Table of Contents
- Water Disaggregation
- Hydrothermal Treatment
- Acid Leaching
- Acid Pug-Water Leach
- Alkaline Roast-Water Leach
- Sulfate Roast-Water Leach
- Chloride Roast-Water Leach
- Multiple-Reagent Roast-Water Leach
- Sulfur Dioxide Atmosphere Roast-Water Leach
- Chlorinating Roast With Hydrochloric Acid
- Limestone-Gypsum Roast-Water Leach
- Hydrochloric Acid Chlorination Roast-Water Leach Process
- Bench-Scale Testing
- Large-Scale Testing
- Process Evaluation and Discussion
- Limestone-Gypsum Roast-Water Leach Process
- Batch Roast Tests
- Raw Materials Test Procedures and Equipment
- Exploratory Studies To Develop Basic Data
- Determination of Preroast Mixture
- Charge Preparation Methods
- Determination of Reaction Mechanism
- Muffle Furnace Tests to Determine Optimum Roast Conditions
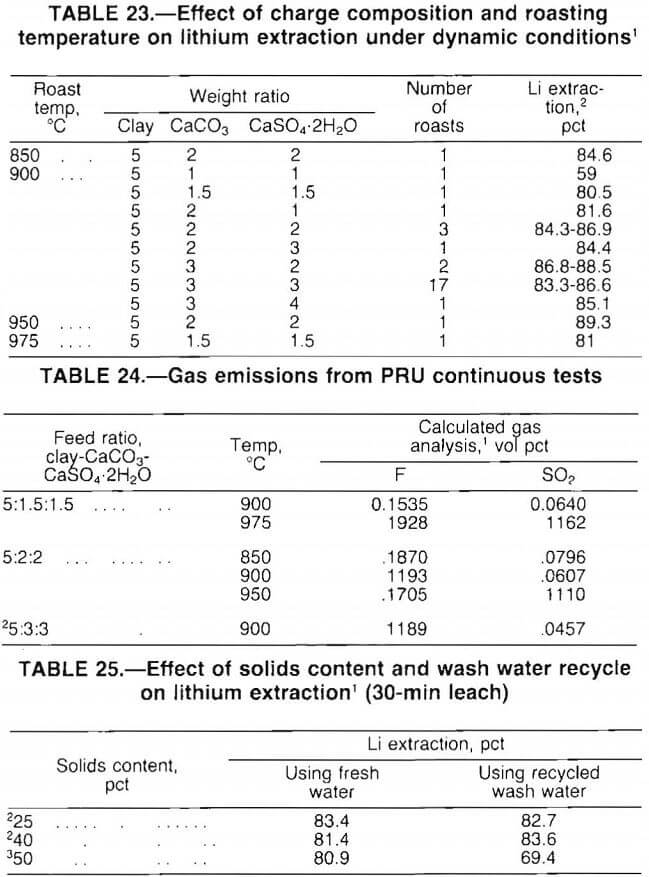
- Effect of Charge Composition and Roast Temperature on Lithium Extraction
- Effect of Roast Time and Temperature on Lithium Extraction
- Effect of Additive Type on Lithium Extraction
- Tube Furnace Tests To Investigate Roasting Atmosphere
- Process Research Unit Studies
- Process Description
- Materials
- PRU Equipment
- Operating Conditions and Test Results
- Feed Preparation
- Roasting
- Leaching
- Solids Content and Wash Water Recycle
- Calcine Particle Size and Leach Time
- Evaporation
- Product Precipitation
- Crystallization
- Overall Lithium Recovery
- Process Description
- Economics
Preliminary Lithium Extraction Tests: In the preliminary McDermitt clay studies, several lithium extraction methods were investigated. Water disaggregation, hydrothermal treatment, acid leach, alkaline roast-water leach, sulfate roast-water leach, and chloride roast-water leach were techniques used in the treatment of both McDermitt A and B clays. Only the higher grade McDermitt B clay was treated by the following methods: (1) multiple-reagent roast-water leach, (2) sulfur dioxide (SO2) atmosphere roast-water leach, (3) HCl roast-water leach, and (4) limestone- gypsum roast-water leach.
Water Disaggregation
Samples of both McDermitt clays were slurried with water to determine if the clay would disaggregate and form a lithium-rich fraction, and to determine the amount of water-soluble lithium in the clay. Clay samples of 25 g were slurried with 225 mL of water and agitated for 1 h. The slurry prepared with McDermitt A showed slight gelation; the McDermitt B slurry did not form a gel. The slurries were screened; analyses of the products showed that the clays did not disaggregate to form a lithium-rich fraction and that less than 1 pct of the lithium in each sample was soluble in water.
Hydrothermal Treatment
Hydrothermal tests were conducted to determine if lithium in the clay could be solubilized under relatively mild conditions of heat and pressure. In each test, 40 g of clay was mixed with 360 mL of water and heated to 200° C in an autoclave for 1 h; the steam pressure was 225 psig. Analyses of the solids and solutions showed that less than 1 pct Li was solubilized by this treatment.
Acid Leaching
Both types of McDermitt clay were leached with H2SO4 solution at pH 1. Clay samples of 25 g were added to 225 mL of pH 1 acid, and the resulting slurry was stirred for 3 h; acid was added every 10 min to maintain pH 1.0. The amount of acid required to achieve the pH 1.0 acidity was designated the standard acid addition. The clays were also leached with multiples of the standard acid addition.
Test results, presented in table 6, show that lithium was extracted from McDermitt A clay by direct H2SO4 leaching; however, the acid requirement was excessive. For example, 1,125 lb H2SO4 per short ton clay was required for 89-pct extraction. In contrast, about 2 pct of the lithium was extracted from McDermitt B clay using 1,178 lb H2SO4 per short ton clay.
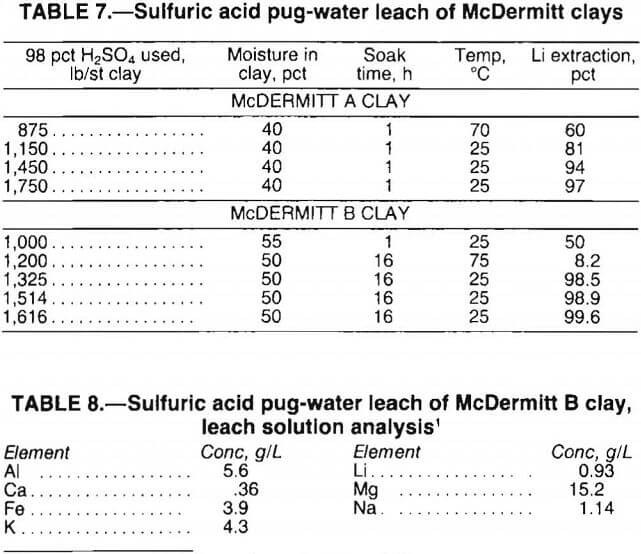
Acid Pug-Water Leach
Sulfuric acid pug-water leach tests were conducted on McDermitt A and B clays to determine the effect on lithium extraction. Finely ground clay samples were well mixed to achieve 40 to 50 pct moisture; 98 pct H2SO4 was added to make a thick paste. After standing 1 h, the McDermitt A paste was water-leached; McDermitt B paste was soaked overnight prior to leaching. Leaching was conducted at about 15 pct solids and ambient temperature for 1 h.
Test results, presented in table 7, show that lithium extraction for McDermitt A clay was slightly lower with the acid pug procedure than with the acid leach. For example, the acid pug method resulted in 81-pct Li extraction with 1,150 lb H2SO4 per short ton clay, whereas leaching with 1,125 lb H2S04 per short ton clay extracted 89 pct of the lithium. Acid pugging of the McDermitt B clay dramatically improved lithium extraction over the diluted acid leach. For example, acid pugging with 1,325 lb H2SO4 per short ton clay extracted 98.5 pct of the lithium; leaching with 1,413 lb H2SO4 per short ton clay yielded 2.6-pct Li extraction.
Lithium was extracted from both types of McDermitt clay by the acid pug-water leach procedure. However, the procedure would not be commercially feasible because (1) the amount of H2SO4 required was excessive, and (2) the leach solution was grossly contaminated with magnesium, potassium, aluminum, and iron (table 8). In addition, leach slurries filtered slowly and were difficult to wash.

Alkaline Roast-Water Leach
The McDermitt clays were roasted with carbonates of sodium, potassium, and calcium, and hydroxides of sodium and potassium; the roasted calcines were leached with water. These tests were conducted to determine if the lithium in the clay could be converted to a water-soluble form by the alkaline roasting reactions.
Finely ground clay samples of 100 g were mixed with 20 to 40 g of potassium carbonate (K2CO3), sodium carbonate (Na2CO3), or calcium carbonate (CaCO3); the mixtures were roasted at 1,000° C for 4 h. Less than 10 pct of the lithium in the clay volatilized during these roasts. Water leaching the finely ground calcines resulted in relatively low lithium extractions (less than 45 pct in all cases).
In the hydroxide roasts, 100-g samples of clay were mixed with 20 to 30 g of sodium hydroxide (NaOH) or potassium hydroxide (KOH). Roasting with NaOH at 1,000° C produced a fused mass that attacked the roasting crucible. Over 72 pct of the lithium in both clays volatilized as the oxide during this roast. Roasting with either NaOH or KOH at 500° C volatilized 5 to 8 pct of the lithium from McDermitt B calcine; no lithium was volatilized from the McDermitt A calcine. Water leaching these calcines extracted only 1 or 2 pct of the contained lithium.
Sulfate Roast-Water Leach
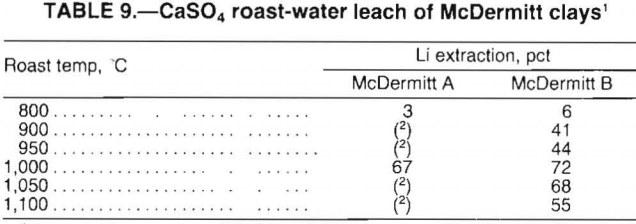
McDermitt A and B clays were roasted with calcium sulfate (CaSO4), sodium sulfate (Na2SO4), and potassium sulfate (K2SO4) to determine if water-soluble Li2SO4 could be formed. The results of the initial roasting tests with 54 g Na2SO4 or K2SO4 per 100 g clay showed that less than 50 pct of the lithium was extracted by water leaching the calcines. Results of CaSO4 testing, presented in table 9, show that roasting at 1,000° C resulted in a 67-pct Li extraction from McDermitt A clay and a 72-pct Li extraction from McDermitt B clay. The total lithium extracted includes lithium in the leach solution as well as a small amount of lithium volatilized.
Chloride Roast-Water Leach
A chloride roast-water leach process has been reported by Peterson and Gloss for extracting lithium from spodumene ores. In this process, 347 parts of potassium chloride (KCl) was roasted with 100 parts of spodumene containing 3.58 pct Li. The ratio of KCl to lithium in the ore was 89:1. When chloride roast-water leach techniques were investigated for extracting lithium from McDermitt B, the same weight ratio was used. This weight ratio was 57 g of KCl to 100 g of the clay. The same amount of KCl was used in the roasting tests with McDermitt A; in these tests, the weight ratio of KCl to lithium in the clay was 158:1.
The mixtures of clay and KCl were roasted at 1,000° C for 4 h. The calcines produced were leached with water to extract water-soluble LiCl. For McDermitt A, 27 pct of the lithium was volatilized during roasting, but no lithium was extracted from the calcine by water leaching; the total lithium extraction was 27 pct. For McDermitt B, 39 pct of the lithium was volatilized during roasting; 19 pct of the lithium was recovered by water leaching the calcine. The total lithium extraction was 58 pct.
Multiple-Reagent Roast-Water Leach
The purpose of multiple-reagent roasts was to achieve greater lithium extraction by combining the effects of chloride roasting with those obtained by a roast-water leach treatment. The reagent combinations tested were KCl plus CaCO3 and KCl plus CaSO4. The tests were conducted with McDermitt B clay only. Roasting temperature and reagent additions were studied. Roasting time was 4 h, and the finely ground calcines were leached in water at 10 pct solids. Test results are shown in tables 10 and 11.
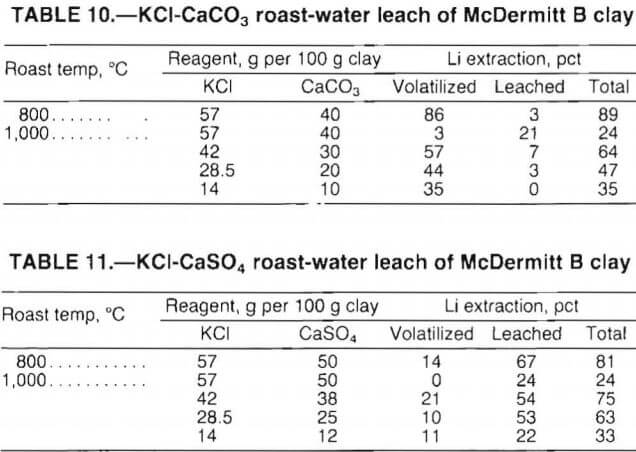
Best lithium extraction by the KCl-CaCO3 roast- water leach procedure was 89 pct. Water leaching recovered 3 pct of the lithium; 86 pct of the lithium was volatilized and not recovered. The highest extraction obtained with the KCl-CaSO4 procedure was 81 pct; 67 pct of the lithium was recovered in the leach, and 14 pct of the lithium was volatilized.
Overall, the test data indicate that multiple-reagent roasting improved lithium recovery over roasting with a single reagent.
Sulfur Dioxide Atmosphere Roast-Water Leach
A series of roast tests was conducted in a 2-in-diam vertical tube furnace to determine if the lithium in McDermitt B clay could be sulfated directly using SO2 gas. Variables investigated included roast temperature, SO2 concentration, and SO2 usage. Calcines were water-leached to determine the effectiveness of each set of operating conditions. Test data indicated 700 C and 20 pct SO2 to be optimum. However, the most important variable was the amount of excess SO2 used. At optimum operating conditions, 903 lb SO2 per short ton clay was required for 85.8-pct Li extraction. Extraction decreased to 58 pct using 450 lb SO2 per short ton clay at similar operating conditions.
Overall, the test results showed that lithium can be extracted from McDermitt B clay by direct sulfation; however, an excessive quantity of SO2 was required. In addition, this roast procedure sulfated most of the magnesium and calcium in the clay, resulting in a highly contaminated leach solution.
Chlorinating Roast With Hydrochloric Acid
Extensive research was conducted to study -lithium extraction from McDermitt B clay by roasting in an HCl-H2O atmosphere. The research was prompted by several chloride roasting techniques described in the literature. For example, a method for chlorinating lithium by mixing lithium-bearing ore with carbon and roasting with chlorine was proposed by MacDougall. Another method involving the chlorination of lepidolite with HCl was investigated by Lof and Lewis, who found that lithium extraction increased with the chlorination temperature up to 950 C, whereupon the lepidolite began to melt and the reaction rate decreased rapidly. Above 900° C, a large part of the LiCl was recovered as a volatile product. Additions of water vapor or air to the HCl gas resulted in decreased lithium extraction.
Bureau research found that proper control of HCl-H2O ratios and roast conditions minimized the extraction of impurities from chlorinated calcines. Also, data showed that the formation of soluble lithium was greatly enhanced when the McDermitt clay was mixed with CaCO3 before chlorination. The research was reported by Davidson and May, and a patent was granted on the chlorination roast procedure. Chapter 4 summarizes the chlorination research; capital and operating costs are also included.
Limestone-Gypsum Roast-Water Leach
A limestone-gypsum roast-water leach process was studied in detail. The research was patterned after work conducted by Sternberg, Williams, and Hayes involving lime-gypsum roasting to recover lithium from spodumene. In this process, lime combined with silica to form a calcium silicate; the lithium in the ore reacted with the gypsum to form water-soluble Li2SO4.
Bureau research showed that lithium could be effectively recovered from McDermitt clay by roasting a mixture of clay, limestone, and gypsum, followed by water leaching. A series of purification steps was developed to recover lithium from the leach solution. This work was reported by Edlund and Lien. The research, as well as capital and operating costs, is summarized in chapter 5. A detailed cost evaluation for this lithium recovery technique is given in appendix B.
Hydrochloric Acid Chlorination Roast-Water Leach Process
Bureau researchers investigated two types of chlorination using HCl. Initially, anhydrous chlorination was studied to determine if HCl could be used to effectively extract lithium from McDermitt clay. Subsequently, selective chlorination using HCl-H2O mixtures was investigated to determine if the chlorination of major contaminating elements such as magnesium and calcium could be eliminated.
Bench-Scale Testing
Equipment and Procedure
Chlorination experiments were conducted in a 1-in- diam tube furnace using approximately 10-g samples contained in ceramic boats. The furnace tube was Vycor glass. A schematic of the furnace setup is given in figure 2. The setup consists of a chlorinating agent supply system, the reaction zone, and a scrubbing system for volatile
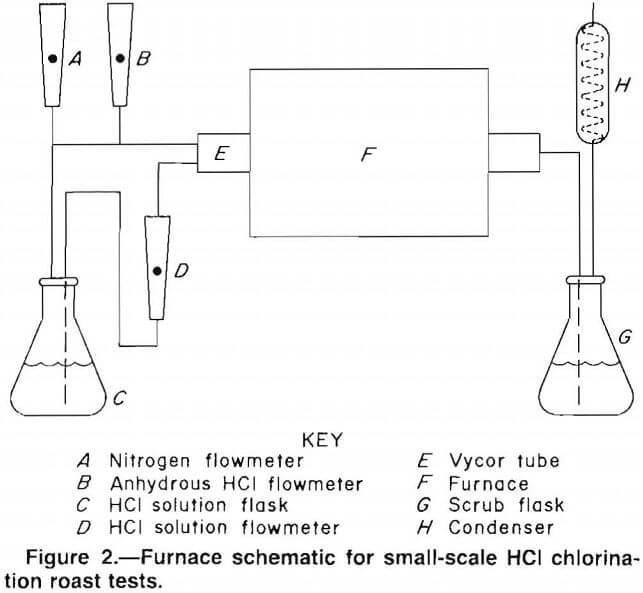
products. Anhydrous HCl and nitrogen (N2) were delivered to the furnace tube by metering through gas flowmeters. Mixtures of HCl-H2O were obtained by forcing an HCl solution from a flask into the furnace tube by pressuring the flask with N2. The HCl solution, which was measured by the flowmeter, flowed through a capillary tube directly into the furnace hot zone.
The furnace temperature was controlled by a Bristol on-off-type controller. A thermocouple was placed on the outside of the furnace tube directly over the sample. Any gaseous products flowed through a water scrubber and condenser system to collect the volatile products.
Samples were weighed into ceramic boats and placed in the furnace tube; then the system was purged with N2. The furnace was heated under N2 to 700° C and held for 15 min to dehydrate the clay and any other materials being reacted. After dehydration, the samples were brought to the desired reaction temperature and the chlorination was started. When the experiment was finished, the furnace was cooled under N2 atmosphere.
After chlorination, the calcined sample was water- leached using 50 mL of water at 80° C. The leach solution, residue, and any volatile condensate were analyzed. A mass balance, computed between the input and output materials, accounted for all elements within ± 5 pct.
Chlorination with Anhydrous HCl
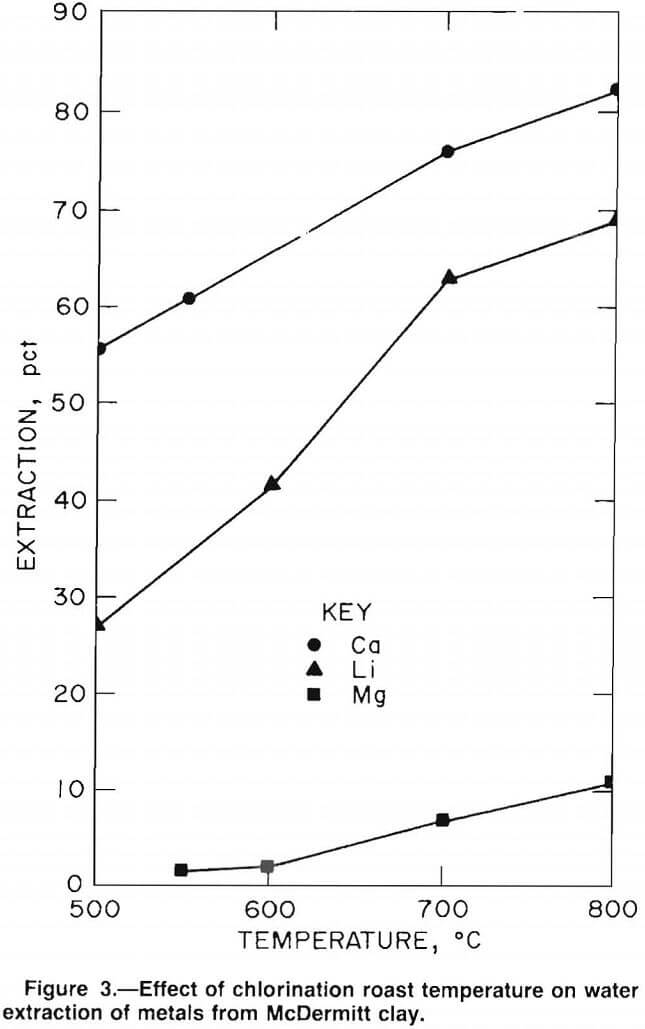
The general effects of chlorination on lithium extraction from clays were measured by chlorinating McDermitt B clay samples with anhydrous HCl. The effect of temperature on lithium, calcium, and magnesium extraction is shown in figure 3. Clay samples were chlorinated for 30 min using 70 cm³/min of anhydrous HCl. As the reaction temperature was increased, the extraction of all elements increased. However, the lithium and calcium extraction rates increased faster than did the magnesium extraction rate.
The lower extraction for magnesium, as compared with lithium, can be explained by the difference in the free
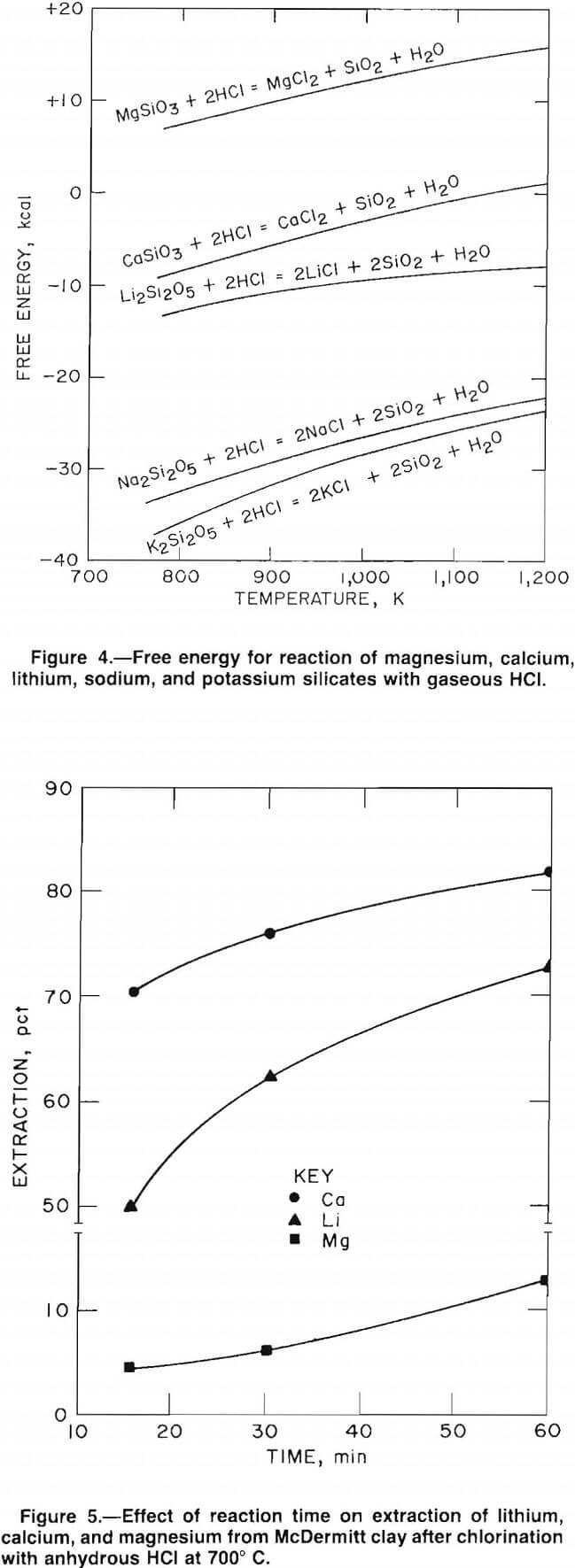
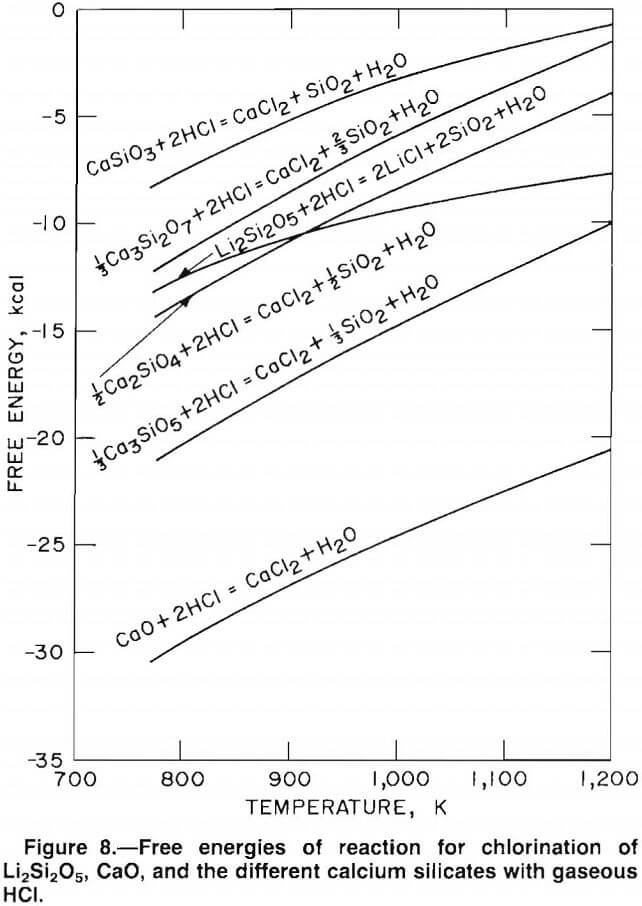
energies of chlorination. The Gibbs free-energy curves, plotted in figure 4, show that lithium, potassium, sodium, and calcium have negative free energies of chlorination. However, the free energy of chlorination for magnesium is positive; therefore, the extent of magnesium chlorination is relatively low.
The chlorination free-energy curves are based on the assumption that metallic ions are usually associated with silica in a clay material. Thus, chlorination of the clay should follow the general reaction 2M (silicate) + 2HCl = 2MCl + 2SiO2 + H2O where M is a metallic cation.
The effect of reaction time on lithium, calcium, and magnesium extraction is shown in figure 5. McDermitt clay was chlorinated at 700° C with 70 cm³/min anhydrous HCl; reaction times of 15, 30, and 60 min were investigated. Total extraction of each element increased with increased reaction time; however, the rate of extraction increased for magnesium while decreasing for lithium and calcium. Chlorination rates (as determined by extraction) may be estimated by the slope of each curve at any point. The increased extraction rate for magnesium was actually a function of the decreased rates of extraction for lithium and calcium, wherein more HCl was available to react with the magnesium as the lithium and calcium chlorination neared completion. Thus, as lithium extraction became complete, high magnesium extraction resulted. In addition, calcium extraction was greater than
lithium extraction, resulting in a grossly contaminated leach solution. Therefore, continued research concentrated on developing a chlorination technique that would be selective for lithium or, if necessary, all of the alkali metals.
Selective Chlorination With HCl-H2O Mixtures
The high magnesium content of the McDermitt clay and the relatively high percent extraction of calcium result in high consumption of HCl. Since HCl is an expensive reagent, selective chlorination of lithium in preference to the magnesium and calcium is desirable. The selective chlorination process is based on the greater stability of alkali metal chlorides versus other metal chlorides when they are exposed to an H2O-HCl atmosphere. The chlorination of a particular cation can be prevented by maintaining a water concentration in the chlorinating gas that is greater than the equilibrium concentration.
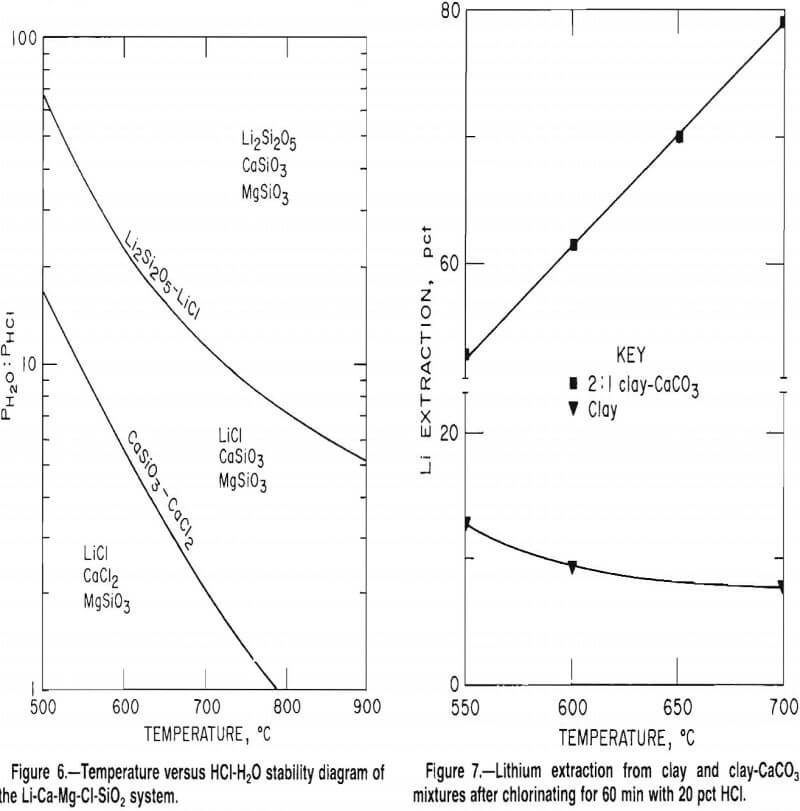
A temperature-composition phase diagram relating the equilibrium H2O-HCl ratio to the chloride-silicate phase boundary between 500° and 900° C is shown in figure 6. From this figure, the stable phase of each element at a given temperature and gas composition can be predicted. For example, to avoid the chlorination of magnesium silicate (MgSiO3) and calcium silicate (CaSiO3) when using an H2O-HCl mole ratio of 8:1 (20 wt pct HCl), the reaction temperature must be above 615° C. If the H2O-HCl mole ratio is decreased to 4:1 (33 wt pct HCl), the temperature must be increased to 700° C to avoid chlorination of MgSiO3 or CaSiO3. The trends predicted in figure 6 were investigated by chlorinating various silicate mixtures.
In the initial tests, finely ground CaSiO3, MgSiO3, and Li2SiO3 (lithium silicate) were mixed in equal weights and chlorinated. Test conditions were chosen to ensure lithium chlorination while preventing magnesium chlorination. The HCl concentrations used were 20 and 33 wt pct; the reaction temperature ranged from 550° to 850° C. Chlorinated samples were water-leached and analyzed for soluble chlorides.
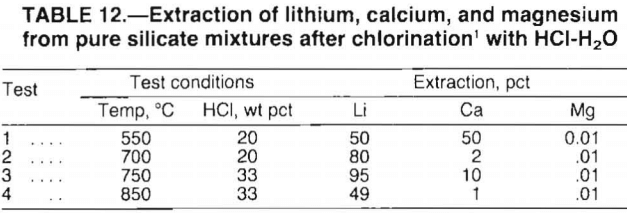
Test results, presented in table 12, verify the trends predicted in figure 6. For the conditions tested, magnesium extraction was no greater than 0.01 pct, whereas lithium extraction ranged from 49 to 95 pct. Calcium extraction was most affected by the chlorination conditions. As predicted in figure 6, increased temperature caused dramatic decreases in calcium extraction. Overall, the test data confirmed that selective chlorination is dependent on reaction temperature and HCl concentration.
An extensive series of tests indicated that the selective chlorination of lithium in McDermitt clay was not as effective as anticipated. Several variables were investigated including (1) roast temperatures of 450° to 700° C, (2) HCl concentrations of 20 to 33 wt pct, and (3) reaction times of 30 to 120 min. The best lithium extraction of 22 pct was obtained at 500° C when chlorinating with 33 wt pct HCl for 30 min. The lower curve in figure 7 shows the results of 60-min chlorination tests conducted at 550° to 700° C using 20 wt pct HCl. The maximum lithium extraction was only 13 pct.
Chemical analysis of the leached calcine showed large amounts of insoluble chloride. The insoluble chloride was assumed to be a chlorosilicate that was formed by the reaction of chloride with free silica (SiO2) present in the clay. The chlorosilicate compound would have a structure similar to that of sodalite [Na8(AlSiO4)6Cl2]. Chlorosilicate formation would tie up the alkali metal chlorides and reduce their solubility in water. Thus, lithium extraction was much lower than anticipated.
Selective Chlorination of Clay-CaCO3 Mixtures With HCl-H2O
The formation of chlorosilicate might be avoided if the SiO2 in the clay was combined into a nonreactive compound. Thermodynamic calculations show that SiO2 should react with lime (CaO) at 700° C to form CaSiO3. Therefore, the addition of CaO as CaCO3 should improve the lithium recovery by decreasing the amount of free silica.
This theory was supported by tests which showed that lithium extraction was much improved by adding CaCO3 to the charge. Mixtures of McDermitt clay and CaCO3 in a 2:1 ratio were chlorinated with 20 wt pct HCl for 1 h at 550° to 700° C. The upper curve in figure 7 shows that lithium extractions were as high as 80 pct. These recoveries, which represent a dramatic improvement over extractions obtained with chlorination of clay alone, indicate that SiO2 reacted with CaO to form a calcium silicate.
Several types of calcium silicates can be formed by the reaction of CaO and SiO2. The ratio of CaO to SiO2 determines which calcium silicates form. A clay-carbonate ratio of 4:1 corresponds to a 1:1 ratio of CaO to SiO2, which yields the silicate, CaSiO3. As the CaO-SiO2 ratio is increased, the more basic silicates will be formed. Ratios of 3:2, 2:1, and 3:1 of CaO-SiO2 produce the silicates Ca3Si2O7, Ca2SiO4, and Ca3SiO5, respectively. Ratios of CaO-SiO2 higher than 3:1 form Ca3SiO5 plus CaO.
Free-energy values for chlorination of the various calcium silicates and CaO are compared with the free energy of chlorination for Li2Si2O5 (the lithium silicate found in McDermitt clay) in figure 8. Selective chlorination between compounds with large differences in free energy is theoretically possible. Therefore, Li2Si2O5 can be selectively chlorinated in mixtures containing CaSiO3, Ca3Si2O7, and Ca2SiO4. Clay-carbonate mixtures greater than 2:1 will form Ca3SiO5, which has a free energy of chlorination about equal to that of Li2Si2O5. Thus, selective chlorination between Li2Si2O5 and Ca3SiO5 is thermodynamically impossible. The free energy of chlorination of CaO is less than the free energy of chlorination of Li2Si2O5; therefore, CaO will chlorinate in preference to Li2Si2O5. Based on these observations, selective chlorination between lithium and calcium is possible using clay-carbonate mixtures greater than 2:1.
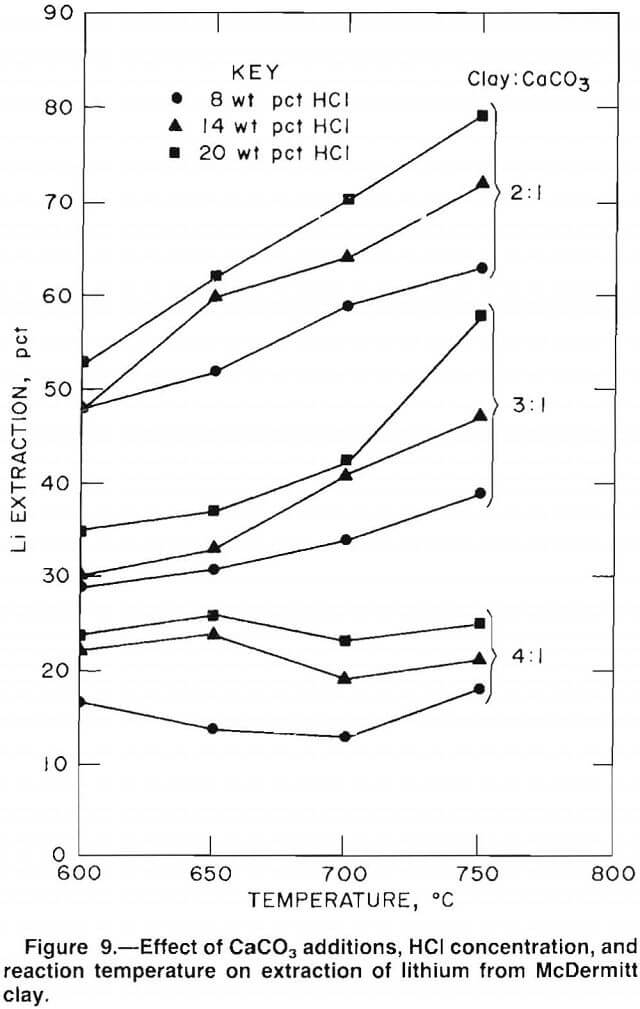
Thermodynamic calculations, together with preliminary test data, indicated that selective extraction of lithium would depend on the HCl-H2O ratio, reaction time, and CaCO3 addition. Therefore, an extensive test series was conducted to study the effects of the following chlorination conditions on lithium extraction: (1) HCl concentrations of 8 to 20 wt pct, (2) reaction temperatures of 600° to 750° C, and (3) clay-CaCO3 ratios of 4:1 (the minimum amount needed to tie up the free silica), 3:1, and 2:1 (the maximum amount that will yield a calcium silicate that can be selectively chlorinated). Test results, presented in figure 9, show that the order in which chlorination conditions improved lithium extraction was first, the clay-CaCO3 ratio; second, reaction temperature; and third, HCl concentration. Also, the data indicate that HCl concentration and reaction temperature may be interacting variables. Increasing either HCl concentration or temperature improves the effect of the other variable on lithium extraction.
Figure 9 indicates that the optimum selective chlorination conditions for lithium recovery from McDermitt clay were 2:1 clay-CaCO3 (at ratios lower than 2:1 calcium begins to chlorinate), 20 wt pct HCl (higher HCl concentrations are expensive to obtain), and 750° C (higher temperatures cause fusion of the charge, which decreases lithium extraction).
Large-Scale Testing
Large-scale chlorination testing was conducted to (1) determine if the operating conditions established in bench-scale studies would be applicable to larger scale operations, and (2) provide information for a material balance and subsequent cost evaluation of the process.
Roasting Equipment and Procedure
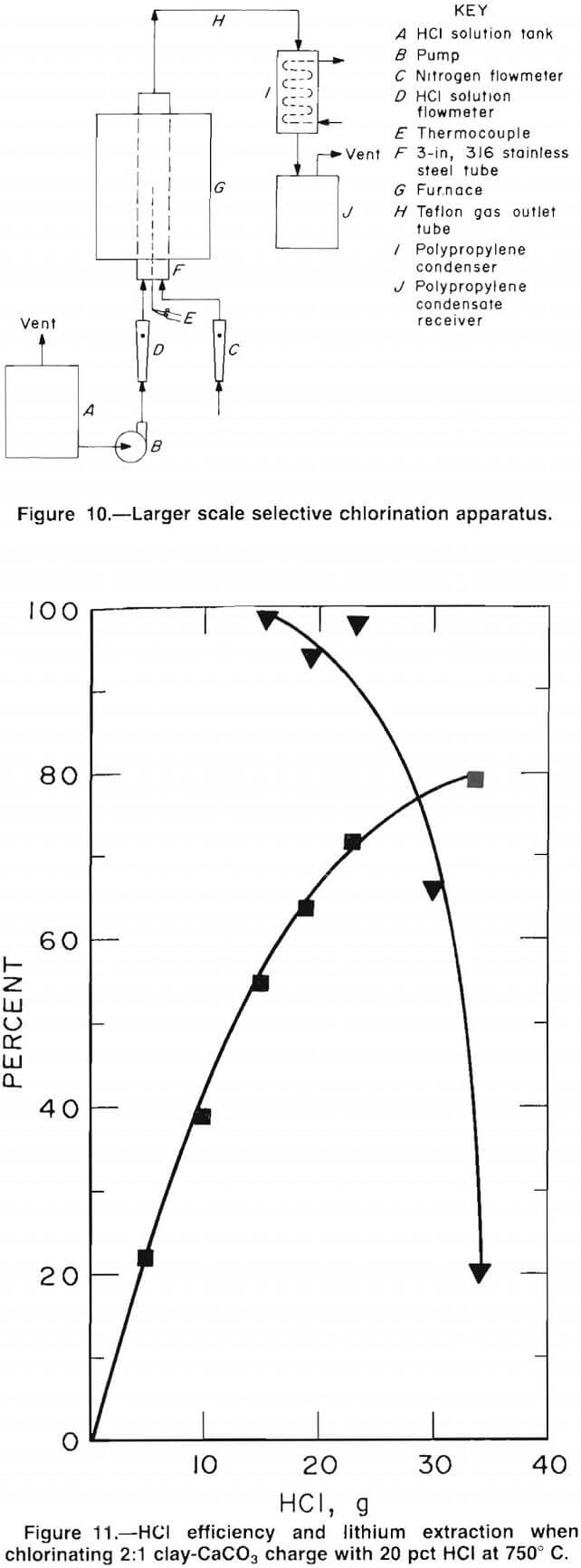
Roast tests were conducted in a 3.5-in vertical tube furnace. The reaction vessel was a 3-in-diam, 316 stainless steel pipe. The 450-g samples of pelletized
clay-CaCO3 mixtures were charged directly to the furnace tube. Figure 10 shows a schematic of the experimental apparatus. The HCl-H2O mixture was pumped into the bottom of the furnace tube through a Pyrex-lined stainless steel tube that served as a vaporizer. Nitrogen gas was fed through the charge during heat up and cool down. The reactor outlet tube was Teflon polymer because of the extremely corrosive nature of the hot offgases. The water-cooled condenser and the condensate receiver were also constructed of corrosion-resistant plastic. The HCl solution was generally fed at a rate of 1 to 2 mL/min, and the roasting time was varied from 30 min to 3 h.
Roast Tests
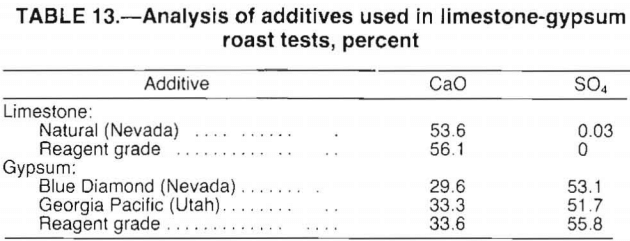
Chlorination roasts were conducted at 750° C using 20 wt pct HCl and a clay-carbonate ratio of 2:1 to determine the efficiency of HCl consumption. The efficiency was defined as the amount of HCl consumed by the chlorination reaction divided by the amount of HCl introduced to the test. Test results, presented in figure 11, show that 70 pct of the lithium in the clay was extracted with an overall HCl efficiency of over 90 pct. The HCl efficiency decreased rapidly for lithium extractions above 70 pct. Nearly twice as much HCl was required to obtain 80-pct Li extraction as for 70-pct extraction. Also, test data showed that the
quantity of HCl used determined the percent lithium extraction regardless of HCl flow rate or HCl consumption.
Overall, the test results indicate that the realistic level of lithium extraction using selective chlorination was about 70 pct; HCl consumption at this point was 140 lb HCl per short ton clay. Extractions above 70 pct would be costly, in terms of both HCl used and processing time.
Lithium Recovery From Calcine
Recovering lithium from the calcine required a series of unit operations. The calcine was ground to minus 100 mesh and leached for 1 h with either fresh water or recycle solution from the product precipitation step. Filtration and washing of the residue produced a dilute solution containing lithium, sodium, potassium, and a small amount of calcium, all as chlorides. The calcium was removed by adding a stoichiometric amount of soda ash to the dilute solution. The calcium-free solution was concentrated by evaporation, causing KCl and NaCl to crystallize. These salts were separated from the solution. Soda ash was then added to the concentrated brine to precipitate Li2CO3, which was washed and dried. The product filtrate and product wash were recycled to the leach step.
Process Evaluation and Discussion
The Bureau’s headquarters Process Evaluation Office prepared a cost evaluation based on the larger scale testing. The capital cost estimate was of the general type called a study estimate by Weaver and Bauman. This type of estimate, prepared from a flowsheet and a minimum of equipment data, should be within 30 pct of the actual plant cost.
The estimated capital cost on a third quarter 1983 basis (Marshall and Swift index of 781.7) for a plant processing 1,000 st/d McDermitt clay was $121 million. The plant would operate 330 d/yr with an annual estimated operating cost of $68 million. Assuming that 69 pct of the lithium in the clay was recovered (recovery based on large-scale test data), the plant would produce 27 st/d Li2CO3 at a cost of $3.85/lb. In contrast, the selling price of Li2CO3 is $1.50/lb (as of July 1987).
The unfavorable economics are primarily due to the relatively low rate of lithium recovery and to the high energy costs associated with evaporation; over 50 pct of the operating costs involved leach solution concentration. To improve the economics, kinetic studies should be conducted on the chlorination step to determine if higher chlorination rates and efficiencies can be attained using a continuous countercurrent flow furnace. Water consumption in the leaching and washing of the calcine should be minimized to reduce the evaporation load.
Limestone-Gypsum Roast-Water Leach Process
The limestone-gypsum roast-water leach extraction technique evolved from exploratory roast tests designed to develop basic lithium extraction data and to establish the reaction mechanism. The success of these preliminary tests led to extensive batch-roast studies to establish optimum roast conditions and to investigate roasting atmosphere. Subsequently, larger scale roasting in a rotary furnace was conducted to study the dynamic variables affecting the calcine. These tests generated a sufficient quantity of calcine for process research unit (PRU) studies in which leach solution purification and product (Li2CO3) precipitation were investigated.
Batch Roast Tests
Raw Materials Test Procedures and Equipment
The limestone-gypsum roast tests were conducted with McDermitt B clay. Chemical analysis of a typical clay sample is given in table 5. The roast feed was a mixture of clay, limestone, and gypsum. Both reagent-grade and naturally occurring limestone and gypsum were used; the CaO and SO4 analyses of the natural materials are presented in table 13.
The general test procedure involved (1) thoroughly mixing selected portions of the dry ingredients, (2) pelletizing the mixture, (3) roasting the pelletized mix, and (4) water leaching the calcines to extract the lithium. The calcine was ground to minus 100 mesh before leaching.
The preroast charge was mixed in a “V” blender. A revolving pan mixer (fig. 12) was used to pelletize the mix. Roasts were conducted in a muffle furnace (fig. 13) and in a tube furnace (fig. 14).
Exploratory Studies To Develop Basic Data
Exploratory roasting studies were conducted to determine the approximate ingredient mix required to effectively extract lithium from McDermitt clay. Subsequently, a test series was conducted to determine the effect of preparation and physical character of the preroast charge on lithium extraction. In addition, tests were conducted to determine the reaction mechanism.
Determination of Preroast Mixture
Preliminary roasting studies were conducted to determine the approximate ingredient mix that would result in the best lithium extraction. Pelletized charges of 40 g were roasted in a muffle furnace for 1 h at 1,000° C under oxidizing conditions. The weight ratios of the clay-CaCO3-CaSO4-2H2O mixes ranged from 5:0:6 to 5:6:0. The calcines were allowed to cool and were then water-leached to determine lithium extraction. A 5:3:3 mix was found to be optimum (table 14); lithium extraction from this mix was 90 pct. Extraction of potassium, sodium, and fluorine from the same mix was 95, 85, and 2 pct, respectively. Lithium extraction from clay roasted alone was only 0.1 pct.



Charge Preparation Methods
A test series was conducted to determine the effect of preparation and the physical character of the preroast charge on lithium extraction. In each test, a 5:3:3 mix was roasted for 2 h in a muffle furnace, and the resultant calcine was leached. Using a pebble mill, the mix samples were prepared by (1) dry mixing only, for roasting without agglomeration, (2) dry mixing followed by pelletizing with water and drying of the pellets, (3) wet mixing followed by filtering, drying of the filter cake, and grinding of the dried filter cake to minus 100 mesh, and (4) wet mixing followed by filtering, then drying and breaking of the filter cake (but without grinding). The pebble mill ensured good mixing while minimizing grinding of the mix ingredients. Test results, shown in table 15, indicate that the best lithium extraction, 91 pet, resulted from wet mixing followed by filtering and roasting of the dried (and broken) filter cake. Using the dry-mix, pelletized-feed method, 89-pct extraction was obtained. The advantage of the slightly higher lithium extraction made possible by wet mixing was more than offset by the disadvantage of slow filtering. Thus, pelletized mixtures were used in subsequent roasting studies. However, in a large-scale operation, feeding the slurry directly to a drying or roasting kiln might be feasible.
Determination of Reaction Mechanism
Exploratory roasting tests were conducted to determine if Li2SO4 from the roasting operation forms as a solid-to-solid or gas-to-solid reaction. Pelletized samples of different clay-CaCO3 and CaSO4·2H2O-SiO2 mixtures were placed in separate boats and then heated together in a tube furnace for 1 h at 1,000° C. The furnace tube was sealed at both ends with rubber stoppers to maintain a static atmosphere of air. At the conclusion of the run, the boats were allowed to cool in the furnace to ambient temperature. A sample of the clay-CaCO3 calcine was leached, and the products were analyzed.
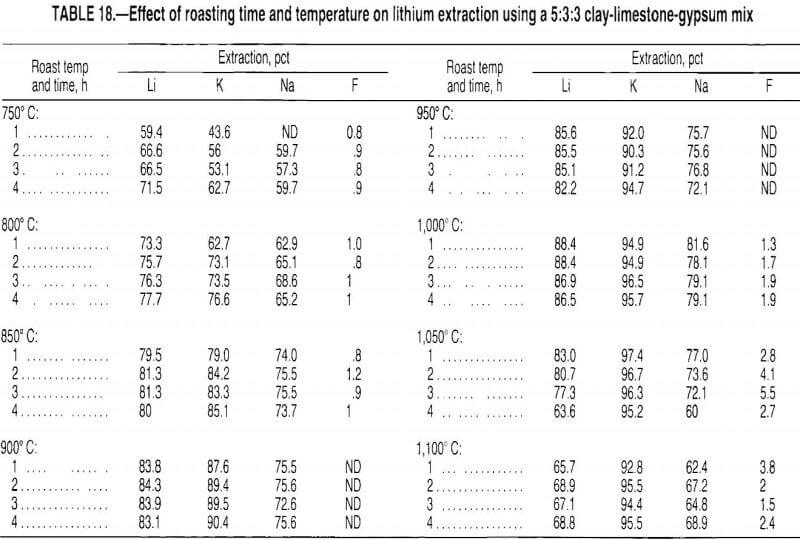
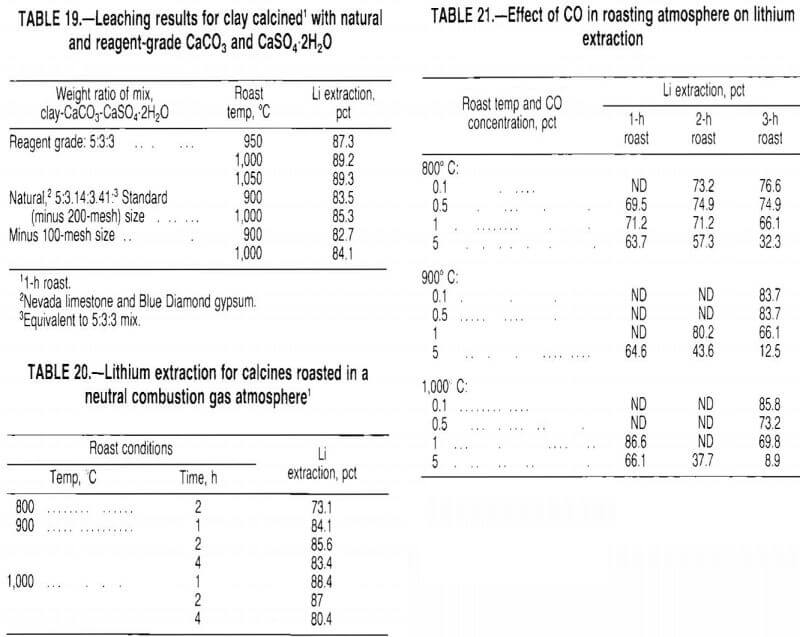
Test results, presented in table 16, show that more lithium was extracted from the calcines roasted in the presence of the CaSO4·2H2O-SiO2 mix than from the clay-CaCO3 calcines roasted alone. These results indicate that SO2 from the CaSO4·2H2O-SiO2 reacted with the Li2Si2O5 in the clay to form Li2SO4. The lithium extracted from the clay-CaCO3 mix calcined alone was attributed to the small amount of sulfur contained in the clay. In addition, leach solution analyses showed that the lithium was extracted as Li2SO4. Therefore, the test data indicate that Li2SO4 was formed by the following reactions:
CaSO4·2H2O + SiO2 → CaSiO3 + SO2 + ½ O2 + 2H2O……………………………………………..(A)
and
Li2Si2O5 + SO2 + ½ O2 → Li2SO4 + 2SiO2………………………………………………………………(B)
Although the reaction of limestone is not shown, limestone is a necessary additive for attaining high lithium extraction. Limestone in the charge limits the back reaction of Li2SO4 with free silica by reacting with the silica to form a calcium silicate.
Muffle Furnace Tests to Determine Optimum Roast Conditions
The exploratory roast-leach studies indicated that lithium extractions approaching 90 pct can be realized in the limestone-gypsum processing of McDermitt clay. A pelletized 5:3:3 clay mix was found to be about optimum when batch-roasted under oxidizing conditions for 1 h at 1,000° C. An expanded laboratory study was undertaken to verify these results and better determine the best conditions for calcine production.
Effect of Charge Composition and Roast Temperature on Lithium Extraction
Tests were conducted to determine the leaching responses of various clay mixtures roasted at 900° to 1,050° C. Pelletized mix samples were placed in open refractory boats and roasted for 1 h at different temperatures in a muffle furnace. The calcines were then leached to determine lithium extraction. Table 17 shows that the best overall results were achieved with a 5:3:3 mix at 1,000° C. These data, together with test results presented in table 14, show that excellent lithium extraction was obtained over a fairly wide range of charge ratios and roast temperatures; the optimum charge ratio appears to be 5:3:3.

Effect of Roast Time and Temperature on Lithium Extraction
Several series of tests were conducted in a muffle furnace to define the influence of time and temperature on lithium extraction. The test conditions and results for a 5:3:3 mix are presented in table 18.
Test data show that increasing the roast temperature from 750° to 1,000° C increased lithium extraction; however, above 1,000° C, lithium extraction decreased. A 1-h roasting period was sufficient to convert the lithium in the clay to a water-soluble sulfate. Generally, prolonged roasting for 4 h reduced lithium recovery. Extraction of potassium, sodium, and fluorine followed the same general trends as lithium extraction.
Effect of Additive Type on Lithium Extraction
A test series was conducted in a muffle furnace to determine if additive type (naturally occurring versus reagent-grade chemicals) would affect lithium extraction. Test results, presented in table 19, show that slightly higher extractions were obtained with reagent-grade chemicals than with naturally occurring materials. The difference in extraction may have been due to impurities in the natural materials. Also, the natural materials were not as finely ground as the reagent-grade chemicals. Table 19 shows that finer grinding of the natural material increased the lithium extraction.
Reagent-grade additives were used for subsequet batch tests; natural materials were used in the larger scale continuous tests in the rotary roaster.
Tube Furnace Tests To Investigate Roasting Atmosphere
Muffle furnace roasts were conducted in an oxidizing atmosphere employing indirect heating. Scaleup of the limestone-gypsum roast would probably involve using a direct-fired roaster in which the roasting atmosphere would be neutral. Thus, testing was conducted to determine if clay mixtures roasted in neutral combustion atmospheres would leach as effectively as those roasted under oxidizing conditions. In this test series, samples of standard 5:3:3 mix were roasted in a simulated gas combustion atmosphere analyzing, in volume percent, 10 CO2, 18 H2O, and 72 N2; this mixture approximated the composition of natural gas combustion products.
Pelletized samples were roasted at various temperatures for different time periods in a tube furnace assembly (fig. 14), Metered amounts of CO2 and N2 were introduced through a glass dispersion tube into a flask partially filled with hot water. Here the gaseous components intermixed and were preheated to the temperature required to establish the desired concentration of water vapor in the gas flow. At the conclusion of each run, the furnace was cooled to 600° C, the gas flow was discontinued, and the calcine was cooled to ambient temperature inside the furnace. The roasted product was then leached and the filtrate residue analyzed. Comparison of the results presented in tables 18 and 20 revealed that roasting in neutral combustion atmospheres did not adversely affect the conversion of lithium in the clay to the water-soluble sulfate.
In addition, tests were conducted to study the effect of low carbon monoxide (CO) concentrations on lithium extraction. The roasting atmosphere in a direct-fired roaster may contain CO since all commercial fuels contain hydrocarbons that may give off CO in the initial or early stages of combustion.
Several tests were conducted to define the influence of variations in the concentration of CO, temperature, and time on the production of a leachable calcine. Samples of pelletized 5:3:3 mix were placed in Alundum combustion boats and then into a tube furnace (fig. 14). An inert N2 atmosphere was maintained during heating and cooling. Metered flows from compressed cylinders of CO and N2
were combined in the proper ratio to give the desired gas mixture; mixtures containing less than 1 pct CO were obtained by blending a calibrated 1-pct CO-in-N2 premix with N2. Samples of the roasted products were leached, and the leach products were analyzed.
Test results, presented in table 21, show that lithium extraction decreased with prolonged roasting when the CO concentration was increased to 1 pct or greater. Comparison of the data with those presented in table 20 indicates that the decrease in lithium recovery was minimal for roasting temperatures lower than 1,000° C in furnace atmospheres containing less than about 0.5 pct CO. (This CO concentration represents a natural gas combustion efficiency of approximately 98 pct.)
Process Research Unit Studies
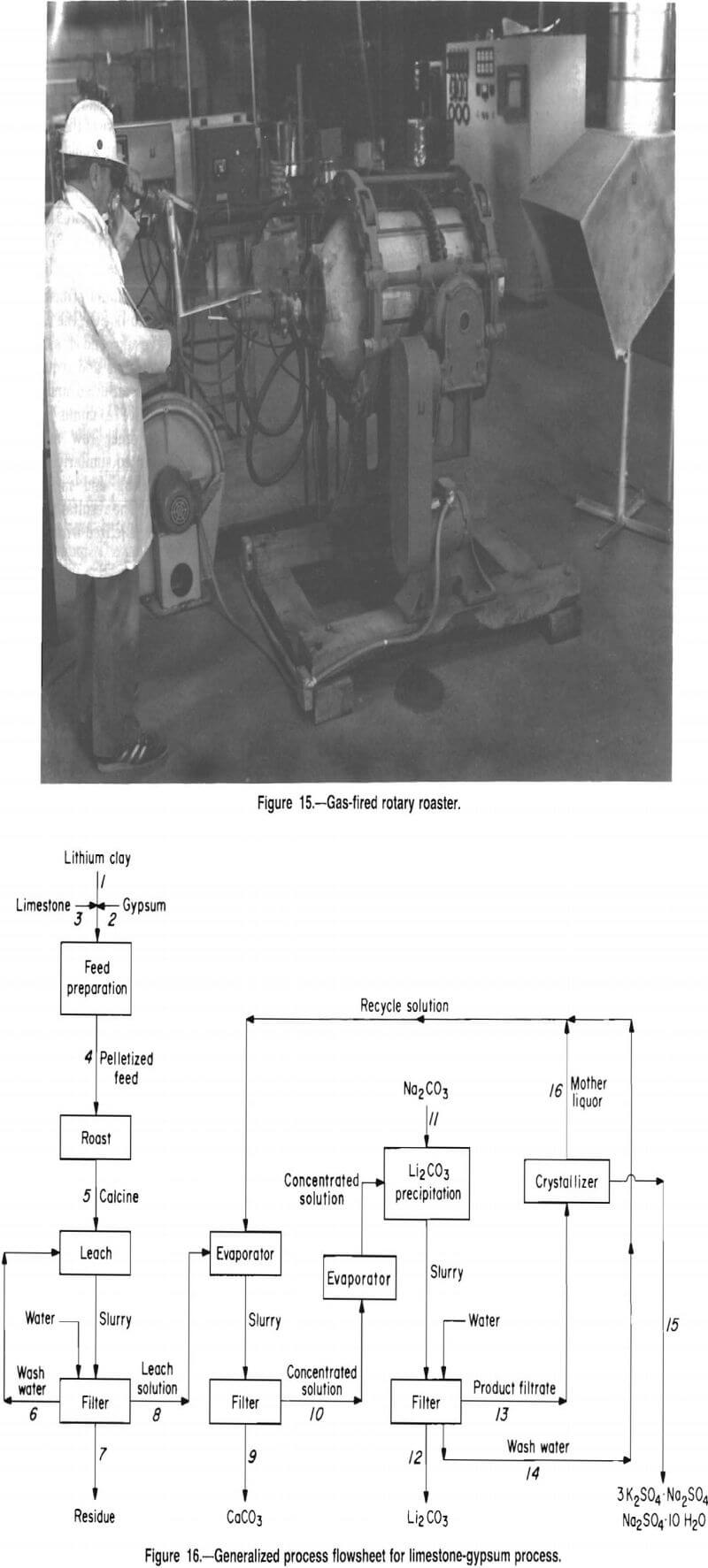
A PRU was constructed to investigate lithium recovery from McDermitt clay on a larger scale. Roasting was conducted in a gas-fired rotary roaster (fig. 15) to study the dynamic variables affecting the calcine and to confirm batch test results. Also, the larger-scale roast operation generated calcine for leaching, solution purification, and product recovery tests.
A process for recovering lithium from McDermitt clay evolved from this research; the process flowsheet is shown in figure 16. The process involves several unit operations including feed preparation, roasting, leaching, evaporation, precipitation, and crystallization.
Appendix A presents a material balance for a typical PRU test; appendix B presents a cost evaluation based on this material balance.
Process Description
This process is designed to recover lithium, as Li2CO3, from McDermitt clay. A mixture of clay, limestone, and gypsum is pelletized, dried, and fed to the roasting furnace. Roasting the pellets at 900° C converts the lithium in the clay to water-soluble Li2SO4. Water leaching the calcine produces a solution containing lithium, potassium, sodium, and a small amount of calcium, all as sulfates. The leach solution, together with recycled product wash and mother liquor, is concentrated by evaporation. During this concentration operation, carbonate ion, present in the recycle solutions, causes calcium to precipitate as CaCO3, which is removed by filtration. The concentrated solution is then heated to boiling, and Na2CO3 is added to precipitate Li2CO3 product.
The product filtrate contains about 3 g/L Li. To recover this lithium, the solution is cooled to crystallize glaserite (3K2SO4-Na2SO4) and glauber salt (Na2SO4-10H2O) and then recycled to the evaporation step.
Removing calcium from the leach solution as CaCO3 prevents calcium contamination of the product. The solution recycled to the evaporation step contains sufficient carbonate ion, as either Li2CO3 or excess Na2CO3, to precipitate over 99 pct of the calcium contained in the leach solution.
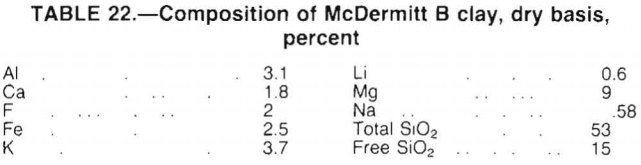
Materials
Analysis of the clay used for the PRU tests is shown in table 22. Agricultural-grade gypsum and natural limestone from Nevada were used in making up the charge. Analysis of these materials is shown in table 13. Simulated solutions used in laboratory evaporation and product purification tests were made up with reagent-grade chemicals.
PRU Equipment
Feed for the roast was ground and mixed in a ball mill (16-in diam by 24-in length). The minus 100-mesh mixture was pelletized with water in a 20-in-diam drum pelletizer (fig. 12).
The pellets were roasted in a direct-fired rotary roaster (fig. 15) that was fired with natural gas and had a working capacity of 0.5 ft³. The roast product (calcine) was leached with water in a 55-gal, baffled, polyethylene vessel. A propeller mixer agitated the slurry.
Figure 17 shows the evaporation, filtration, and product precipitation equipment. To recover the leach solution, the slurry from the leach tank was transferred
by gravity flow to a pan filter with a surface area of 9 ft². A tubing pump transferred the leach solution to a 55-gal stainless steel tank for concentration. The tank was equipped with a 9,000-W over-the-side immersion heater. The concentrated solution was filtered on a tabletop Buchner funnel to remove CaCO3. A tubing pump transferred the concentrated solution to the product precipitation unit, which consisted of a 6.5-gal stainless steel vessel equipped with a 1,000-W over-the-side immersion heater. An impeller mixer agitated the solution. The Li2CO3 product was recovered and washed on a Buchner funnel.
The product filtrate was cooled by refrigeration to crystallize glauber salt and glaserite.
Operating Conditions and Test Results
Each process unit operation including feed preparation, roasting, leaching, evaporation, product precipitation, and crystallization was studied in detail both in the PRU and in the laboratory. The objective of the work was to determine operating conditions that would (1) maximize lithium recovery, (2) minimize process operating costs, and (3) produce a high-purity product.
Feed Preparation
The McDermitt clay contains lithium principally as hectorite. To convert the lithium to Li2SO4, the clay was mixed with limestone and gypsum and roasted. As received, the clay was soft and friable and required no heavy crushing. However, it was air-dried and passed through a jaw crusher to produce a minus 10-mesh material before blending. The other raw materials, limestone and gypsum , were treated similarly. Further feed preparation entailed grinding and mixing the ingredients for 1 h in a ball mill. The resultant mixture (80 pct finer than 200 mesh) was pelletized with water to

produce nominal 6.5-mm-diam pellets. These pellets contained up to 20 pct moisture and were dried at 70° C before roasting.
Roasting
The objectives of the roasting tests were to (1) generate calcine for leaching, purification, and product recovery studies, (2) determine optimum roasting conditions, and (3) determine typical gas emissions.
Initially, a series of batch tests was conducted in the rotary roaster to determine optimum retention time and roast temperature. In these tests, small charges (500 g) of pelletized 5:3:3 mix were roasted. The test results showed a 2-h retention time and 900° C to be optimum. This retention time was used throughout the rotary roaster studies; the temperature was varied in a few tests in which the effect of temperature on lithium extraction was investigated.
To generate calcine for use in PRU solution studies, an equivalent 5:3:3 mixture of clay, limestone, and gypsum was used; batch testing had established the 5:3:3 mix as optimum. The pelletized feed was charged to the roaster in 600-g increments every 5 min to simulate continuous operation. Generally, each test produced 80 lb of calcine in 6.5 h operating time.
The final phase of the PRU roast studies involved investigating the effects of charge composition and roast temperature on lithium extraction. A test series was conducted in which various mixes were roasted; lithium extraction was determined by water-leaching composite samples of the calcines.
Test results, presented in table 23, show that lithium extractions of at least 80 pct were attained with a wide range of clay-limestone-gypsum ratios. Also, the data indicate that good lithium extraction was achieved over a temperature range of 850° to 975° C. The 5:2:2 mix was chosen for cost evaluation because this mix provided high extraction with a relatively low reagent addition.
Emissions of SO2 and fluorine were calculated from material balances for two-thirds of the tests listed in table 23. The calculated emissions from representative roasts are presented in table 24. In a commercial operation, these offgases would require scrubbing before being vented to the atmosphere.
Leaching
The objective of the PRU leach tests was to determine the relationship between leach-system variables and optimal lithium extraction. The following variables were studied: (1) leach pulp percent solids, (2) wash water recycle, (3) calcine particle size, and (4) leach time.
The calcines leached in these tests were produced by roasting 5:3:3 mixtures of clay, limestone, and gypsum. Generally, 70 lb of calcine was water-leached in each test. A slurry filter recovered the leach solution. The filter cake was washed and discarded.
Solids Content and Wash Water Recycle
A series of 30-min leach tests was conducted at ambient temperature to study the effect of percent solids and wash water recycle on lithium extraction. The test results, presented in table 25, show that the calcine was leached effectively at 40 pct solids with recycled wash water. At 50 pct solids, the lithium extraction decreased.
Since the wash water was recycled to the leach step, the volume of wash water used was equal to the volume of makeup water required for the next leach. In the material balance calculations, 40 pet solids was used as the optimum.
Calcine Particle Size and Leach Time
The calcine pellets do not break apart during the leach. If a coarse particle could be leached effectively, grinding requirements would be minimized. Therefore, a test series was conducted to study the effect of calcine particle size on lithium extraction.
The calcine was leached for 30 min at 40 pct solids using recycled wash water. Test results, given in table 26, show that the 30-min leach extracted the lithium equally well from all particle sizes tested. To determine the effect of leach time on lithium extraction, a test series was conducted with coarse-crushed and whole pellets. The pellets were leached at 40 pct solids in recycled wash water. Test results, presented in table 27, show that lithium was extracted from coarse-crushed pellets with a 5-min leach; whole pellets were not effectively leached in 5 min.
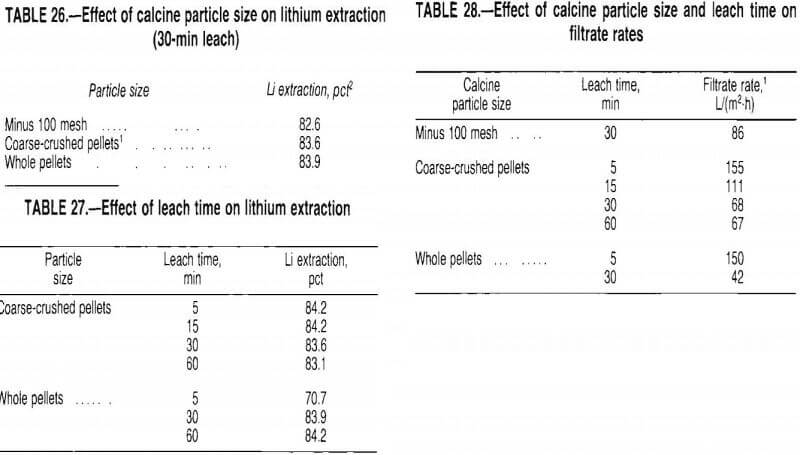
Although the pellets did not break apart during the leach, prolonged agitation generated fines which affected filtration rates. The data presented in table 28 show that filtrate rates decreased with increased leach time and increased particle size. For 30-min leaches, the whole pellet slurry filtered slowly because the filter cloth was blinded with fines. As the calcine particle size decreased, the fines tended to remain on top of the filter cake, allowing faster filtration. Overall, the test results indicate that coarse-grinding the calcine and leaching it for 5 min at 40 pct solids provided good extraction and high filtration rates. Under these conditions, lithium extractions of 82 to 84 pct could be expected; the leach solution generally contained 2.5 to 3.0 g/L Li.
Evaporation
As figure 16 shows, the evaporator was fed with leach solution and solution recycled from the previous test. The recycled solution (mother liquor plus product wash) accounted for about 20 pet of the total volume in the evaporator.
In addition to concentrating the solution, calcium as CaCO3 was removed from the leach solution in this step of the process. The leach solution was saturated with CaSO4 (about 0.6 g/L Ca²-). Extensive laboratory testing showed that reducing the calcium ion concentration to about 0.015 g/L prevented calcium contamination of the product.
The evaporation procedure involved the following steps:
- The solution (leach plus recycle) was evaporated to about 50 pct of its original volume and then filtered to remove CaCO3. Carbonate ion (approximately 15 g/L) present in the recycled solution precipitated over 99 pct of the calcium contained in the leach solution.
- The filtrate was returned to the evaporator. Evaporation continued until the solution was reduced to 20 pct of its original volume.
- The hot concentrated solution, containing 12 to 13 g/L Li, was transferred to the product precipitation step. Generally, this concentrated solution was cloudy because a small amount of Li2CO3 precipitated during evaporation.
Product Precipitation
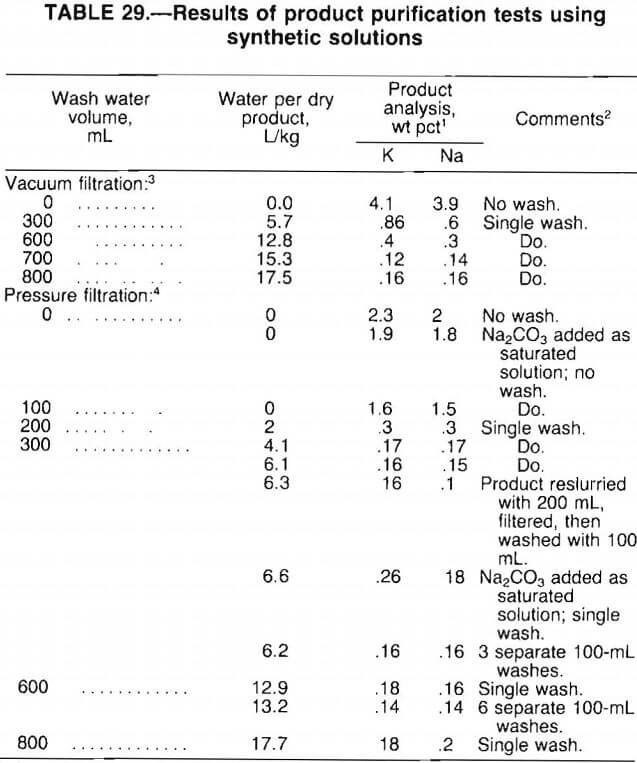
Lithium recovery involved heating the concentrated solution to boiling and adding a stoichiometric amount of Na2CO3 to precipitate a Li2CO3 product. The objective of this step was to recover a product of at least 99-pct purity.
Initially, the product was recovered from the hot solution by vacuum filtration and then dried. This procedure yielded a product of about 80-pct purity with the principal contaminants being Na2SO4 and K2SO4. Numerous tests were conducted in the PRU and laboratory (using PRU leach solution) to investigate product purification techniques. Test results were erratic because precise control of solution concentration was difficult; therefore, synthetic solutions were used to study operating variables.
A series of laboratory tests was conducted using 1-L batches of synthetic concentrated solution (made up with reagent chemicals) containing 97 g/L Li2SO4, 158 g/L K2SO4, and 87 g/L Na2SO4. Adding a stoichiometric amount of Na2CO3 to the hot solution precipitated Li2CO3. Product filtration and washing procedures were then studied.
From the test results presented in table 29, the following observations were made:
- Pressure filtration yielded a product of higher purity than vacuum filtration by reducing the moisture content of the filter cake.
- With pressure filtration, 4 to 6 L of wash water per kilogram of dry product was required to produce a 99-pct-pure product. A much higher volume of water was needed to produce a comparable product by vacuum filtration.
- For pressure filtration, wash water volumes above 6 L/kg of dry product did not further improve product purity. Also, single-stage washing was as effective as either multistage washing or product reslurry.
- Adding Na2CO3 as a saturated solution, rather than as a dry powder, had little effect on product purity. However, this procedure generated a coarse grainy product, in contrast to the fine powdery product obtained by adding dry Na2CO3.
The wash water and product filtrate recovered in these tests contained 14 to 16 g/L Li2CO3. The wash was recycled to the evaporator. After a crystallization step, the mother liquor was also recycled.
Crystallization
In addition to residual Li2CO3, the product filtrate contained high concentrations of K2SO4 and Na2SO4 (over 150 g/L of each). If the solution is to be recycled, the buildup of these salts must be prevented.
Laboratory and PRU tests showed that the most effective method for reducing the sulfate concentration involved crystallizing the salts by chilling the product filtrate to between 0° and -4° C; below -4° C, the filtrate freezes. The mother liquor, which contained 70 g/L Na2SO4 and 100 g/L K2SO4, was recovered by either vacuum or pressure filtration. Pressure filtration tended to reduce lithium loss by decreasing the amount of mother liquor present in the filter cake. The filter cake was a mixture of glauber salt and glaserite.
Laboratory tests showed that, if desired, glaserite and glauber salt could be recovered separately by a two-step crystallization procedure. At product filtrate temperatures down to about 17° C, glaserite crystallized. The salt was recovered by vacuum filtration and analyzed as 33 wt pct K, 8 wt pct Na, and <0.1 wt pct Li. Further cooling of the product filtrate (to as low as -4° C) crystallized glauber salt. These salts were recovered by pressure filtration and dried. The dried salts contained 28 wt pct Na, 6 wt pct K (a small amount of glaserite crystallized with the glauber salt), and 0.15 wt pct Li.
Overall Lithium Recovery
PRU roast-leach test results (tables 25-27) indicate 82- to 84-pct Li extraction as optimum. Treating the leach solution by the methods specified resulted in 95- to 98-pct recovery of the contained lithium. Losses occurred in CaCO3 filtration (0.5-pct loss) and in the crystallization step (2- to 5-pct loss, depending on the filtration method used to separate the mother liquor from the salts). Overall, 78 to 82 pct of the lithium contained in the clay was recovered as 99-pct-pure Li2CO3.
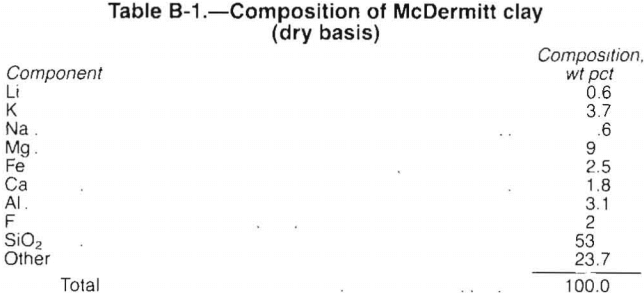
Process Description
The proposed plant is designed to process 1,000 st/d of McDermitt clay and is based on the material balance and research data provided by the researchers at the Salt Lake City Research Center. Table B-1 presents the composition of dry McDermitt clay, which contains 0.6 wt pct Li, found as Li2Si2O5. This process recovers 82 pct of the lithium, producing 27 st/d Li2CO3.
In the process, the clay, limestone, and gypsum are ground, mixed, and pelletized. Lithium in the pellets is converted by roasting from Li2Si2O5 to Li2SO4. Soluble Li2SO4 is extracted by leaching the roasted pellets with water. Concentration of the leach solution by evaporation precipitates some carbonate impurities. Soda ash is added to the concentrated solution, precipitating a Li2CO3 product from the now barren solution. Undesired sulfates are crystallized by cooling the barren solution before recycling it to the evaporator to recover any remaining lithium. For description purposes, the plant is arbitrarily divided into six sections: feed preparation, roasting, leaching, evaporation, lithium recovery, and crystallization.
Feed Preparation Section
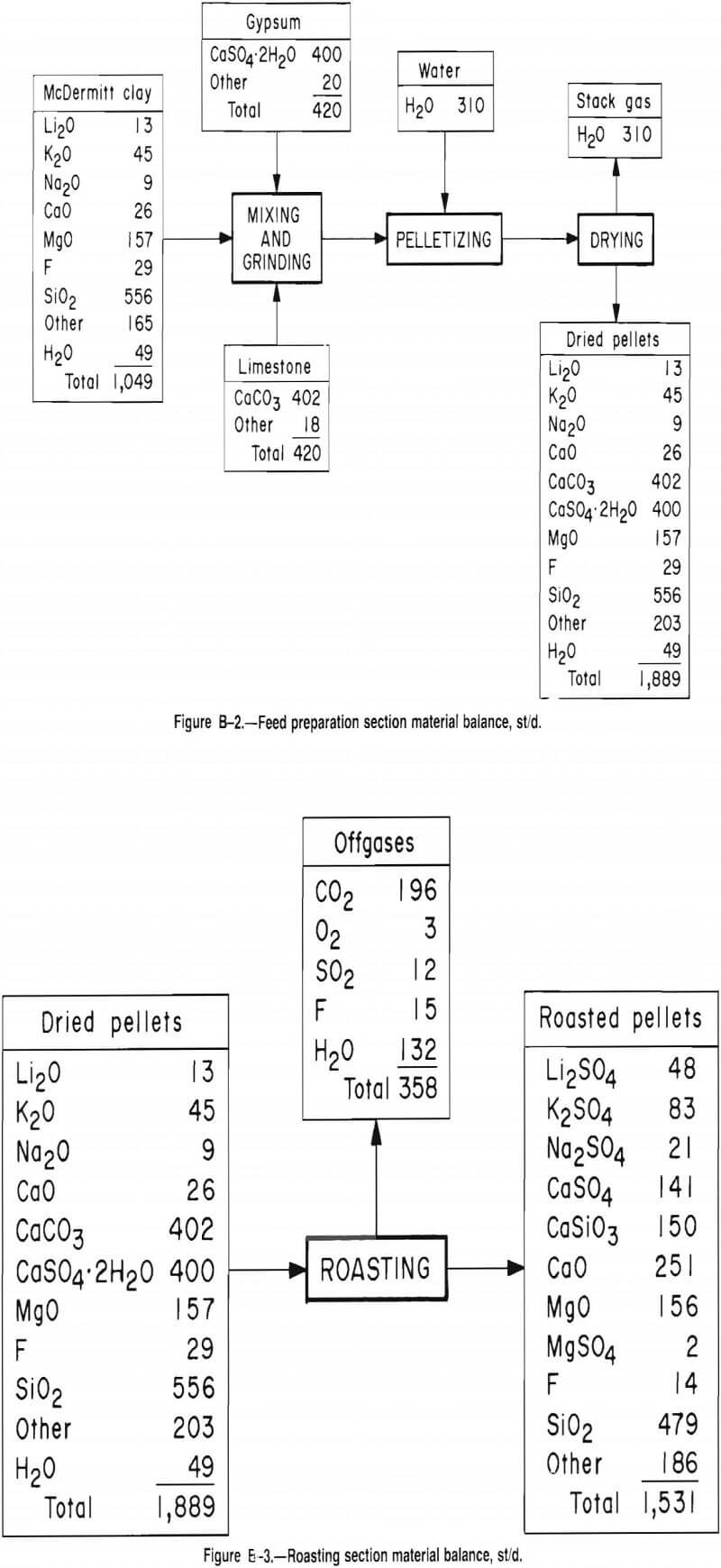
The plant can store a 60-day supply of as-mined clay and a 30-day supply of both limestone and gypsum. Each raw material is screened at 3/16 in, and the oversize fractions are crushed in hammer mills.
Wet clay (1,050 st/d) is mixed with 420 st/d of both gypsum and limestone. The three components are fed directly to dry ball mills, where they are mixed and ground to minus 100 mesh. The ball mill fines are pelletized with water on pelletizing disks and dried in a rotary dryer.
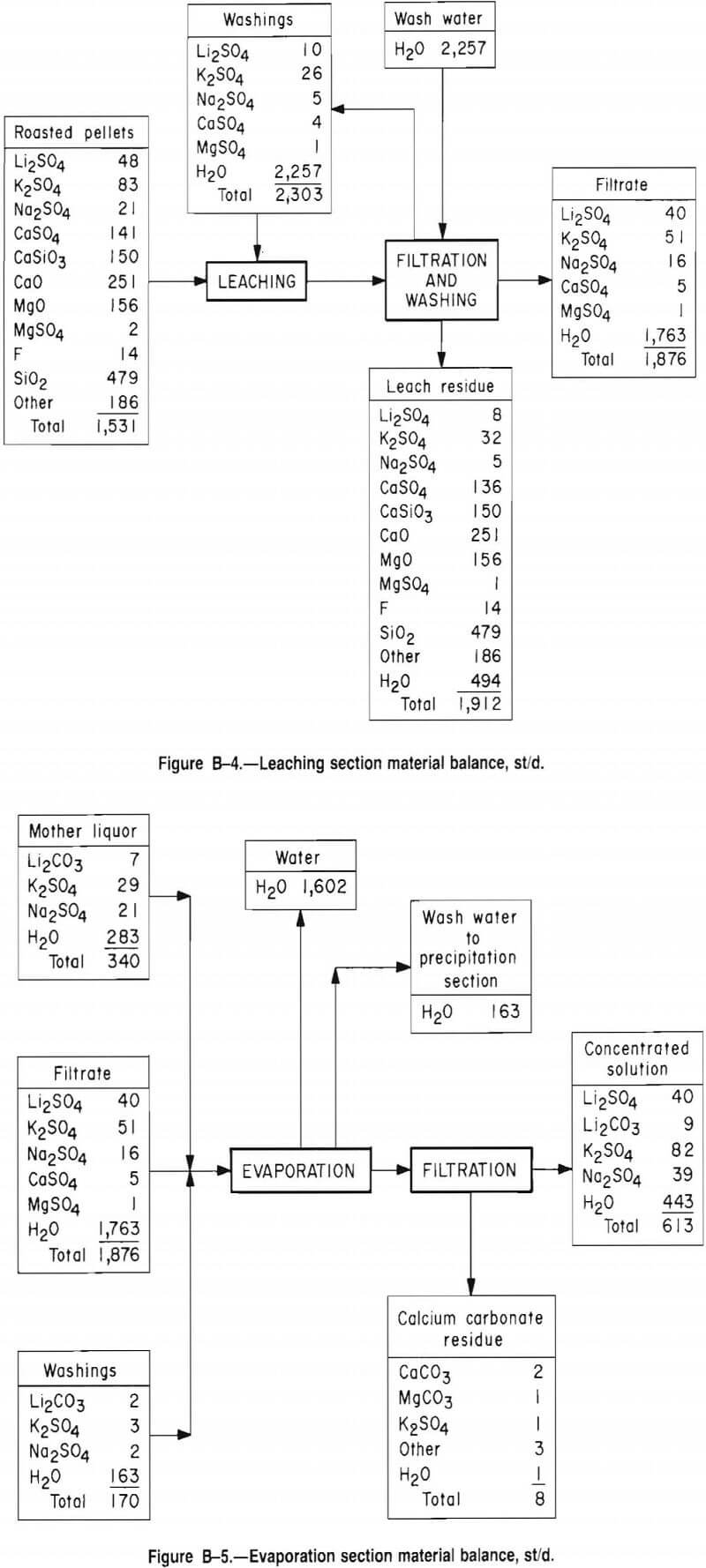
Roasting Section
The dry pellets are roasted at 900° C in a rotary kiln where the gypsum reacts with the Li2Si2O5 to produce Li2SO4. The overall reactions are
2CaSO4·2H2O + 2SiO2 → 2CaSiO3 + 2SO2 + O2 + 4H2O
and 2Li2Si2O5 + 2SO2 + O2 → 2Li2SO4 + 4SiO2.
To ensure complete reaction, the kiln is designed to provide a 2-h residence time. Limestone is converted to lime, and many of the minor salts are converted into sulfates. After roasting, the pellets are cooled to 60° C in a rotary cooler and conveyed to the leaching section.
The kiln flue gases pass through cyclones, which remove entrained solids. Heat is removed from the gas with a waste-heat boiler. The cooled gases are further cleaned in baghouses, and finally scrubbed to remove SO2 and hydrogen fluoride (HF).
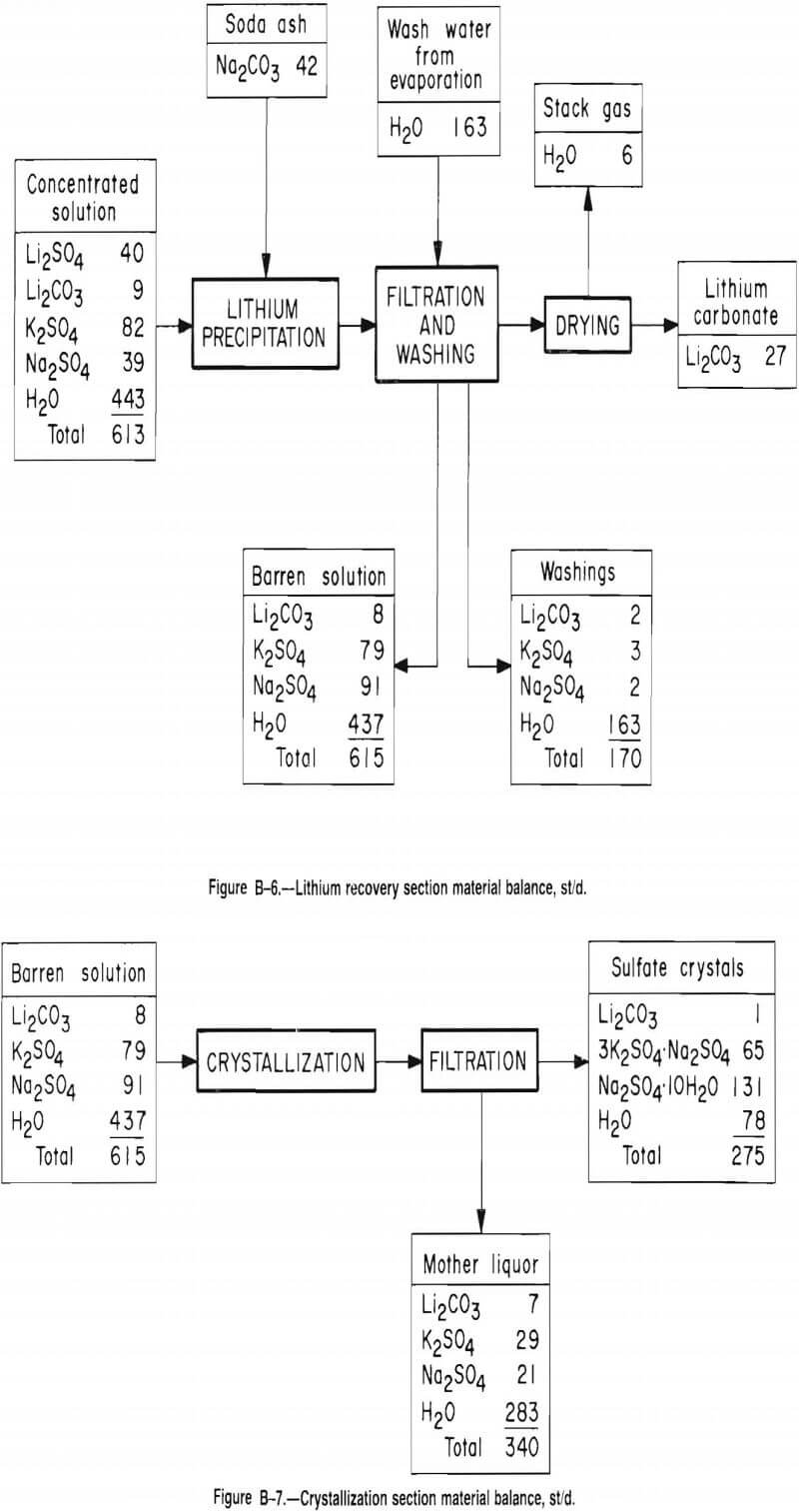
Leaching Section
Pellets from the roasting section are leached for 5 min in an agitated tank. During this time, 84 pct of the Li2SO4 and 30 pct of the other sulfates are solubilized. The pregnant solution is then separated from the leach slurry with belt filters and pumped to the evaporation section for concentration. The remaining 1,900 st/d of wet solids are washed with water and conveyed to a tailings pond. After washing the solids, the water is used as leach solution.
Evaporation Section
Pregnant solution from the leaching section is collected in a surge tank and mixed with washings from the lithium recovery section and recycled mother liquor from the crystallization section. This mixed solution is then concentrated in a five-effect evaporator from 1.7 pct by weight Li2SO4 to 6.5 pct. At the same time, CaSO4 converts to CaCO3 by reaction with other aqueous salts and precipitates from solution. The CaCO3 solids represent less than 0.2 pct of the concentrated effluent and are removed with pressure-leaf filters. The filtrate is collected in a sump and pumped to the lithium recovery section.
Lithium Recovery Section
Concentrated solution (615 st/d) from the evaporation section is heated to 95° C in agitated tanks and mixed with 42 st d of soda ash. Soda ash reacts with the aqueous Li2SO4 and generates 27 st/d of Li2CO3 precipitate. The overall reaction is
Li2SO4 + Na2CO3 → Li2CO3 + Na2SO4.
The product slurry is separated with two pressure- leaf filters into Li2CO3 solids and barren filtrate. The solids are dried at 300° C, cooled, and conveyed to storage silos.
Crystallization Section
Potassium and sodium sulfates are removed from the hot barren solution in the crystallization section. The hot solution is first pumped through a water-cooled heat exchanger and reduced to 40° C. Warm solution is then pumped through a two-stage crystallizer and cooled to 0 C. Stage 1 of the crystallizer cools the solution to 25° C by recycling the mother liquor from stage 2 as coolant. Stage 2 is cooled by refrigeration. Seventy-six percent of the sodium and 64 pct of the potassium in solution are removed by the crystallizer. Potassium crystallizes as glaserite, and the remaining sodium as glauber salt.
The crystal slurry is pumped to pressure-leaf filters, where the crystals are separated from the mother liquor and sent to the tailings pond. Mother liquor is pumped to the evaporation section for recovery of its lithium content.
Economics
The economics in this study are based on a plant processing 1,000 st/d of McDermitt clay and designed from a material balance and research data supplied by the Salt Lake City Research Center. The proposed plant produced 27 st/d of Li2CO3, which on an annual basis equals 70 pet of the estimated North American consumption of lithium during 1984.
The expense of developing and operating the McDermitt clay surface mining operation is excluded from this evaluation. Charges for this operation cannot be assigned with confidence but are expected to have a small effect on the overall economics. Nevertheless, these costs will have to be included in the sale price of the Li2CO3.
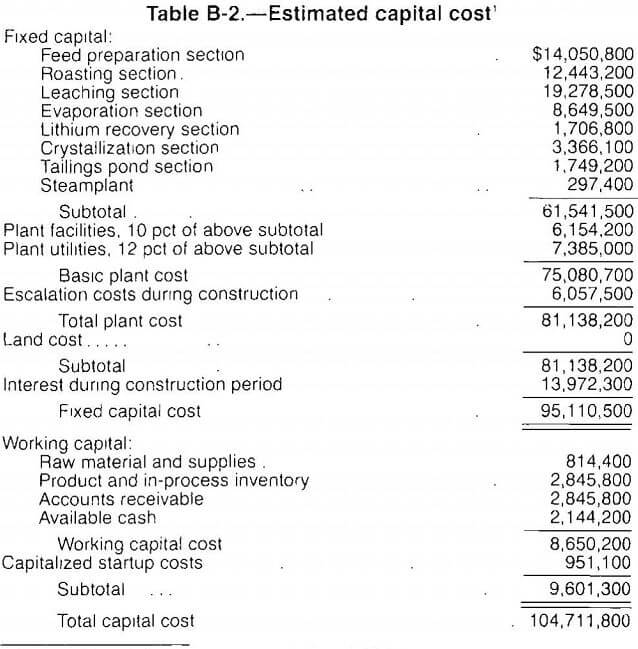
Capital Costs
Equipment designs in this evaluation are based on data extrapolated from bench-scale research. The capital costs in this study estimate are expected to fall within 30 pct of the actual costs.
The estimated fixed capital costs, based on first quarter 1987 prices (Marshall and Swift [M and S] index of 803.7), total $95.1 million and are presented in table B-2. This index is used to update manufacturers informal cost quotations and capacity-cost data used in this study. Construction materials are selected to optimize a unit’s service life. The tailings pond is designed with a 2-yr capacity. It is assumed that after 2 yr, process residues may be disposed of as backfill at the mine site.
Factors for piping, etc., except for the foundation and electrical factors, are assigned to each section, using as a basis the effect fluids, solids, or a combination of fluids and solids may have on the process equipment. The foundation factor is estimated for each piece of equipment individually, and a factor for the entire section is calculated from the totals. The electrical factor is based on the motor horsepower requirements for each section. A factor of 10 pct, referred to as miscellaneous, is added to each section to cover minor equipment and construction costs that are not shown with the equipment listed.
For each section, the field indirect cost, which covers field supervision, inspection, temporary construction, equipment rental, and payroll overhead, is estimated at 10 pet of the direct cost. Engineering cost is estimated at 5 pct, and administration and overhead cost is estimated at 5 pct of the construction cost. A contingency allowance of 10 pct and a contractor’s fee of 5 pct are included in the section costs.
The costs of plant facilities and plant utilities are estimated as 10 and 12 pct, respectively, of the total process section costs and include the same field indirect costs, engineering, administration and overhead, contingency allowance, and contractor’s fee as are included in the section costs. Included under plant facilities are the cost of material and labor for auxiliary buildings such as offices, shops, laboratories, and cafeterias, and the cost of non process equipment such as office furniture, and safety, shop, and laboratory equipment. Also included are labor and material costs for site preparation such as clearing, grading, drainage, roads, and fences. The costs of water,
power, and steam distribution systems are included under plant utilities.
Working capital is defined as the funds in addition to fixed capital, land investment, and startup costs that must be provided to operate the plant. Working capital, shown in table B-2, is estimated from the following items: (1) raw material and supplies inventory (cost of raw material and operating supplies for 30 days), (2) product and in-process inventory (total operating cost for 30 days), (3) accounts receivable (total operating cost for 30 days), and (4) available cash (direct expenses for 30 days).
Capitalized startup costs are estimated as 1 pet of the fixed capital costs. Land investment is not included in this estimate.
Operating Costs
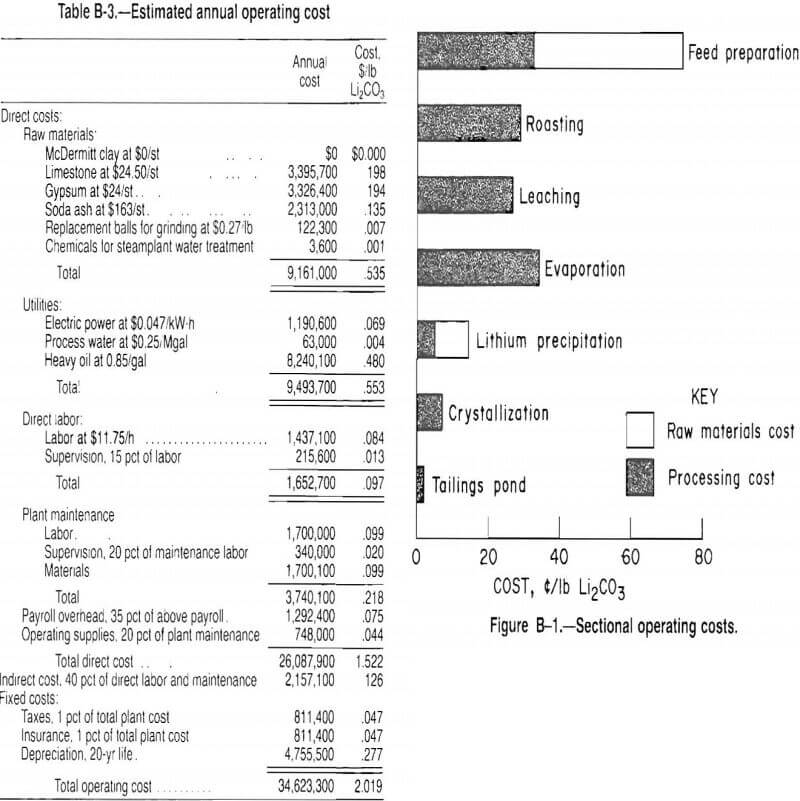
Estimated operating costs are based on a schedule of 3 shifts per day, 7 days per week, and 330 days of operation per year. The remaining time is allotted for scheduled or unscheduled downtime. Operating costs are divided into direct, indirect, and fixed costs and are presented in table B-3. The total estimated operating cost is about $2.02 lb Li2CO3; the current selling price (July 1987) of Li2CO3 is $1.50/lb.
Direct costs include raw materials, utilities, direct labor, plant maintenance, payroll overhead, and operating supplies. Raw materials and utility requirements per pound of Li2CO3 are shown in table B-3. The direct labor cost is estimated on the basis of assigning 4.2 employees for each position that operates 24 h/d, 7 days per week, and 1.4 employees for each position that operates 8 h/d, 7 days per week. The cost of labor supervision is estimated as 15 pct of the labor cost.
Plant maintenance is separately estimated for each piece of equipment and for the buildings, electrical system, piping, plant utility distribution systems, and plant facilities.
Payroll overhead, estimated as 35 pct of direct labor and maintenance labor, includes vacation, sick leave, social security, and fringe benefits. The cost of operating supplies is estimated as 20 pct of the cost of plant maintenance.
Indirect costs are estimated as 40 pct of the direct labor and maintenance costs. The indirect costs include the expenses of control laboratories, accounting, plant protection and safety, plant administration, marketing, and company overhead. Research and overall company administrative costs outside the plant are not included.
Fixed costs include the cost of taxes (excluding income taxes), insurance, and depreciation. The annual costs of both taxes and insurance are each estimated as 1 pct of the plant construction cost. Depreciation is based on a straightline, 20-yr period.
Evaluation
By reducing the McDermitt clay-limestone-gypsum ratio from 5:3:3 to 5:2:2, 19 pct less material is handled by the process. This translates to 441 st/d less gypsum and limestone going through the plant which results in a substantial cost savings.
Figure B-1 shows the breakdown of operating costs by section. The raw material costs are presented separately by the light regions in the graph. Gypsum and limestone costs are added to the feed preparation section even though they are essential to the entire process. The cost of soda ash is added to the fifth section, where it is used to precipitate the product.
Ninety percent of the operating costs, excluding raw materials, are shared almost equally by the first four sections. The last three sections handle much less material and have correspondingly smaller costs. Limestone, gypsum, and soda ash purchase costs contribute $0.53/lb Li2CO3, which represents 26 pet of the total operating budget.
Figures B-2 through B-7 and tables B-4 through B-11 give additional details on the economic evaluation of recovering lithium from McDermitt clay.