Table of Contents
Two materials critical to the construction of several advanced photoelectric devices are cadmium and tellurium. CdTe is used in thin-film photovoltaic (PV) devices to absorb electromagnetic radiation (visible sunlight) and convert the energy into an electric current. The Solar Energy Research Institute (SERI) estimates that demand for CdTe in these PV devices could reach 260 t/y to generate approximately 0.2% of United States’ consumption of electricity in 1989. Single-crystal CdTe wafers are used as infrared detectors for night vision devices. In addition, CdS thin-film electrodes are used in newly developed CuInSe2 PV cells (1,3). The installation of two 20-kW PV power systems using polycrystalline thin-film CdTe and CuInSe2 devices was begun in 1990. Thus, use of cadmium chalcogenides (compounds containing sulfur-group elements) can be expected to grow as solar power generation becomes more common.
Extremely high-purity materials are required for semiconductors; thus, direct reuse even of the PV manufacturing scrap is impossible with current technology. For example, cut CdTe wafers cannot be remelted and grown into new crystals because of oxygen and other atmospheric contaminants that accumulate on the wafer surfaces during cutting. Because of this difficulty in directly reusing PV scrap, this research focused on separating the elements into metallurgical grades for reintroduction into existing processing streams.
The USBM’s interest in treating this scrap stems from both material supply and environmental concerns. Although the actual amount of material currently used in these devices is small in comparison with other domestic uses, the value of the cadmium and tellurium in these devices is significant. If consumption of the PV devices increases as expected, the supply of tellurium could become limiting; hence, recycling of the PV scrap should help to assure an adequate supply of materials.
Another concern, however, is the potential for environmental damage through disposal of this scrap. Cadmium is regulated by the U.S. Environmental Protection Agency (EPA) as potentially hazardous. Tellurium poses similar health concerns, although not covered by EPA regulations as is cadmium. Soluble and volatile tellurium compounds, which may form during improper handling of the scrap, are considered toxic. By making technology available to recover hazardous elements from PV scrap, the USBM may help in limiting environmental damage that could occur through improper disposal of scrap.
A major objective of the research was to avoid generation of process wastes. Thus, each of the procedures described below was intended to produce only usable products.
Research Approach
Very little information has been published about the chemistry and behavior of tellurides. However, tellurium is in the same chemical group as sulfur; thus, metal tellurides should respond much like metal sulfides. Leaching chemistry of sulfide ores is well known. Expected responses of CdTe during hydrometallurgical testing were derived from the available thermodynamic data and from published reports of sulfide mineral processing. Forward, for example, demonstrated oxidative leaching of ZnS to produce elemental sulfur and zinc sulfate solutions. Likewise, oxidative leaching of CdTe was expected to produce elemental tellurium and solubilized cadmium sulfate.
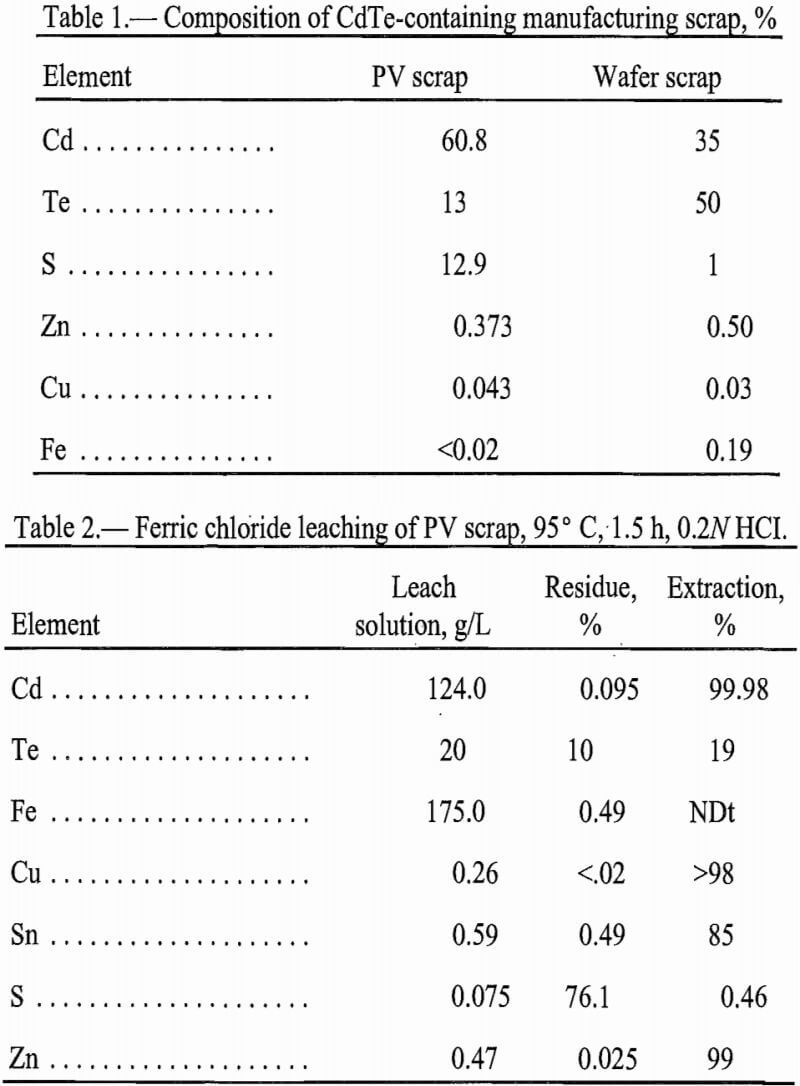
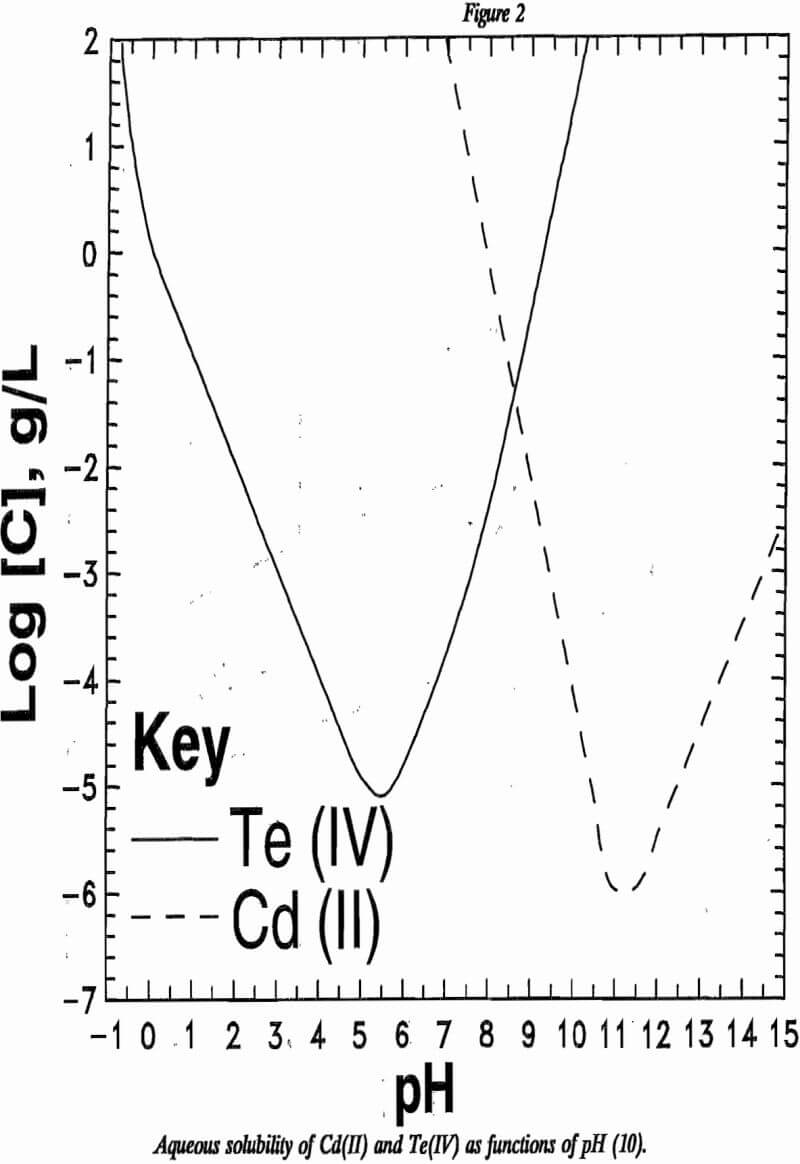
Pourbaix developed pH-potential (Eh-pH) and solubility diagrams for cadmium and tellurium from thermodynamic data. The superimposed Eh-pH diagrams for cadmium and tellurium are shown in figure 1. Solubilities of oxidized forms of these elements are plotted against pH in figure 2. Both tellurium(IV) and cadmium(II) are soluble in strongly acidic and strongly basic solutions. The minimum solubility for each element approaches 10 -5 g/L in aqueous solutions. The plots in these two figures indicate that the separation of cadmium and tellurium should be possible through careful control of oxidation potential during leaching and acid concentration during solution purification.
Experimental
Test Materials
CdTe/CdS PV Scrap
The primary material studied in this research was semiconductor scrap from the manufacture of CdTe thin-film PV devices. The PV scrap contained CdTe (the photosensitive component) and CdS (used as a current conductor). This scrap was a polycrystalline powder. Through microscopy, the crystal size was estimated to be smaller than 200 mesh, although the scrap was agglomerated. X-ray diffraction (XRD) showed that the CdTe and the CdS were present in separate phases. The scrap was contaminated with fragments of glass from the plate glass support used in making the PV devices. An elemental analysis of the material is reported in table 1.
CdTe Wafer Scrap
No published information was available about the leaching behavior of CdTe. In the absence of this information, leaching tests using CdTe free of other cadmium-containing material were conducted. Zinc-doped CdTe single crystal wafer scrap was used to obtain these kinetic data. Elemental composition of the scrap is reported in table 1. XRD showed CdTe as the major phase with lesser amounts of elemental tellurium and silicon carbide.
Equipment
Ferric chloride and H2O2 leaches were done in beakers on a combination hot plate and magnetic stirrer. A mercury thermometer in the leach solution was used to monitor the temperature. The leach slurries were agitated using Teflon-coated stirrer bars. The beakers were covered with watch glasses during leaching to reduce evaporation.
Oxygen-pressure leaches were done in a 300-mL autoclave with a magnetically coupled stirrer. Wetted parts of the autoclave were constructed of 316 stainless steel. The stirrer was operated at the maximum stirring speed (approximately 800 rpm) to thoroughly suspend the solids in the leach liquor. Temperature was monitored with sheathed thermocouples dipped in the leach slurry. Oxygen was supplied from compressed gas cylinders through a two-stage regulator.
Procedures
Three oxidative-leaching procedures were tested as methods to separate cadmium from tellurium in PV scrap. These procedures were chosen with the expectation that each could yield saleable cadmium and tellurium products, and that each would not produce potentially hazardous byproducts. The leach procedures were ferric chloride leaching, hydrogen peroxide leaching, and oxygen-pressure leaching.
Ferric Chloride Leaching
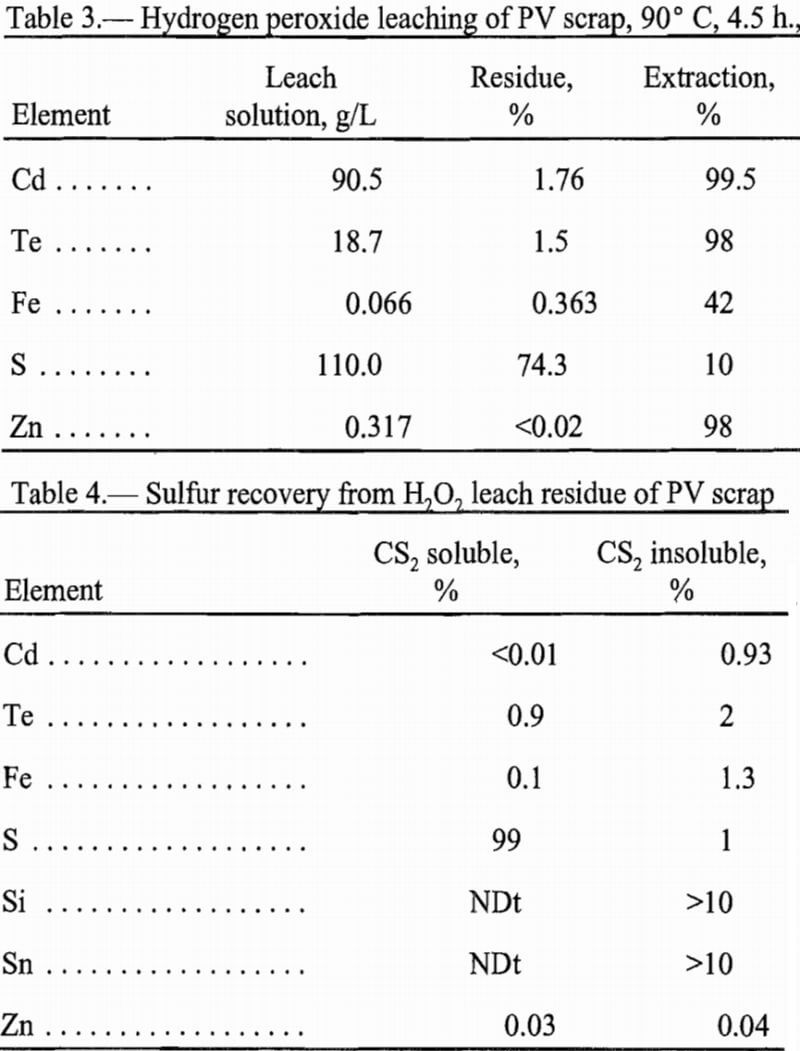
The FeCl3 leach solutions containing 2.5M FeCl3 and 0.2N HCl were prepared individually immediately before each test using anhydrous FeCl3. Solutions were filtered before use to remove any Fe2O3 contaminant that was present in the FeCl3. This filtration had the beneficial effect of reducing contamination of the tellurium product. Leaches were conducted on stiirer-hotplates in beakers covered with watch glasses. Solutions were heated to 80° C before use. Sufficient scrap then was added to provide 112 g Cd for each liter of leach solution. The heat of reaction was enough to raise the temperature to 105° C. After leaching for up to 5 h, the slurry was filtered.
Hydrogen Peroxide Leaching
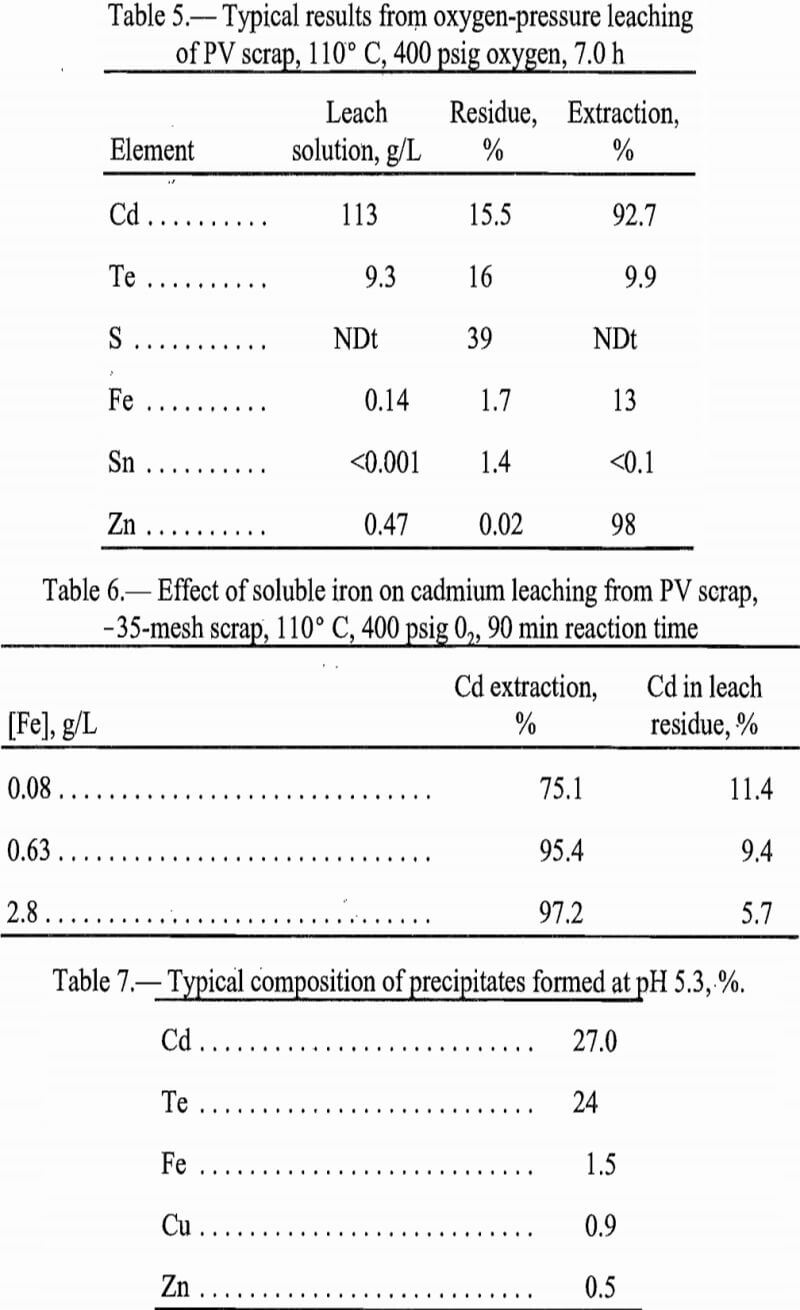
For H2O2 leaching, crushed scrap was slurried with 2.2N HCl or H2SO4. Sufficient scrap then was added to provide 112 g Cd for each liter of leach solution. Aqueous H2O2 (30% solution) was added from a burette over a period of up to 2 h. The heat of reaction provided enough heat to raise the temperature to 100° C. The slurry was allowed 0.5 h further to complete leaching. The slurry then was filtered. The solids were washed with water and dried in air.
Oxygen-Pressure Leaching
For oxygen-pressure leaching, scrap and 2.2N H2SO4 were mixed in a 300-mL, magnetically coupled, stirred autoclave. Sufficient scrap was used to provide 112 g Cd for each liter of leach solution. The vessel was closed, and the air was displaced with oxygen. The outlet valve then was closed, and the vessel was pressurized to the desired oxygen overpressure, the autoclave then was heated to 110° C. Leaching with vigorous agitation extended for as long as 7 h after the slurry reached target temperature, temperature, was monitored with a sheathed thermocouple dipped in the solution. Following leaching, agitation was stopped immediately and the vessel was cooled in air within about 15 min. The slurry then was filtered. The solids were washed with approximately 50 mL of deionized water and air dried. Wash water was combined with leach liquor for analyses.
CdTe Leach Kinetics Tests
The wafer scrap was crushed, blended, and sized before testing. Most leach tests were done with -200-mesh material to assure adequate suspension in the leach slurry. Some material was sized into narrow fractions to allow calculation, of surface area.
Analyses
Solutions were analyzed directly by ICP for cadmium and transition metals, and by AAS for tellurium. Solids were analyzed directly by XRD and XRF. The solids also were fused and digested for analysis by AAS and ICP. Solids were analyzed for sulfur content using wet-chemical techniques.
Results
Leaching Mixed CdTe-CdS PV Scrap
Ferric Chloride Leaching
The USBM has shown the effectiveness of ferric chloride leaching to solubilize metals from sulfide ores. The reaction selectively oxidizes the metal and leaves sulfur in the elemental state. The proposed reactions for leaching of PV scrap with FeCl3 are:
CdTe(s)+2FeCl3(aq)=CdCl2(aq)+Te(s)+2FeCl2(aq)…………………………………………………(1)
CdS(s)+2FeCl3(aq)=CdCl2(aq)+S(s)+2FeCl2(aq)…………………………………………………….(2)
Ferric chloride leaching yielded a cadmium-bearing solution and a solid product containing tellurium and sulfur as reported in table 2. Tellurium was not detected as a separate phase by XRD in the solid product. Sulfur and tellurium form a solid solution when the mixture contains predominantly sulfur. No tellurium oxides or cadmium-containing phases were detected.
Solution Purification
The data in table 2 show partial dissolution of tellurium. This solubilized tellurium cemented readily onto powdered iron, yielding elemental tellurium containing approximately 2% each of Cd and Fe. This loss of cadmium amounted to 0.5% of the cadmium in solution. Optimization of this cementation step probably could improve the selectivity. With proper control of the pH and the reduction potential, coprecipitation of the cadmium likely could be avoided. The resulting solution contained high levels of cadmium and iron chlorides free of tellurium.
Cadmium Recovery
A serious problem was encountered in recovering cadmium from the chloride leach solution. For the leach scheme to be feasible on a commercial scale, the cadmium recovery step should regenerate the FeCl3 lixiviant. During leaching, the ferric chloride was reduced to Fe+², as depicted by equation 1. Precipitation of the iron as FeOOH appeared to be a logical method to separate Cd+² from Fe+². The FeCl3 solution could be regenerated by dissolving the FeOOH precipitate in HCl. However, oxidation of the iron with air at approximately pH 2.6 proved very slow and incomplete. After 42 h of oxidation, less than 50% of the iron was precipitated. Regeneration of the FeCl3 lixiviant by digesting the FeOOH in HCl proved unsatisfactory because dissolution of the FeOOH was slow and incomplete. Because no satisfactory separation of iron from cadmium was found, no cadmium product was made using this leaching technique. Leaching using FeCl3 was discontinued.
Hydrogen Peroxide Leaching in H2SO4
The early leaching on the wafer scrap showed that H2O2 would oxidize both the cadmium and the tellurium. The leaching reactions may be represented as:
CdTe+H2O2+H2SO4=CdSO4+2H2O+Te……………………………………………………………………(3)
Te+2H2O2+2H2SO4=4H2O+Te+4+2SO4-²……………………………………………………………….(4)
CdS+H2O2+H2SO4=CdSO4+2H2O+S……………………………………………………………………….(5)
Leaching the PV scrap with H2O2 is advantageous in separating tellurium from sulfur. Leaching extracted the tellurium and cadmium to leave sulfur as the residue. Extraction results are reported in table 3.
This leach procedure appeared promising as a means of separating cadmium and tellurium from sulfur in the scrap. Sulfur was separated from the glass and tin in leach residue with CS2. The CS2 then was distilled off to leave elemental sulfur as a purified product. Analyses of the products thus separated are tabulated in table 4.
This leach procedure yielded a solution that was compatible with solution treatments described later in this report. This leach procedure combined with solution treatment, however, was poor at separating cadmium from tellurium. As a result, little further effort was expended in studying this leach procedure further.
Oxygen-Pressure Leaching in H2SO4
In this procedure, gaseous oxygen oxidizes both CdTe and CdS. The cadmium dissolution reactions are:
CdTe+½O2+H2SO4(aq)=CdSO4(aq)+Te+H2O…………………………………………………….(6)
CdS+½O2+H2SO4(aq)=CdSO4(aq)+S+H2O………………………………………………………..(7)
The cadmium is solubilized in an acidic solution and removed. Elemental tellurium is produced during leaching of cadmium telluride just as elemental sulfur is produced from the leaching of cadmium sulfide. Tellurium in the elemental form remains as a solid in the leach residue. Conditions for the production of elemental tellurium can be found from the Pourbaix diagram shown in figure 1. Elemental tellurium is thermodynamically stable in neutral to acidic aqueous solutions under reducing conditions.
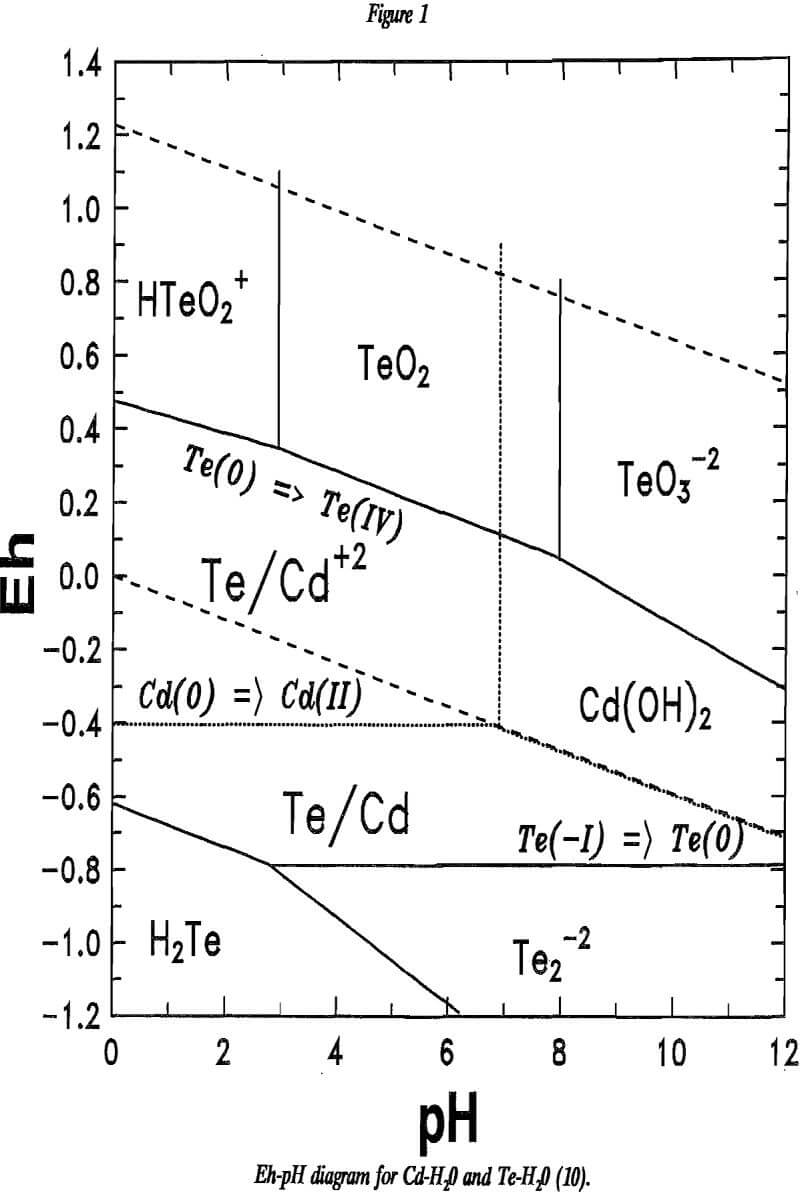
High cadmium extraction was obtained at 110° C and 400 psig O2. The PV scrap was not crushed during this portion of the research because the material dispersed in the leach solution with minimal effort. Typical leaching results are reported in table 5.
Leaching Kinetics of CdTe
To better understand leaching of CdTe from the scrap, the kinetics of CdTe dissolution were examined using synthetic, single-crystal CdTe. Composition of this material is reported in table 1.
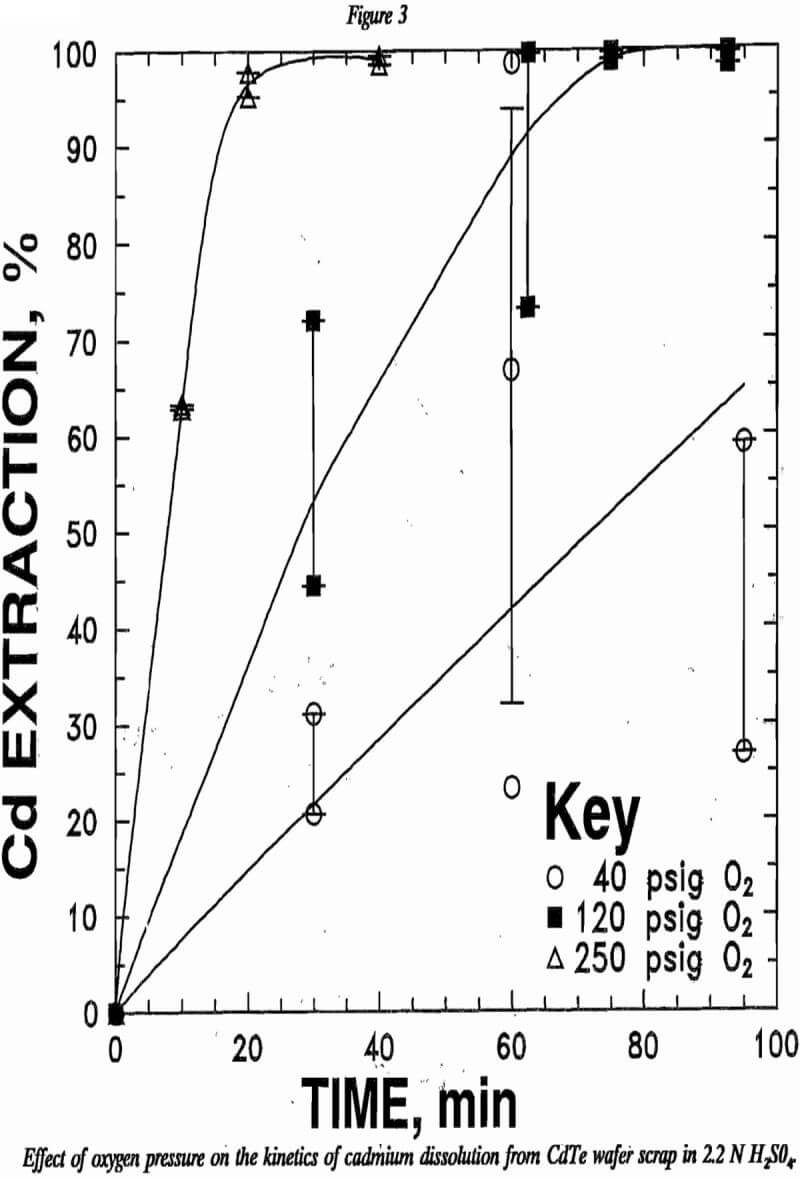
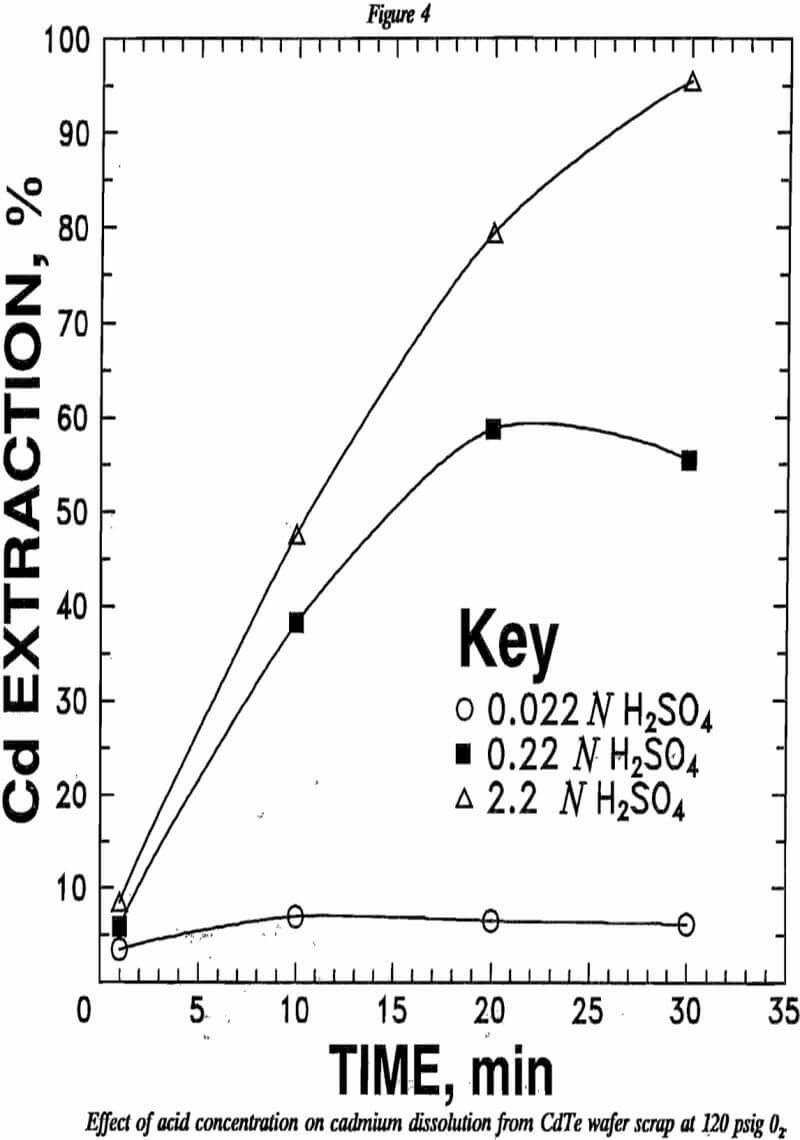
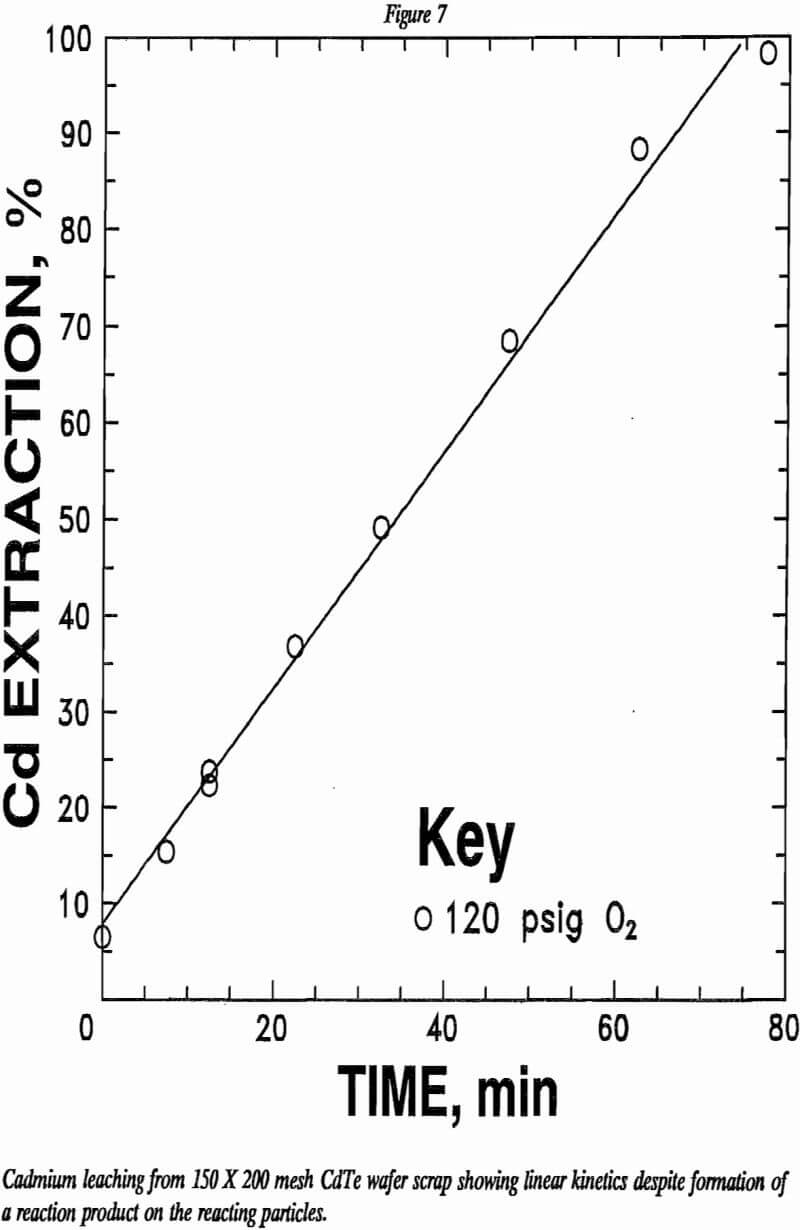
Equation 6 suggests that oxygen concentration, acid concentration, and CdTe surface area may influence the kinetics of CdTe leaching. Increasing the oxygen pressure increased the cadmium leaching rate as indicated by figure 3. Increasing acid concentration likewise increased the cadmium leaching rate as shown in figure 4.
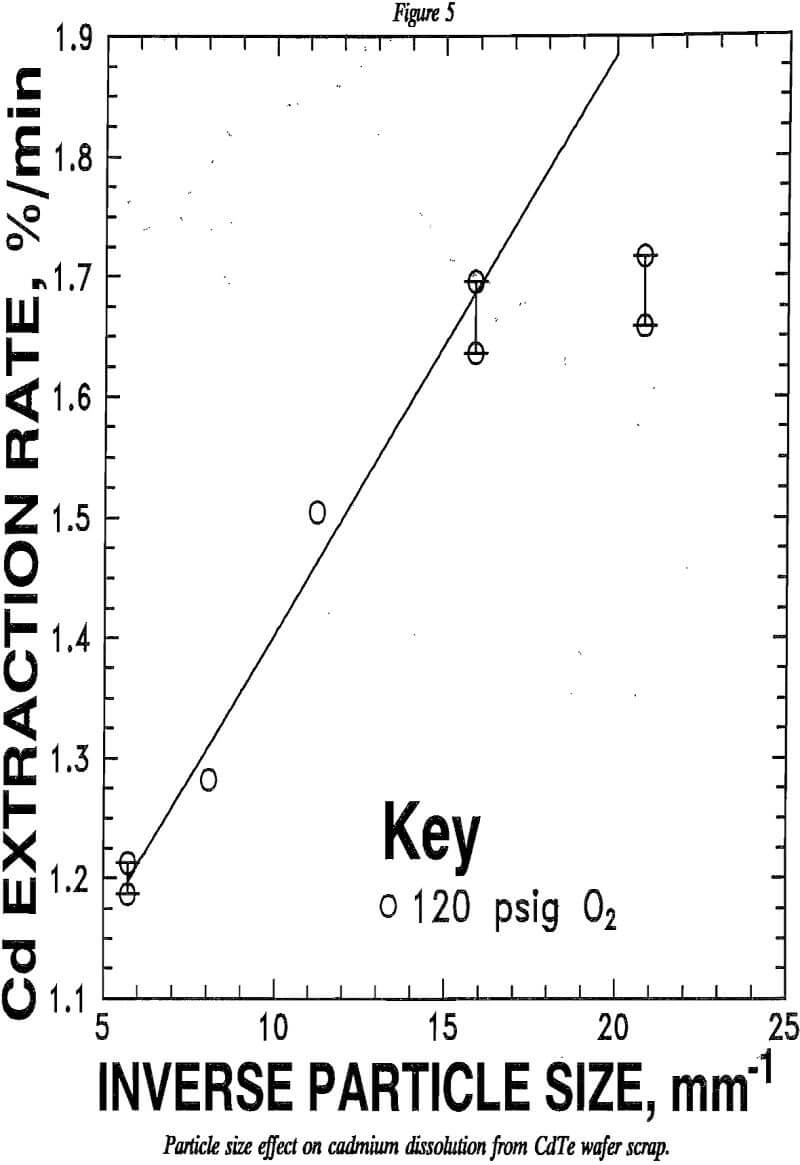
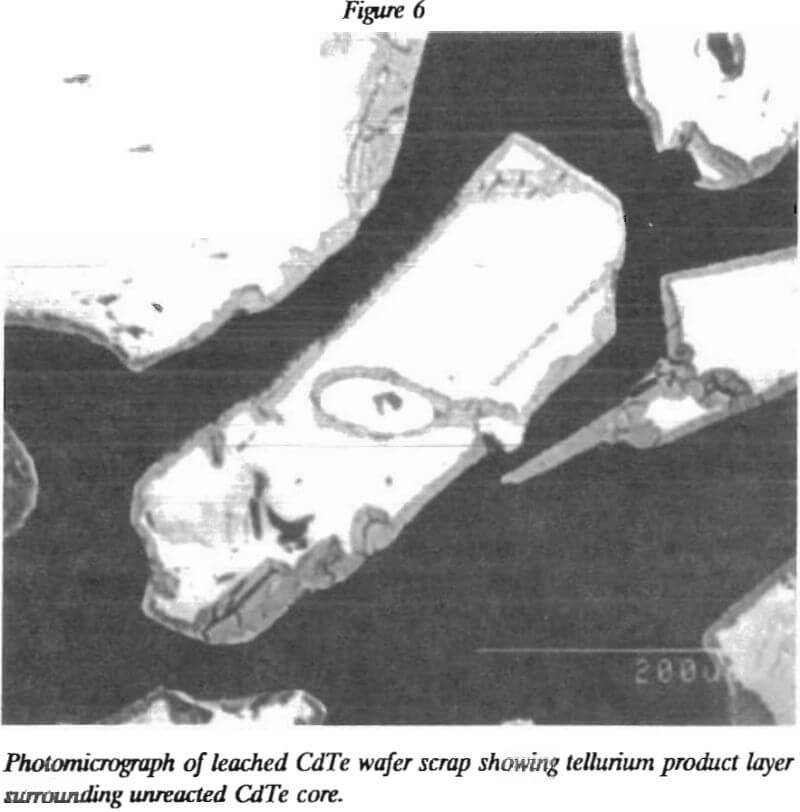
As with many heterogenous reactions, cadmium leaching from CdTe is a function of particle size, as shown in figure 5. Commonly, the initial leach rate is inversely proportional to the particle size. In such a case, the plot should follow the line drawn in figure 5. The curvature in the experimental data plotted in this figure indicates that the leach reaction is unusual. A layer of tellurium accumulates on the unreacted CdTe particles as the leaching progresses. This layer of elemental tellurium is shown.by the photomicrograph in figure 6.
Cadmium dissolution as a function of time in 2.2N H2SO4 is depicted in figure 7. Cadmium extraction appears to increase linearly with time. One normally would expect the leaching rate to slow down as the CdTe is consumed. Possibly, the CdTe particles are cracked enough to maintain a constant surface layer exposed to the leach solution as the reaction . progresses. However, the data are insufficient to explain these results at this time.
Leaching Kinetics of Mixed CdTe and CdS in PV Scrap
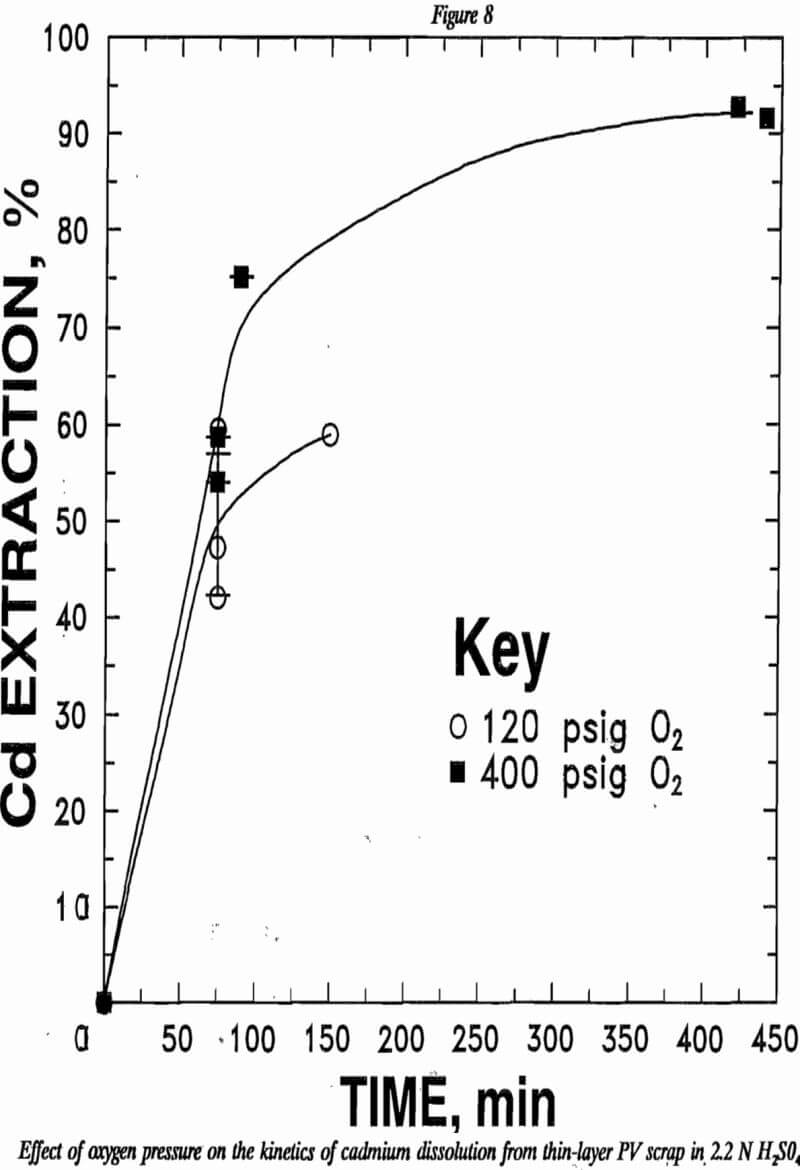
Oxygen-pressure leaching was less rapid with the PV scrap than with wafer scrap. The difficulty was solubilizing cadmium from the CdS phase. The refractory nature of the sulfide required higher oxygen overpressure to improve leaching. The data plotted in figure 8 show that higher oxygen pressure improved the rate of cadmium leaching. While cadmium extraction improved with increased oxygen pressure, oxygen-pressure leaching could not match the extractions obtained with ferric chloride or hydrogen peroxide even at the highest pressures used. The highest cadmium extraction was 92.7% after 7 h of leaching under 400 psig O2. The leach residue contained 15.5% Cd. Leaching with ferric chloride or hydrogen peroxide yielded over 99% cadmium extraction in shorter times.
In an attempt to increase cadmium extraction from PV scrap by oxygen-pressure leaching, the scrap was pulverized to 50% less than 200 mesh. The pulverized scrap then was leached for 90 min at 110 ° C at 400 psig in 2 2N H2SO4. Average extraction for these tests was 93.8% Cd compared with 92.7% Cd extraction for -35-mesh material in a comparable test reported in table 5. Cadmium content of the finer residue was 12.7% compared with 15.5% reported above.
Leaching Catalyzed by Soluble Iron
As a result of inadequate leaching of the PV scrap with oxygen alone, variations of oxygen-pressure leaching were attempted. It is known that soluble iron salts may catalyze oxidation of minerals. The leaching reactions are:
CdTe+2Fe+³=Cd+²+2Fe+²+Te………………………………………………………………………(8)
CdS+2Fe+³=Cd+²+2Fe+²+S………………………………………………………………………….(9)
4Fe+²+O2+4H+=4Fe+³+2H2O…………………………………………………………………….(10)
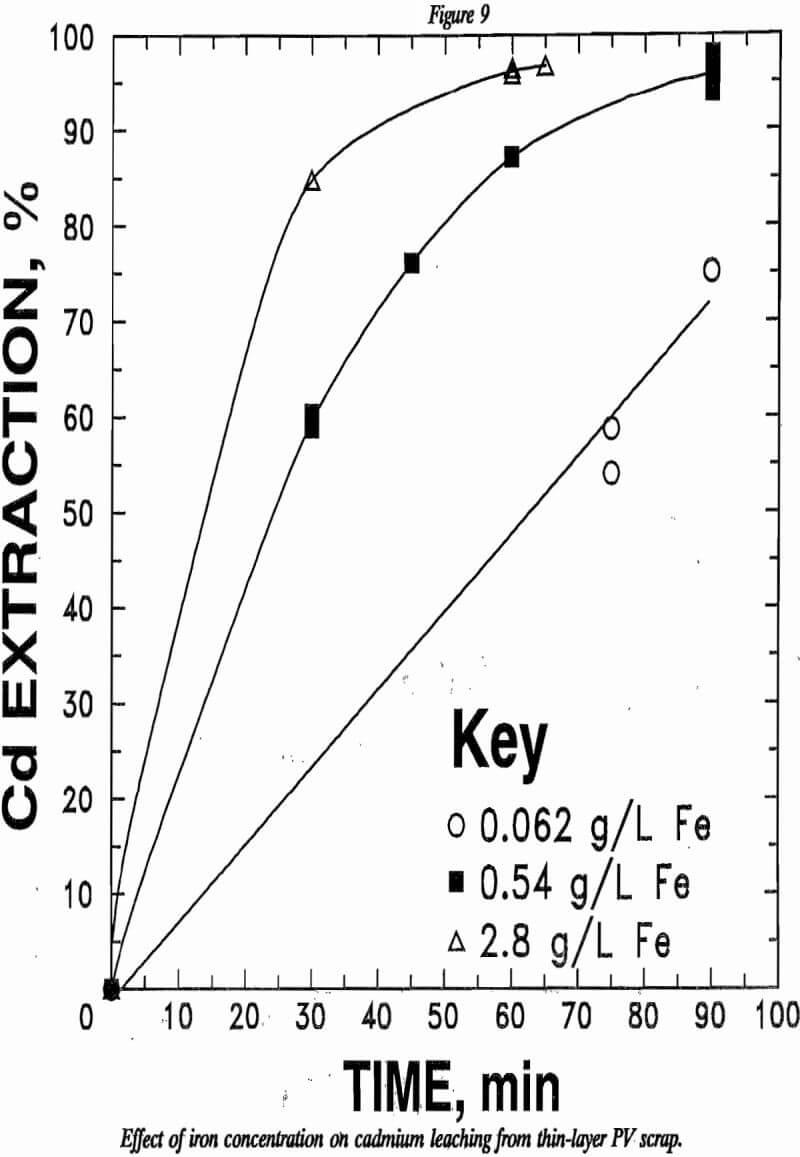
Iron was added as Fe2(SO4)3·4H2O, Fe(NH4)2(SO4)2·6H2O, or as iron that coprecipitated with tellurium during purification of the leach solution as described in the next section of the paper. Results of oxygen-pressure leaching catalyzed with iron are reported in table 6 and plotted in figure 9. Higher iron concentrations yielded faster and more complete cadmium extractions. Leach residues typically contained between 5 and 10% Cd. XRD showed the solids to contain CdS, elemental sulfur, and elemental tellurium. However, it appears unlikely that oxygen-pressure leaching could match the cadmium extractions obtained by the other two procedures.
Product Recovery
Oxygen-pressure leaching appeared the most promising method for separating cadmium and tellurium from PV scrap. Methods for producing saleable cadmium and tellurium products following this leach procedure were investigated. Hydrogen peroxide leaching yielded solutions of similar composition which likely could be treated by methods describe in this section of the paper.
Precipitating Tellurium from Leach Solution by pH Adjustment
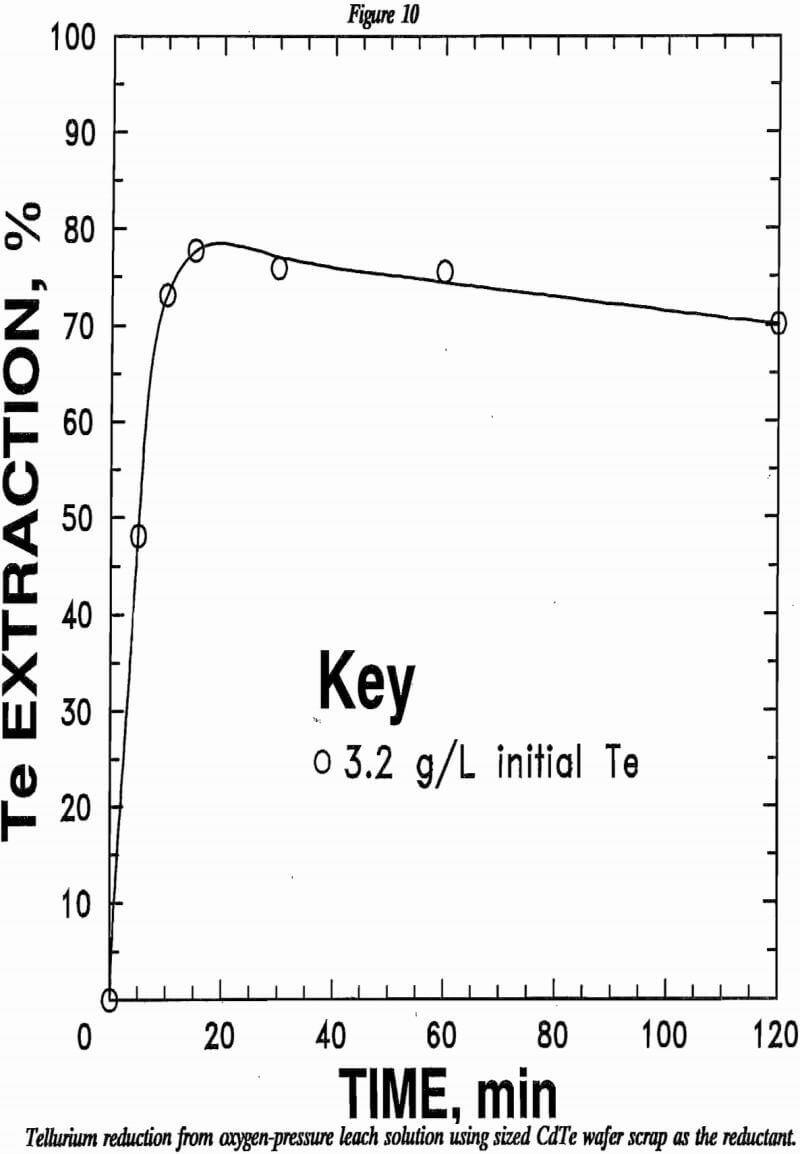
While oxygen-pressure leaching resulted in preferential dissolution of cadmium, codissolved tellurium needed to be removed from solution before purified cadmium could be recovered. One method to remove the tellurium is reduction using CdTe. The reaction resembles the Claus reaction for sulfur species:
Te+4(aq) + 2 CdTe = 3 Te(s) + 2 Cd+²………………………………………………………………(11)
The effectiveness in using CdTe to reduce tellurium from solution was tested using CdTe wafer scrap. Results are shown in figure 10. A leach solution containing 3.2 g/L Te was mixed with four times the stoichiometric requirement of 35×65 mesh CdTe wafer scrap ad allowed to react in a covered beaker at 90° C. Approximately 80% of the tellurium was reduced and precipitated from solution within 15 min.
Alternatively, tellurium may be precipitated as a hydrated oxide. As shown in figure 2, Te(IV) reaches minimum solubility in mildly acidic solutions, while Cd(II) reaches minimum solubility in basic solutions. Tellurium was removed from solution by raising the pH to approximately 5.3 using aqueous NaOH. The concentration of tellurium remaining in solution was less than 0.05 g/L above pH 5.3. The precipitation may be represented as:
Te+4+4NaOH(aq)=TeO2(s)+2H2O+4Na+…………………………………………….(12)
The solid settled readily from solution. Filtration was adequately rapid. Typical composition of the precipitate is reported in table 7.
Separation of soluble cadmium from soluble tellurium through pH adjustment was not as selective as might be predicted from figure 2. The ratio of cadmium to tellurium appeared to change continuously with changing pH. XRD analyses showed the precipitate to be amorphous. For these reasons it was believed that the precipitate was not a specific compound of cadmium and tellurium, but rather a mixture of tellurium and cadmium oxides or basic salts.
The precipitate was added to a subsequent leach to redissolve the cadmium and reduce the oxidized tellurium according to reaction 13:
TeO2+2CdTe+2H2SO4=3Te+2CdSO4+ 2H2O…………………………………………………….(13)
The tellurium-containing leach residue also contained glass, sulfur, and other foreign matter from the scrap. However, standard methods of oxidative leaching followed by selective reduction of tellurium should allow for separation of the tellurium from the sulfur and inert materials.
Cadmium Recovery
Cadmium was recovered most conveniently by evaporating the cadmium sulfate solution. This method avoids creation of a liquid effluent. The high cadmium concentration in the leach solutions, the low volume of solutions, and the environmental consequences of releasing cadmium-contaminated liquids all influenced this processing choice. The evaporite was a crystalline solid of hydrated CdSO4 typically containing less than 1 % Zn and less than 0.05% Te. The solid could be shipped to a cadmium refinery for electrowinning. Samples of this product were sent to a cadmium producer for analysis.
Alternatives to evaporation include precipitating the cadmium as a carbonate and cementing the cadmium with zinc. Sodium carbonate precipitated the cadmium as CdCO3 between pH 9 and 10, which was filtered from solution. Following carbonate treatment, the solutions typically contained 3 mg/L Cd.
Cementation of the cadmium was impeded by the high cadmium concentrations. The cadmium tended to cement into a porous mass of metal rather than remaining a dispersed powder. The cadmium metal also oxidized readily. Effluent solutions contained less than 10 mg/L Cd. Carbonation of the solution would remove the zinc before disposal could be considered.
Purities of these three cadmium products were similar. Tellurium content was below detection limits (<0.05%). The dried sulfate contained all of the alkali metal salt used to control pH. However, the carbonate and cemented metal also contained entrained sodium sulfate. All of the cadmium products contained approximately 1% Zn and Cu.
Summary
Several leach procedures were demonstrated for reclaiming cadmium and tellurium from PV scrap. Oxygen-pressure leaching appeared advantageous for treating PV scrap because the cadmium sulfate and tellurium products are commonly traded and can be purified readily by available technology. The sulfuric acid leaching medium is commonly used commercially for purifying both cadmium and tellurium; thus, this reagent would not contaminate downstream processing steps. These advantages are balanced against the requirement of leaching under oxygen pressure in an autoclave.
Oxygen-pressure leaching at 110° C under 400 psig O2 in 2.2N H2SO4 readily solubilized cadmium from the CdTe phase. Dissolution of cadmium from the CdS phase, however, was incomplete. The addition of soluble iron to the leach solution increased the rate and the extent of cadmium dissolution. With 2.8 g/L Fe in solution, cadmium extraction reached 97% in 90 min. The bulk of the tellurium and sulfur remained in an elemental form in the leach residue. The solution was purified of solubilized tellurium by raising the pH to 5.3. The remaining solution was evaporated to recover crystalline cadmium sulfate. Alternatively, the cadmium was precipitated as CdCO3 or as cemented cadmium metal. This work has shown that CdTe scrap can be treated readily with standard hydrometallurgical methods to produce marketable products.
It is anticipated that technology developed for treating CdTe would prove effective in treating CdS-bearing scrap from the manufacturing of CuInSe2 photovoltaic devices.
Considerations for Future Scrap
If SERI projections are realized, much of the growth in CdTe usage will be in thin-layer PV devices rather than wafers. The thin-layer devices are composite materials containing thin layers of CdTe and CdS on a glass substrate. The objective in treating this composite scrap would be to solubilize totally the cadmium and tellurium to prevent many tons of glass scrap from being classified as hazardous. The leaching data collected in this investigation show that cadmium reached as much as 100 g/L in solution and tellurium reached as much as 5 g/L. SERI projects CdTe coatings containing at most 21 g CdTe/m² glass. Assuming glass panels with a thickness of 3 mm and a density of 2.65 g/cc, the PV panels would contain 0.3% CdTe. Leaching the crushed panels at 30% pulp density would produce a solution containing approximately 0.5 g/L each of cadmium and tellurium. These are much lower than concentrations observed routinely in pressure leaching of the PV scrap. Thus, oxygen-pressure leaching should adequately remove both cadmium and tellurium from the glass substrate. Leaching and subsequent processing should yield saleable tellurium and cadmium, and additionally yield clean, pulverized glass that would not be classified as a hazardous waste.
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
