recovery procedures should be adaptable to high-volume processing technology.
The Federal Bureau of Mines investigated the extraction of aluminum from copper-dump leach liquors by resin ion exchange. Resin ion exchange was selected for the investigation because this technology appeared to have the potential for meeting a significant percentage of the aforementioned processing criteria. The system selected was a single-contact loading followed by a single-contact stripping. This mechanically simple system was investigated because the leach liquor recirculates to the dump; therefore, the aluminum need not be quantitatively extracted in one loading operation. A strongly acidic cation-exchange resin was chosen for the studies, and the application of both hydrogen-form and sodium-form resin was investigated. After the equilibrium and kinetic relationships were developed, multiple-cycle loading-stripping tests were conducted to simulate the conditions of continuous processing.
Four process solutions are reported for ion-exchange tests. The terms used in this report to identify these process solutions are underlined in the following illustration:
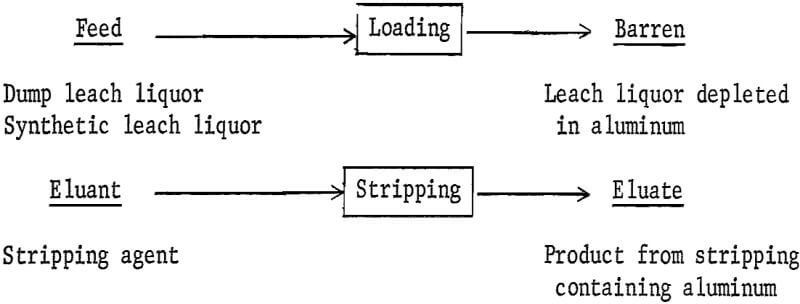
The two different feed solutions are defined as follows:
Dump leach liquor-liquor obtained from an operating copper-waste dump.
Synthetic leach liquor—solution prepared from reagent-grade chemicals containing aluminum, magnesium, and iron sulfates.
This report presents potentials and probable limitations associated with the ion-exchange recovery of aluminum from copper-dump leach liquors.
Raw Materials
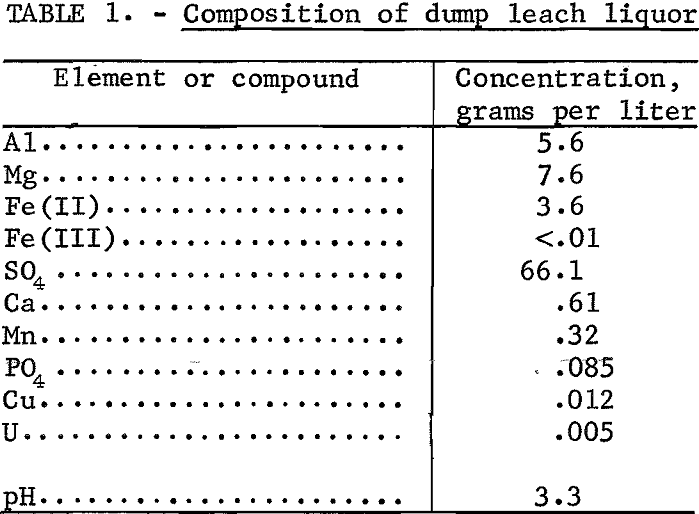
Copper-dump leach liquors taken from the solution overflow at the top of a cone precipitator at a dump-leaching operation were used for the preliminary ion-exchange tests. The composition is shown in table 1. However, the ferrous iron in solution was unstable and oxidized and precipitated as Fe(OH)3 as the solution stood in the laboratory. Within 2 weeks, ferrous iron decreased from 3.6 to 3.25 grams per liter with a proportionate increase of ferric iron. Because it would have been necessary to obtain a fresh sample of dump leach liquor before each series of tests to assure consistent results, a synthetic leach liquor was used as feed solution to ion-exchange tests. This synthetic leach liquor was prepared by dissolving reagent-grade aluminum, magnesium, and iron sulfates in distilled water and contained, in grams per liter, 5-6 Al, 6.0 Mg, 4.7 Fe(II), and 64.6 SO4. After the synthetic leach liquor was adjusted to pH 2, the ferrous iron in solution was stable during the time required for a test series.
At the end of the ion-exchange investigations, a 5-galIon sample of dump leach liquor was obtained from a leaching operation and used as feed in multiple-cycle ion-exchange tests. The purpose of these final tests was to confirm that results of the tests with the synthetic leach liquor were valid for operations with dump leach liquors.
Experimental Procedures and Results
Shaker-scale tests were conducted to establish kinetic and equilibrium data. Multiple-cycle loading and stripping tests were then made to simulate continuous processing.
Single-Contact Tests
Single-contact shaker tests were run to establish fundamental data about the extraction and stripping characteristics of the system consisting of a strongly acidic ion-exchange resin and the synthetic leach liquor.
Amberlite IR-120 resin was used for the investigations. The resin was used in the sodium and hydrogen forms with NaCl and H2SO4 as eluants.
Equilibrium Characteristics of the Resin-Leach Liquor System
The following characteristic properties of the resin-leach liquor system were investigated: (1) Contact times required to reach an equilibrium condition for both extraction and stripping operations; (2) distribution isotherms for extracting aluminum, magnesium, and iron from synthetic leach liquor by the resin in both the sodium and hydrogen forms; (3) distribution isotherms for stripping aluminum, magnesium, and iron from loaded resin with NaCl and H2SO4.
The laboratory procedures used for the tests followed the same general steps. Resin measurements were made in the wet-tamped condition in distilled water. After the distilled water was drawn off, measured amounts of aqueous test solutions, either synthetic leach liquor or eluants, were combined with the resin in polyethylene bottles. The bottles were agitated on a mechanical shaker for the time period set for the test. After the test solution and resin were contacted, the aqueous phase was removed by filtration. The resin was washed to remove remaining aqueous test solution, and the filtrate and wash solutions were analyzed. To determine the concentrations of metals contained in the resin phase, 10 milliliters of resin was measured and contacted two times with 9-N H2SO4, and the two stripping solutions were analyzed. Loaded resin used as feed to stripping tests was prepared by contacting a large batch of resin three times with equal volumes of synthetic leach liquor and then washing the resin until only a trace of sulfate ion remained in the wash water. Before the stripping tests, 10 milliliters of the loaded resin was stripped with acid to determine the metal concentrations on the feed resin.
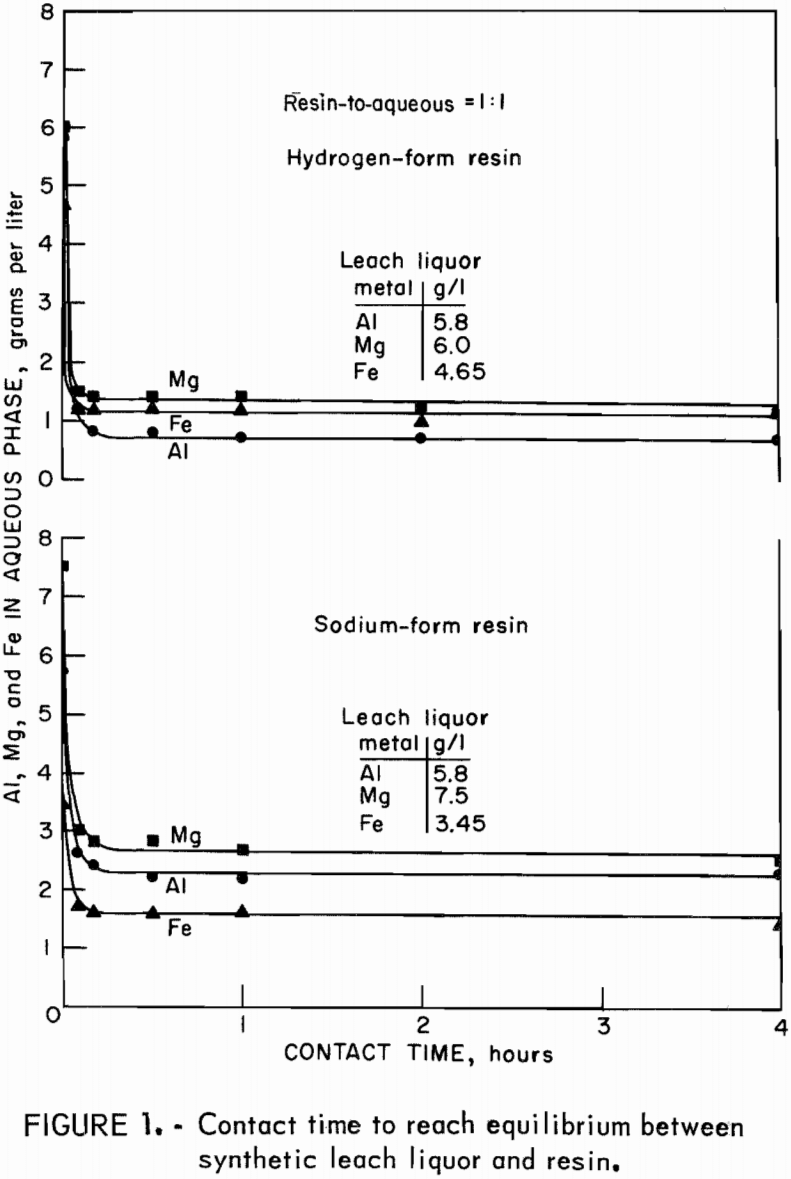
Contact Times
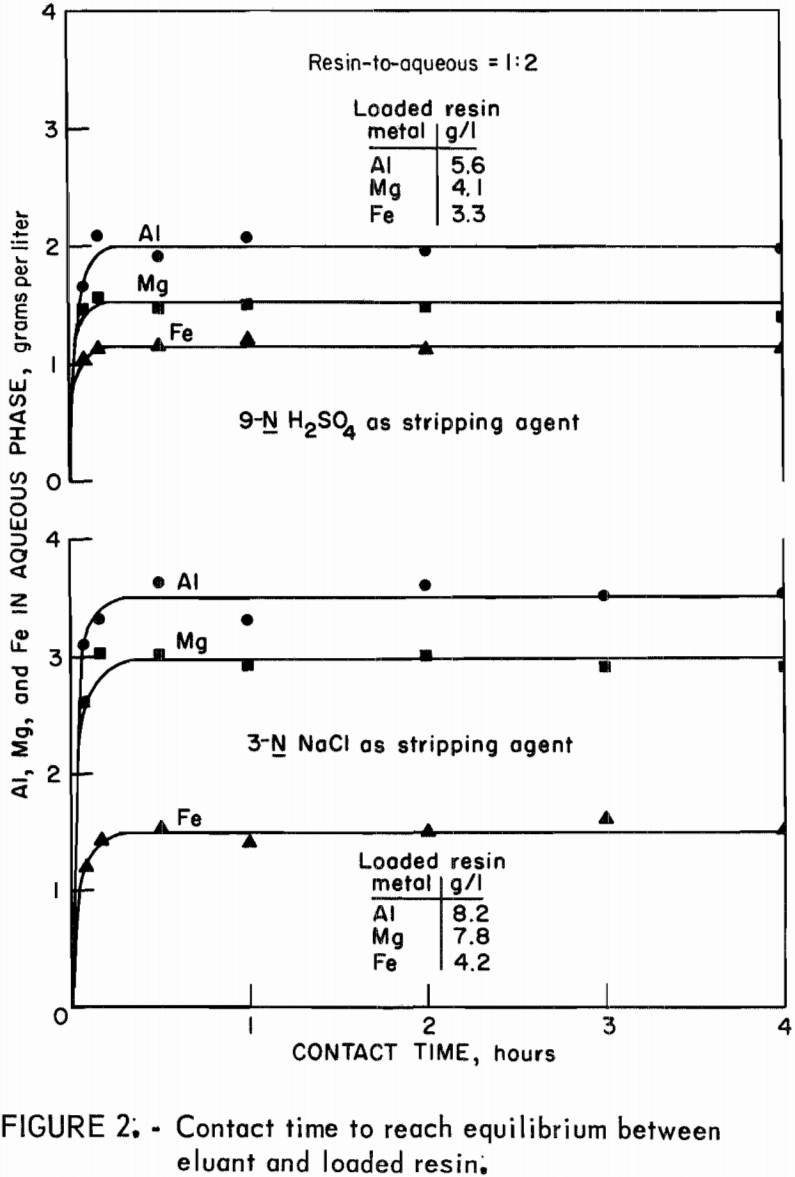
To determine the time required to reach equilibrium between synthetic leach liquor and resin, equal volumes of leach liquor and sodium- or hydrogen-form resin were contacted for time periods from 5 minutes to 4 hours. Concentrations of aluminum, magnesium, and iron in the final aqueous phases are plotted against time of contact in figure 1. Equilibrium for extraction was reached in 10 minutes. Time tests to attain stripping equilibrium were run by contacting 25 milliliters of loaded resin with 50 milliliters of 3-N NaCl or of 9-N H2SO4 for time periods from 5 minutes to 4 hours. Equilibrium was reached within 10 minutes, as shown in figure 2.
Distribution Isotherms
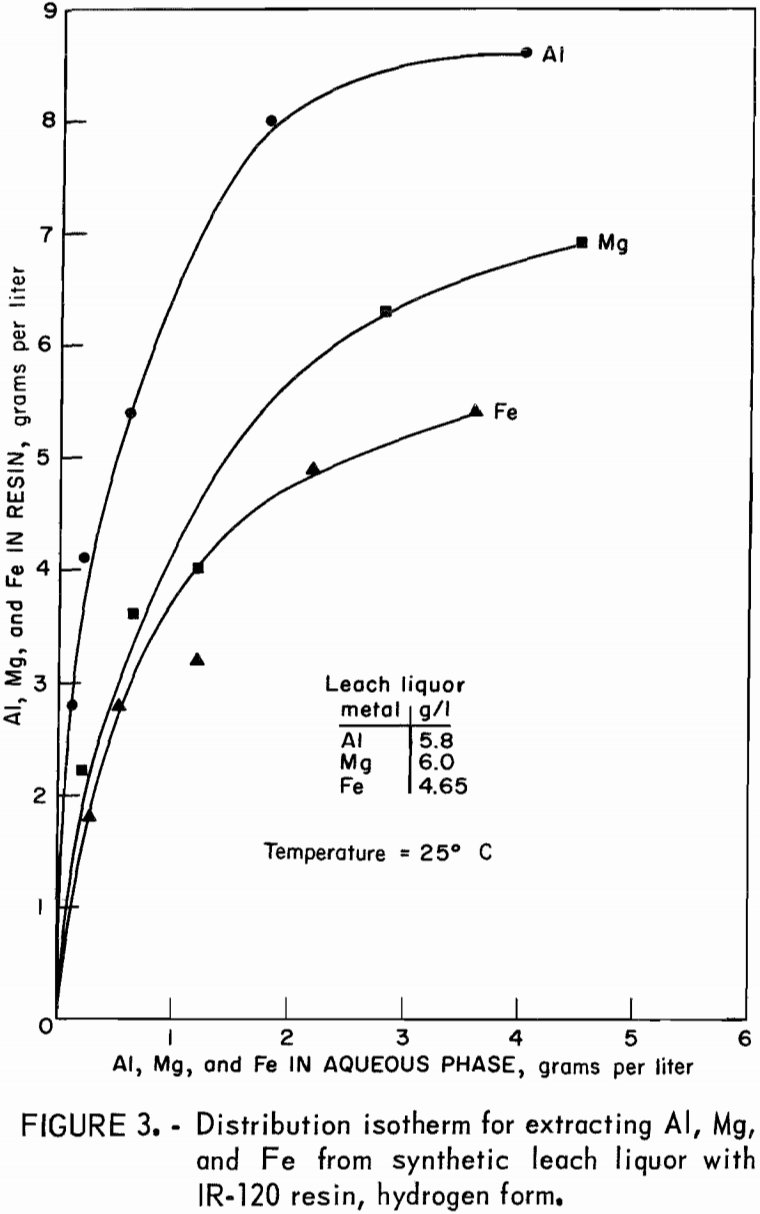
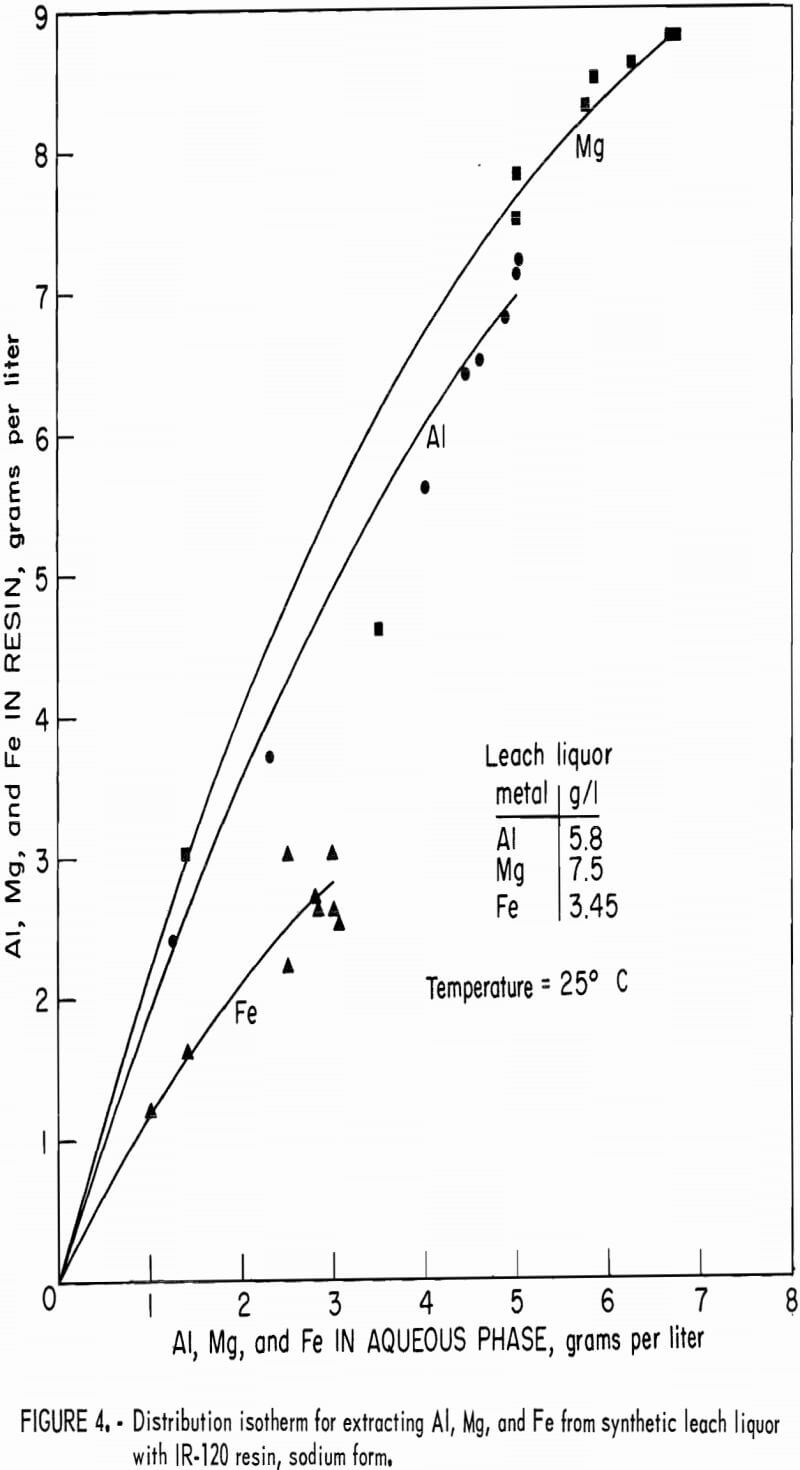
Distribution isotherms for loading were established by contacting resin in the hydrogen and sodium forms with synthetic leach liquor at resin-to-aqueous ratios ranging from 2:1 to 1:10 for 4 hours. Concentrations of aluminum, magnesium, and iron in the equilibrated aqueous and resin phases were determined and plotted on figure 3 for hydrogen-form resin and figure 4 for sodium-form resin.
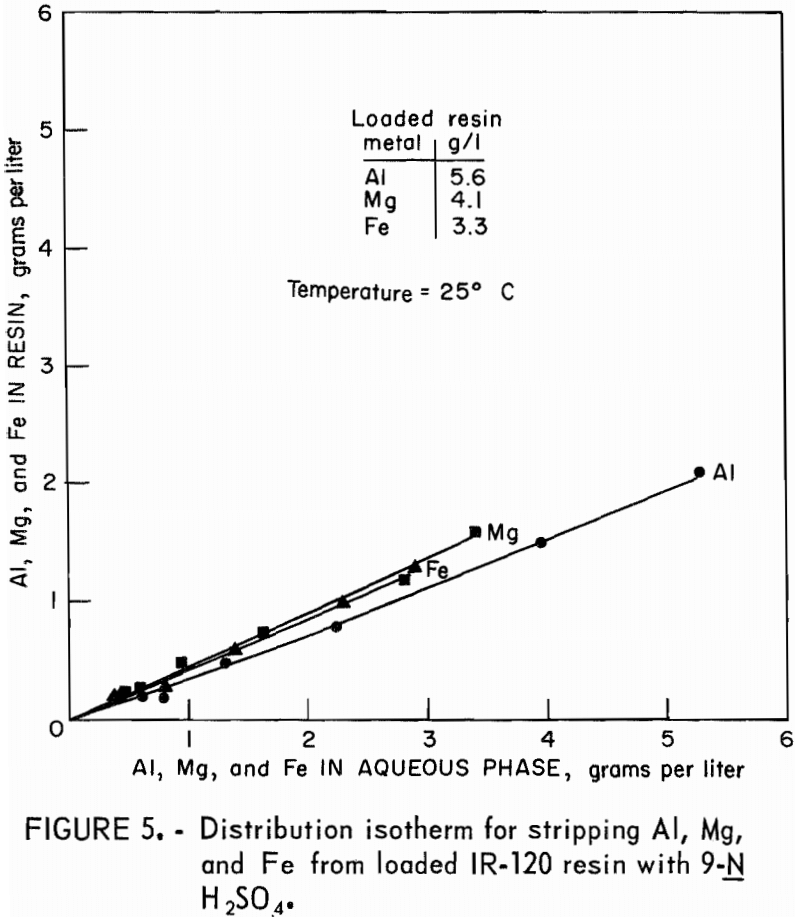
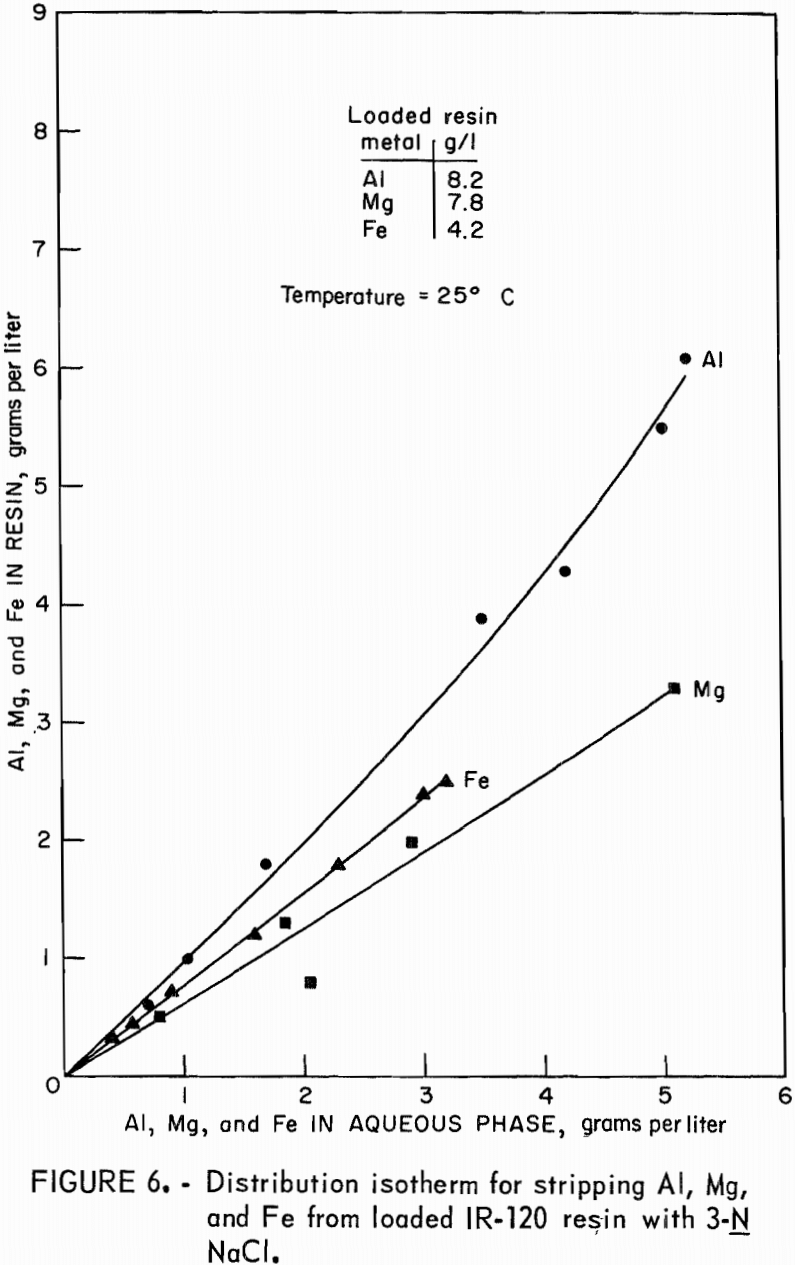
Stripping-distribution isotherms were established by contacting loaded resin for 4 hours with 9-N H2SO4 or with 3N NaCl in resin-to-aqueous ratios that ranged from 2:1 to 1:10. Equilibrium concentrations of aluminum, magnesium, and iron in the eluate and on the stripped resin are shown in figure 5 for hydrogen-form resin and in figure 6 for sodium-form resin.
Loading Characteristics
One series of tests investigated the loading characteristics of resin in the presence of synthetic leach liquor. The capacity of IR-120 resin is given as 2.15 equivalents per liter. Two tests were run to determine if aluminum, a trivalent ion, would eventually displace magnesium and iron, divalent ions, on the resin when the resin was contacted with fresh portions of leach liquor. The sodium and hydrogen forms of resin were used. Portions of the resin were contacted with equal volumes of synthetic leach liquor for 1, 3, 5, and 10 contacts. Analyses of the barrens and final loaded resins showed that both forms of resin were loaded to capacity after five contacts. The form of the starting resin, whether sodium or hydrogen, had no effect on the final concentrations of metals on the loaded resin. Both forms of resin when loaded contained, in grams per liter, 9.7 Al, 9.9 Mg, and 7.5 Fe, which is a total of 2.16 equivalents per liter. The concentration of each metal on the resin was not altered by five additional contacts with fresh
portions of synthetic leach liquor; therefore, magnesium and iron on loaded resin were not displaced by additional amounts of aluminum made available to the resin. The test results are shown in figure 7.
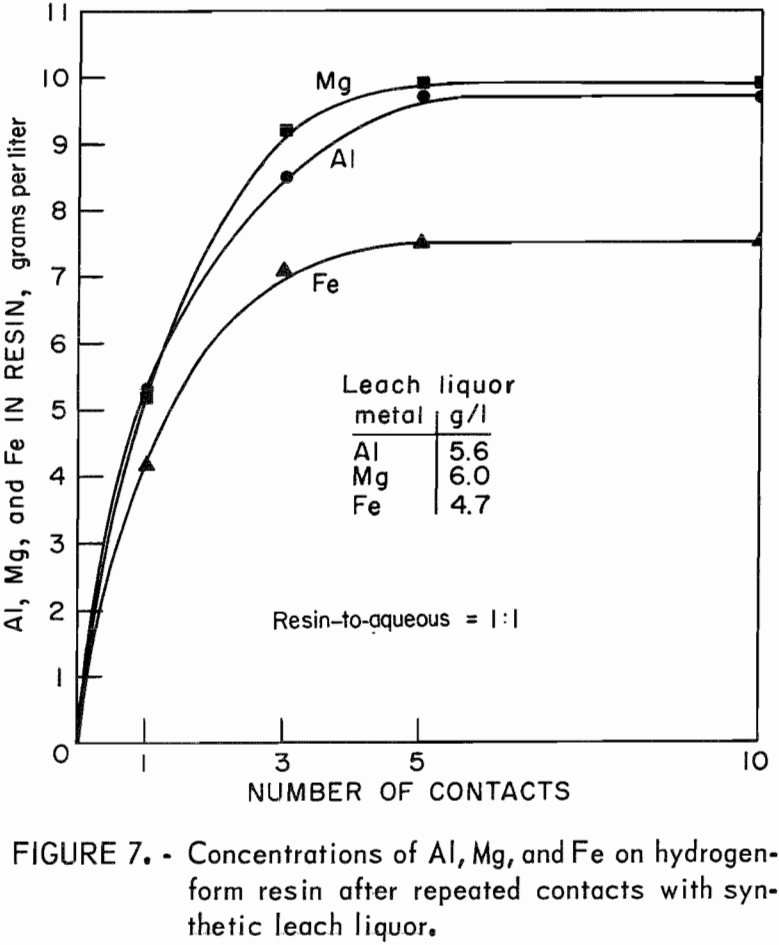
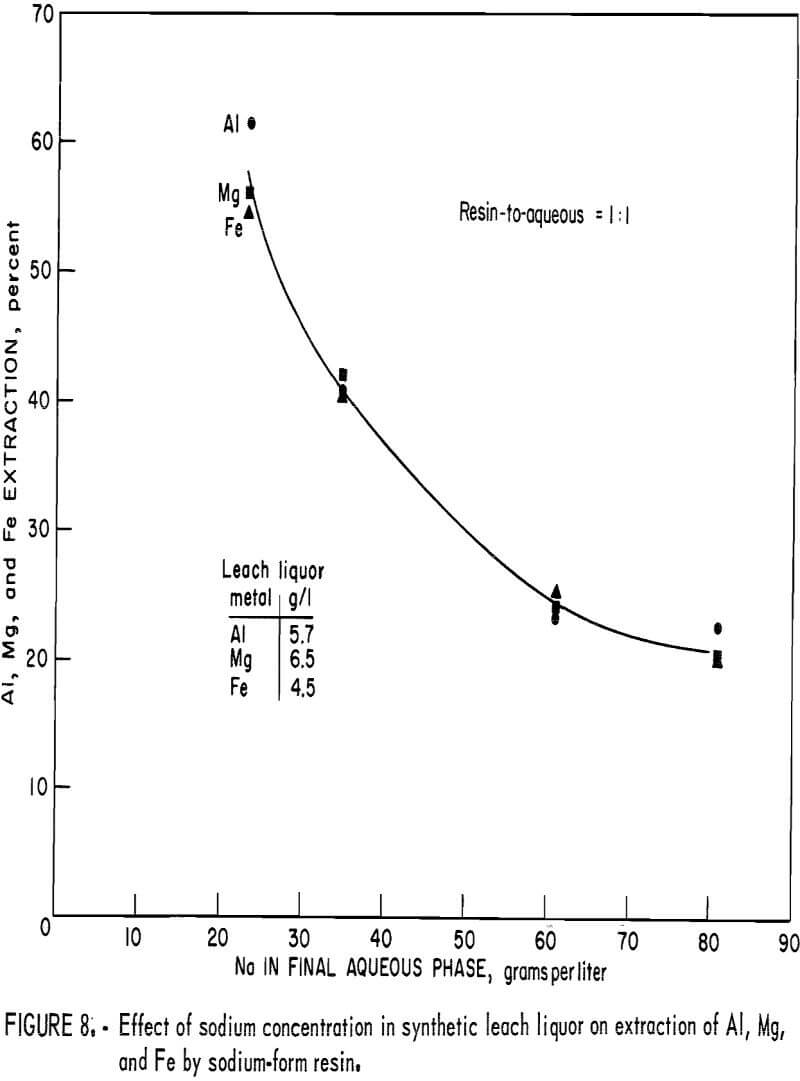
When sodium-form resin is used for extraction and NaCl for stripping in continuous processing, sodium ions are added to the synthetic leach liquor each time it is recirculated through the ion-exchange operation. Single-contact tests were conducted to determine how increasing the concentration of sodium in synthetic leach liquor affects the extraction of aluminum, magnesium, and iron. Solutions were prepared to contain up to 80 grams per liter sodium by addition of Na2SO4. The feed solutions were equilibrated with equal volumes of sodium-form resin. When synthetic leach liquor containing no sodium was contacted with resin to provide a reference extraction, the barren (final aqueous phase) contained 23 grams per liter sodium resulting from the exchange of sodium for extracted metals. At this sodium concentration, about 55 percent of the aluminum, magnesium, and iron was extracted by the resin. At 35 grams per liter sodium, 40 percent of the metals was extracted. The effect of increasing sodium concentration on extraction was not as pronounced after the initial decrease, and even at 80 grams per liter, which is equivalent to near saturation of Na2SO4 in the aqueous phase, slightly over 20 percent of the aluminum, magnesium, and iron was still extracted by the resin. The extraction plotted against the sodium concentration in the barren is shown in figure 8.
Stripping Characteristics
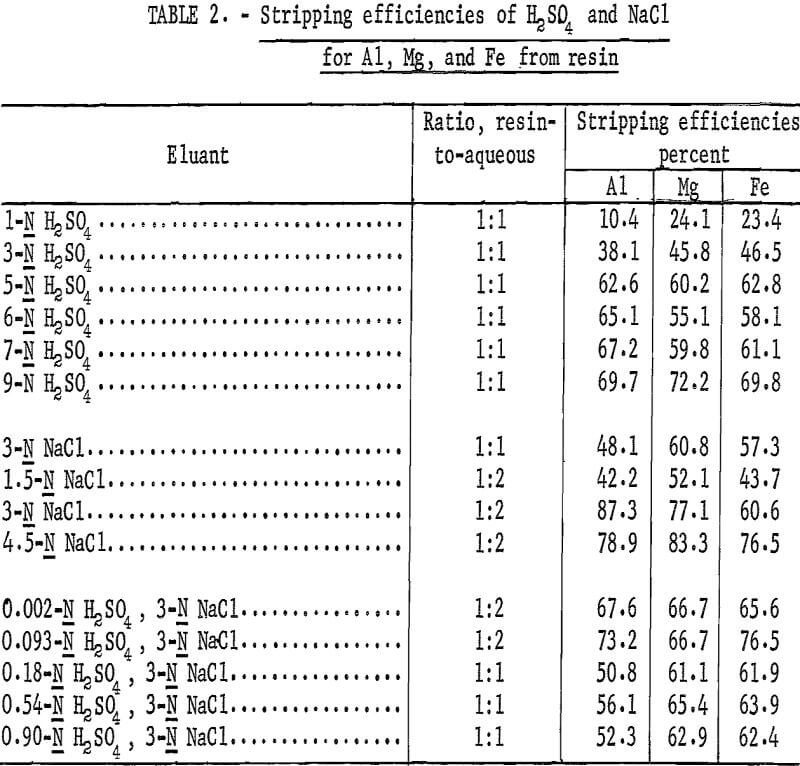
Various concentrations of H2SO4 and NaCl were tested as eluants for aluminum, magnesium, and iron from loaded resin. Resin-to-aqueous ratios of 1:1 were used for the acid tests; ratios of 1:1 and 1:2 were used for the NaCl tests. Analyses of the eluates were used to calculate the stripping efficiencies of the eluant being tested. Results of stripping tests are given
in table 2. It should be rioted that higher stripping efficiencies obtained from resin-to-aqueous ratios of 1:2 are a result of twice the number of sodium ions in the eluant.
Tests were run to determine the effect of increasing metal concentrations in H2SO4 as eluant on removal of additional aluminum, magnesium, and iron from loaded resin. In planning for multiple-cycle tests, one process selected was recycling of a portion of the eluate with a portion of fresh eluant added. The purpose was to increase the aluminum concentration in the final eluate to a level that would enable easier recovery while reducing the total volume of solution that would require treatment.
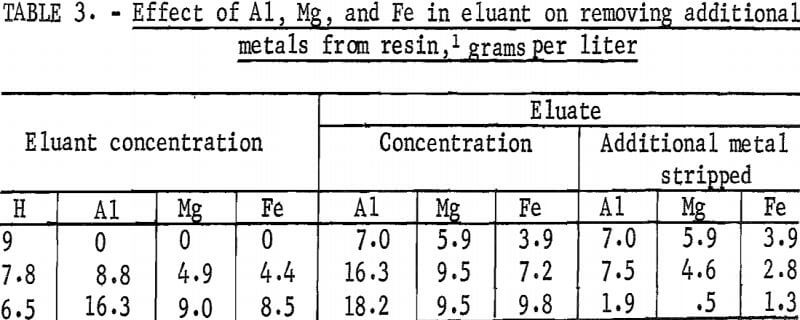
To prepare the test solutions, weighed amounts of aluminum, magnesium, and iron sulfates were added to H2SO4 solutions to simulate values expected in repeated recycles of stripping agent. For each test solution, the acid concentration was adjusted so that the total ion concentration remained at 9 equivalents per liter. To prevent crystallization of metal sulfates at room temperature, concentration of metals in H2SO4 could not exceed 21 grams per liter aluminum and 12 grams per liter each of magnesium and of iron. Equal volumes of loaded resin and test solution were equilibrated for 30 minutes, and the final phases were separated and analyzed. The results are shown in table 3. The additional amounts of metals that were removed from the resin in each recycle decreased as the concentrations of metals in the prepared eluant were increased. The aluminum concentration in the eluate was increased from 7 grams per liter in 9-N. H2SO4 to 18.2 grams per liter when the eluant used contained 16.3 grams per liter aluminum.
Multiple-Cycle Tests
The purpose of multiple-cycle ion-exchange tests was to apply the results of shaker-scale experiments to possible continuous processing of synthetic leach liquor. In continuous processing, dump leach liquor after leaving the copper cementation plant would pass through an ion-exchange operation to extract aluminum, magnesium, and iron and then be recirculated to the copper-waste dump. The ion-exchange operation would consist of two-steps— loading and stripping. In the loading step, dump leach liquor would be mixed with resin in a single-contact batch operation and then be drained from the resin and returned to the dump. In the stripping step, eluant would be mixed with the loaded resin in a single-contact batch operation, and then be separated from the resin for further processing for aluminum recovery. The stripped resin would be mixed with incoming dump leach liquor to repeat the loading step, initiating another cycle. Laboratory tests were designed to simulate these operations. A detailed description of the laboratory procedures is given in the appendix. The cycle of loading-stripping was repeated until steady-state conditions were attained in the resin-aqueous systems, usually at the end of six cycles.
The laboratory investigations were limited to hydrogen- and sodium-form resins with the use of H2SO4 and NaCl as eluants.. The operational variables studied were (1) recycling a portion of the eluate to the stripping stage, (2) inclusion of a washing step, and (3) recycling a portion of the barren to the loading step. Concentrations of H2SO4 eluants were varied from 3 to 9 N; concentration of NaCl eluant was 3 N for all tests. Synthetic leach liquor was used to investigate the effect of variables. Finally, dump leach liquor obtained from an operating dump was used as feed for four multiple-cycle tests with hydrogen- and sodium-form resins run under established conditions.
Hydrogen-Form Resin—H2SO4 Eluant
Two operational stripping procedures were investigated with hydrogen-form resin and 9-N H2SO4 as initial eluant to compare the effects on the concentration of aluminum, magnesium, and iron in the eluate. For the first procedure, loaded resin was stripped with an equal volume of 9-N H2SO4. For the second procedure, loaded resin was stripped with recycle eluant prepared by mixing 80 volume-percent of the eluate from the previous cycle with 20 volume-percent of 9-N H2SO4. In this procedure, the concentrations of aluminum , magnesium, and iron were increased in the eluate until a steady-state condition was attained between the recycling eluant and the loaded resin. In each test, the resin was washed between the stripping step and subsequent loading step by contact with an equal volume of water. The twofold purpose of recycling a portion of eluate was to obtain a high concentration of aluminum, magnesium, and iron, and to reduce the volume.of product solution, thus, facilitating further treatment for aluminum recovery. Conditions and results of these tests are shown as tests 1 and 2 in table 4. In the test in which 80 volume-percent of the eluate was recycled, the eluate contained 11.0 grams per liter aluminum; without recycle, the eluate contained 5.0 grams per liter aluminum. In all subsequent tests with hydrogen-form resin, 80 volume-percent of the eluate was recycled to the stripping step.
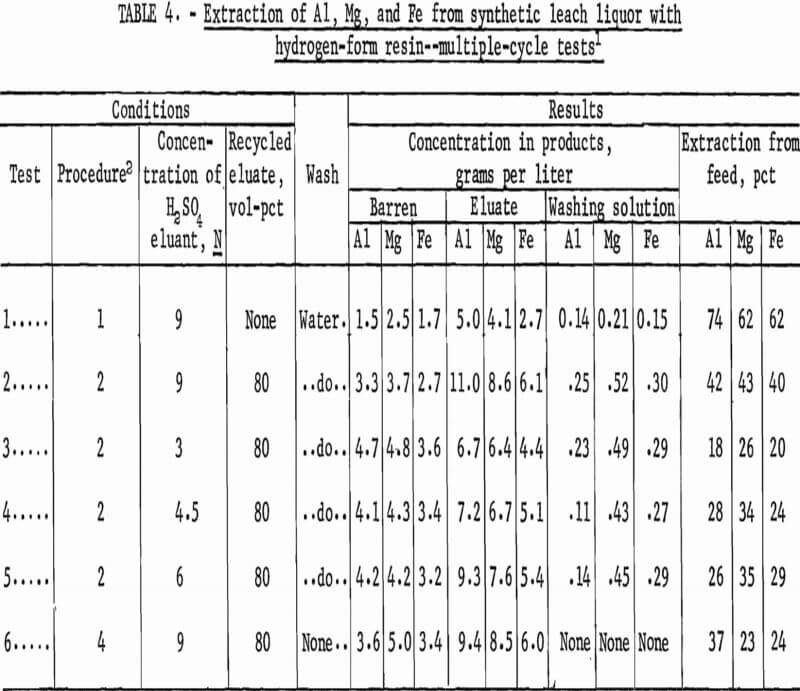
Three concentrations of H2SO4 as eluant, 3, 4.5, and 6 N, were used in multiple-cycle tests to determine the effect of acid concentration on stripping efficiency. In continuous processing, the efficiency of stripping, in turn, influences the extraction of metals from synthetic leach liquor. The loaded resin was stripped by contact with an equal volume of recycle eluant, which was a mixture of 20 volume-percent of the acid being tested and 80 volume-percent of the eluate from the previous cycle. The resin was washed with an equal volume of water between the stripping and loading steps. Conditions and results of these tests are shown as tests 3, 4, and 5 in table 4. Comparison of results from these tests with those for test 2 run with 9-N H2SO4 shows that use of 9-N H2SO4 results in the highest extraction of aluminum from synthetic leach liquor and the highest concentration of aluminum in the eluate.
The final multiple-cycle test with hydrogen-form resin and synthetic leach liquor was conducted to determine the overall effect of eliminating the washing step. The washing step was included initially to remove the remaining eluate from drained resin. If not removed, metal and hydrogen ions carried on drained resin would mix with the metals in synthetic leach liquor and decrease extraction during the loading step. For the final test, the loaded resin was stripped with an equal volume of recycle eluant, which was a mixture of 20 volume-percent of 9-N H2SO4 and 80 volume-percent of eluate from the previous cycle, drained, and returned directly to the loading step. Conditions and results of this test are shown as test 6 in table 4. The pH of the barren drained from the loading step was 0.38 for test 6, in which the washing step was eliminated, and 0.59 for test 2, which included a washing step. Extraction of aluminum was reduced from 42 to 37 percent by eliminating the washing step. Because this lower level of extraction was still acceptable, the washing step was not included in subsequent tests.
Sodium-Form Resin—NaCl Eluant
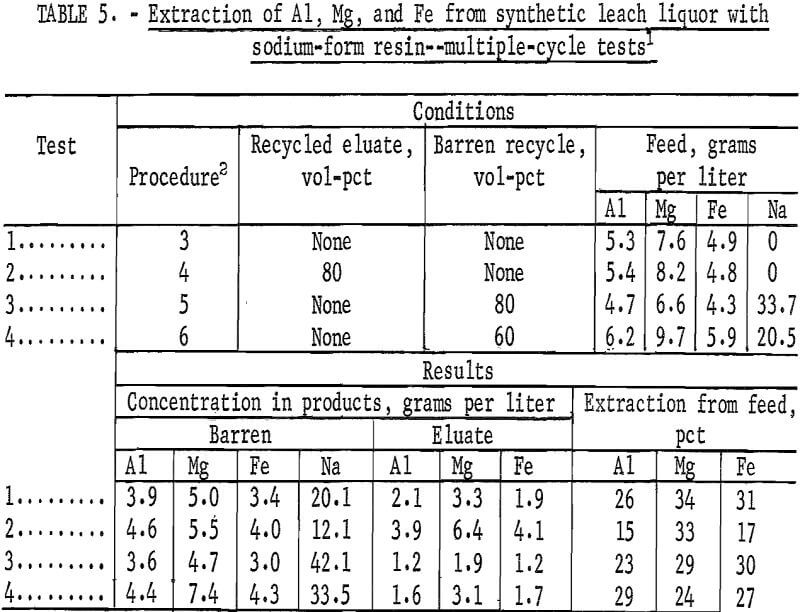
The two stripping procedures described, without a recycled eluate and with a recycled eluate, were tested with sodium-form resin and 3-N NaCl as eluant. In the first procedure, loaded resin was stripped with an equal volume of 3-N NaCl; in the second procedure, loaded resin was stripped with a mixture of 80 volume-percent of the eluate from the previous cycle and 20 volume-percent of 3-N NaCl. No washing step was used. Conditions and results are shown as tests 1 and 2 in table 5. The eluate from the recycle test contained about twice the concentration of aluminum as the eluate from the test with no recycle.
Two operational procedures for loading were tested to determine the overall effect of high sodium concentrations in recirculating synthetic leach liquor on the loading and stripping operations. Single-contact tests showed that extraction of aluminum was depressed by high concentrations of sodium in synthetic leach liquor. If increased sodium concentrations depressed extraction below a practical level, a portion of the recirculating leach liquor would have to be removed from the system during continuous processing. To simulate this condition, portions of the barren from the loading step were bled from the system; and the remaining barren was recycled to the next loading cycle. It was then necessary to add concentrated solutions of aluminum, magnesium, and iron sulfates to the recycled barren to replace the metals extracted and make up feed for the next cycle. In two variations of this procedure, 80 and 60 volume-percent of the barren from one cycle were reserved for use as feed to the next cycle. In each test, the loaded resin was stripped with an equal volume of 3-N NaCl, and no washing step was used. Conditions and results are shown as tests 3 and 4 in table 5. At steady-state conditions, the barren contained 42 grams per liter sodium with 80 volume-percent recycle, representing a bleed stream of 20 volume-percent of barren, and 34 grams per liter sodium with 60 volume-percent recycle , representing a bleed stream of 40 volume-percent of barren. Comparison of results from these tests with the one run under the same conditions (test 1) shows that these levels of sodium concentration do not significantly depress extraction.
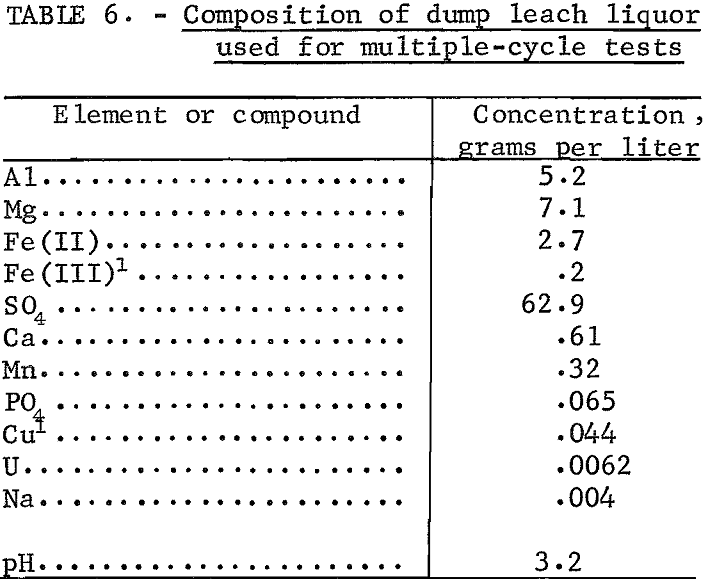
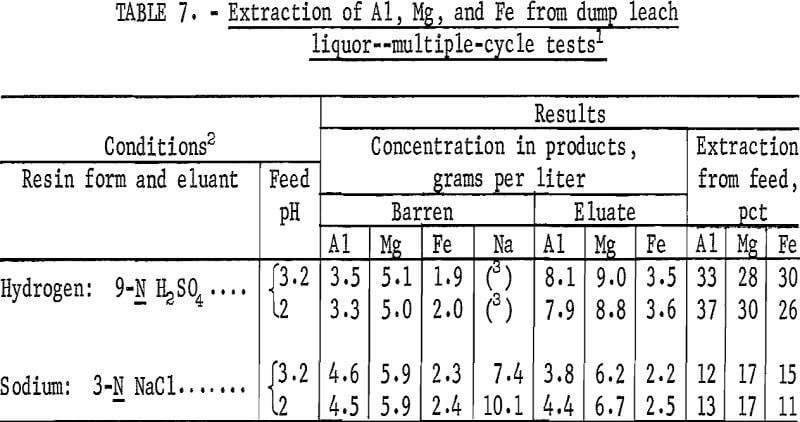
Established Conditions with Dump Leach Liquor
Liquor obtained from a copper-waste dump leaching operation was used as feed for multiple-cycle tests. The dump leach liquor contained minor amounts of calcium, manganese, phosphate, copper, sodium, and uranium in addition to aluminum, magnesium, and iron. The composition is shown in table 6. A 5- gallon sample of dump leach liquor was taken from the solution overflow at the top of a cone precipitator at a cementation plant. The sample was divided into two portions; one portion was used as feed without alteration, and the second was acidified to pH 2 to prevent precipitation of Fe(OH)3. Two tests were run with each feed; one was run with sodium-form resin and 3-N NaCl as eluant, and the second with hydrogen-form resin and 9-N H2SO4 as eluant. For these tests, 80 volume-percent of the eluate was recycled; no washing step was used. Conditions and results of these tests are shown in table 7.
Extraction of aluminum, magnesium, and iron was nearly the same for dump leach liquor at pH 3-2 and at pH 2. Results of these multiple-cycle tests verified the results of ion-exchange tests conducted with synthetic leach liquor under the same operating conditions.
Selectivity Tests With Specialty Resins
Two specialty resins, BioRex-63 and Amberlite XE-318, were tested for selective extraction of aluminum from synthetic copper-waste leach liquor. BioRex-63 is an intermediate acid resin containing phosphonic acid difunctional groups on a styrene-divinylbenzene lattice. The capacity of the resin is reported to be 3.1 equivalents per liter. Amberlite XE-318 is an experimental chelating resin based upon a macroreticular matrix. The capacity and selectivity of this resin is dependent upon pH of the aqueous phase in the resin-aqueous mixture.
Extraction coefficients were determined for aluminum, magnesium, and iron from synthetic leach liquor for these two resins and for IR-120 to provide a basis for comparison. Each resin was tested in both the sodium and hydrogen forms. In shaker tests, measured portions of wet-tamped resin were contacted with equal volumes of the synthetic leach liquor for 30 minutes to attain equilibrium conditions. The final aqueous solutions were separated from the resins, and the resins were washed with water. The filtrates and wash solutions were combined and analyzed. To determine the metal concentrations on the final resins, 10 milliliters of resin were contacted twice with 50 milliliters of 9-N H2SO4. The two acid solutions were combined and analyzed. The concentrations of metals in the final aqueous and resin phases are shown in table 8. Table 9 shows the extraction coefficients for aluminum, magnesium, and iron from the synthetic leach liquor and the resulting separation factors for aluminum from magnesium and aluminum from iron.
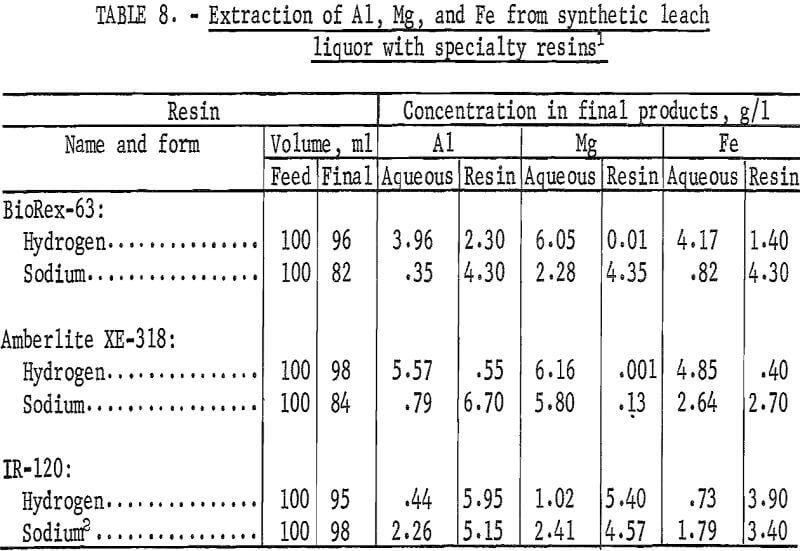
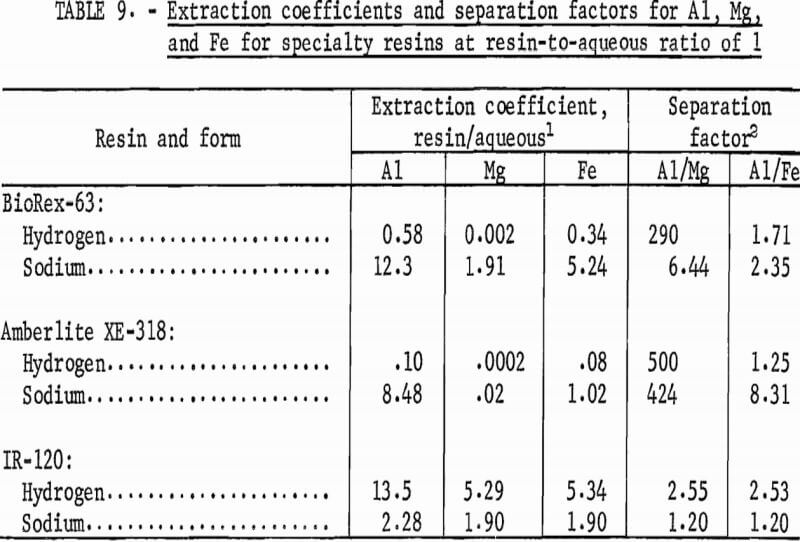
The extraction coefficients show that aluminum was extracted from the synthetic leach liquor by BioRex-63 and by Amberlite XE-318 in the sodium form, but not by these resins in the hydrogen form. In contrast, aluminum was extracted more effectively by IR-120 in the hydrogen form than in the sodium form. Amberlite XE-318 exhibited a high selectivity for extraction of aluminum over extraction of magnesium and iron, as shown by the two separation factors. Although the separation factors determined for BioRex-63 were not as favorable, the separation for aluminum from magnesium was higher than the separation factors determined for IR-120. The high separation factors for the hydrogen forms of the specialty resins are not of practical interest because the corresponding extraction coefficients are extremely low.
Discussion
Resin ion-exchange technology presented several limitations when it was operated under the proposed processing criteria. First, strongly acidic cation-exchange resins are not selective for extraction of aluminum over magnesium or iron. The resin was loaded with all three metals in about the same proportion as these metals were present in the leach liquor feed solution. Consequently, when the resin was stripped, all three metals were eluted, requiring further processing of the eluate to separate and recover aluminum only. Second, the capacity of this resin, which is 2.15 equivalents per liter, limited the highest possible loading concentration to 19.3 grams aluminum per liter of resin if it were loaded exclusively with aluminum. In the presence of magnesium and iron in the leach liquor, the highest aluminum loading obtained was 9.6 grams per liter, equal to 1.07 equivalents per liter. The third limitation of strongly acidic cation-exchange resin is the need for excess of eluant to remove aluminum, magnesium, and iron because of the preference of this resin for divalent and trivalent ions over monovalent ions. Only hydrogen and sodium ions were used in stripping agents in order to comply with the criterion that any ions added to the recirculating leach liquor will not be detrimental to the copper-leaching operation. Eluants of H2SO4 or NaCl were chosen because they are low in cost and readily available. The excess of eluant required was demonstrated in the multiple-cycle tests. With 9-N H2SO4 and 80 volume-percent of recycled eluate 7-5 equivalents of hydrogen ion are required to recover 1 equivalent of aluminum. With 3-N NaCl as eluant and 80 volume-percent of recycled eluate, 7 equivalents of sodium is required to recover 1 equivalent of aluminum. This requirement for excess eluant resulted in low concentration factors by ion exchange. Under the best condition, aluminum was concentrated from 5.7 grams per liter in the synthetic leach liquor to 11 grams per liter in the eluate, a concentration factor of 2.
Use of alternate ion-exchange resins presents different problems. Weakly acidic carboxylic resins are not effective for extracting aluminum below pH 4 or 5. Use of these resins would, therefore, require a partial neutralization of copper-waste leach liquor before the extraction operation and reacidification of leach liquor after the extraction, before it is recirculated to the dump. The two specialty resins investigated were effective for extracting aluminum preferentially to magnesium and iron when used in the sodium form, but not when used in the hydrogen form. However, these resins, when loaded, could be stripped with acid, but not with NaCl. Therefore, a third operational step would have to be added to the simple load-strip operation to regenerate the resin from hydrogen to sodium form by use of NaOH. After regeneration, the resin would have to be washed extensively to remove hydroxyl ion, further complicating the operations, before the resin was contacted with the leach liquor in the subsequent loading cycle.
Conclusions
This report presents the information obtained during investigations of ion-exchange technology for extracting and recovering aluminum from copper-waste leach liquor. At the start of the investigations, preferable processing criteria were specified that restricted the conditions tested to the use of a strongly acidic cation-exchange resin and H2SO4 or NaCl as eluants in a simple load-strip operation. With hydrogen-form resin, 35 percent of the aluminum contained in the leach liquor was extracted in each loading cycle in a single contact. Extracted metals were stripped from the resin by a single contact with 9-N H2SO4. Recycle of 80 volume-percent of the eluate resulted in an increase in the aluminum concentration in this product to 11 grams per liter. Use of sodium-form resin and 3-N NaCl for stripping resulted in 12-percent extraction of aluminum and 4 grams per liter aluminum in the eluate.
Although the ion-exchange extraction of aluminum described in this report is technically feasible, the potential application of ion-exchange technology to copper-waste leach liquors would depend on one or more of the following conditions: (1) High concentration of copper in the leach dump justifying high consumption of acid; (2) availability of a resin with a high loading capacity that is selective for aluminum over magnesium and iron; or (3) an efficient eluant resulting in a favorable concentration ratio for aluminum from dump leach liquor to eluate.
Appendix
Preparation of Synthetic Leach Liquor
Dissolve 691.6 grams Al2(SO4)3 · 18H2O, 608.3 grams MgSO4·7H2O, and 234.0 grams FeSO4·7H2O in about 7 liters of distilled water; bring the solution to a total volume of 10 liters; adjust to pH 2 by dropwise addition of concentrated H2SO4.
Procedure for Multiple-Cycle Tests
Procedure 1—Load-strip-wash
Resin preparation:
- Measure 100 milliliters of wet-tamped resin in graduated cylinder.
- Transfer resin to 250-milliliter, widemouth, polyethylene bottle with distilled water.
- Set filtering device in place on bottle; invert over 250-milliliter filter flask and apply vacuum to draw off water; discard water.
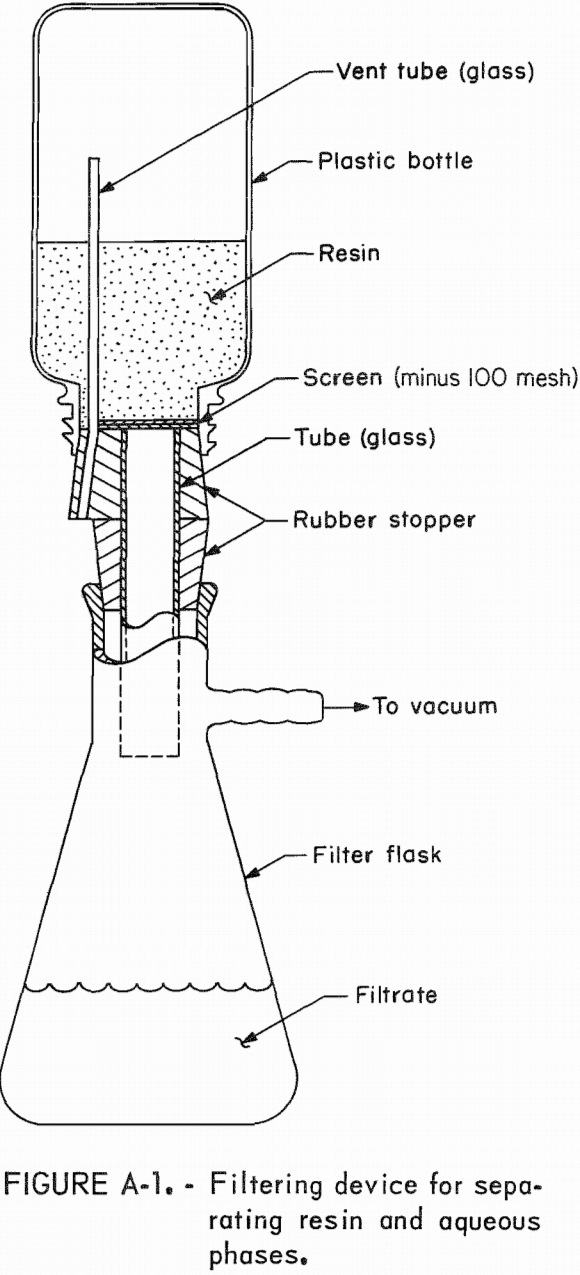
(This filtering device assembled from two rubber stoppers, glass tubing, and minus 100-mesh stainless steel screen is illustrated in figure A-1. Use of this device made it possible to separate aqueous and resin phases without either losing resin on filter paper or losing solution by entrainment in the resin phase.)
Loading steps:
4. Add 100 milliliters of feed to drained resin in bottle.
5- Cap and shake 30 minutes on a mechanical shaker.
6. Filter resin (see step 3) and sample filtrate, which is barren.
Stripping steps:
7. Add 100 milliliters of eluant to drained resin in bottle.
8. Cap and shake 30 minutes.
9. Filter resin and sample filtrate, which is eluate.
Washing steps:
10. Add 100 milliliters of distilled water to drained resin in bottle.
11. Cap and shake 30 minutes.
12. Filter resin and sample filtrate, which is washing solution. Repeat loading, stripping, and washing steps six times.
Test results:
13. Analyze samples of barren (step 6), eluate (step 9), and washing solution (step 12) for aluminum, magnesium, and iron. In addition, analyze these samples for sodium in tests with sodium-form resin.
14. Plot concentrations of each metal against the cycle number for each test solution. The test is considered to be at steady-state conditions when metal concentrations in each test solution remain constant for three cycles.
15. Report metal concentrations in test solutions at steady-state conditions.
Procedure 2-Recycling of 80 volume-percent of eluate
Follow resin preparation, loading, and washing steps given in procedure 1. For stripping step, use the following procedure:
Stripping steps:
- For the first cycle only, add 100 milliliters of eluant to drained resin from loading step.
- Cap and shake for 30 minutes.
- Filter resin; measure and record volume of eluate. Save 80 milliliters for use in next cycle. Dilute remaining volume of eluate to 100 milliliters to be used for sample.
- Follow washing steps.
- For the second and subsequent cycles, mix 20 milliliters of eluant and 80 milliliters of eluate saved from the previous cycle, and add mixture to drained resin from loading step.
Procedure 3—Elimination of washing step
Follow resin preparation, loading, and stripping steps given in procedure 1, but go directly to the loading step from the stripping step.
Procedure 4—Elimination of washing step—Recycle of 80 volume-percent of eluate
Follow resin preparation, loading and stripping steps given in procedure 2, but go directly to loading step from the stripping step.
Procedure 5-Recycle of 80 volume-percent of barren
Follow resin preparation and stripping steps given in procedure 1, but eliminate the washing steps. (See procedure 3.) For the loading step, use the following procedure:
Loading steps:
- For the first cycle only, add 100 milliliters of feed to drained resin in the bottle.
- Cap and shake 30 minutes.
- Filter resin; measure and record volume of barren. Save 80 milliliters of barren for use in next cycle. Dilute the remaining volume of barren to 100 milliliters to be used for sample.
- Follow stripping steps.
- For the second and subsequent cycles, mix 20 milliliters of a solution of metal sulfates prepared to contain, in grams per liter, 8.7 Al, 14.1 Mg, and 9.0 Fe at pH 2 with the 80 milliliters of barren saved from the previous cycle and add the mixture to drained resin from the stripping step.
Procedure 6—Recycle of 60 volume-percent of barren
Follow resin preparation and stripping steps given in procedure 1, but eliminate the washing steps. (See procedure 3) For the loading step, use the following procedure:
Loading steps:
- For the first cycle only, add 100 milliliters of feed to drained resin in bottle.
- Cap and shake 30 minutes.
- Filter resin; measure and record volume of barren. Save 60 milliliters of barren for use in next cycle. Dilute the remaining volume of barren to 100 milliliters to be used for the sample.
- Follow stripping steps.
- For the second and subsequent cycles, mix 40 milliliters of a solution of metal sulfates prepared to contain, in grams per liter, 9.3 Al, 14.9 Mg, and 8.4 Fe at pH 2 with the 60 milliliters of barren saved from the previous cycle. Add the mixture to drained resin from the stripping step.

