Table of Contents
The manufacture and use of nickel-cadmium alkaline storage batteries in the United States began to grow at a rapid pace in the late 1950’s. The batteries have found use in a wide variety of consumer appliances, electronic units, industrial equipment, communication devices, and space applications. The U.S. Department of Defense is the biggest single user of the nickel-cadmium cells. The importance of developing a method for recovering nickel, and particularly cadmium, became evident when it was reported in 1963 that 300,000 to 400,000 pounds of cadmium were being consumed in the United States by battery manufacturers. This represents approximately 3 percent of the total primary and secondary production of cadmium in the United States for that year.
As part of its program in Secondary Metals Research, the Bureau of Mines included this research, which led to the development of a hydrometallurgical process for selectively leaching the cadmium from battery plates using an ammonium-nitrate leaching solution. Scrap nickel-cadmium alkaline storage batteries were obtained from the National Bureau of Standards, U.S. Department of Commerce, which had made some preliminary tests on recovery methods for the Bureau of Naval Weapons but had abandoned the work because an early solution was not in sight.
The recovery of cadmium from secondary sources represents a small portion of the overall cadmium production. Techniques that have been developed for the recovery of secondary cadmium at the Bureau of Mines included vacuum distillation and amalgam electrolysis. At the time of this investigation there were no known processes in use for recovering cadmium and nickel from scrap storage batteries. The value of these metals warranted more costly methods of recovery than are presently used by secondary metal plants, as well as the use of more highly skilled operating personnel.
Scrap Battery Composition and Construction
The scrap batteries used in this investigation were manufactured by several American firms and were for the most part of similar construction. They had been used in aircraft, and weighed approximately 80 pounds. The batteries differed in the type of material used for the housing, cell cases, and grid material. The battery housing was made of steel or aluminum, and in some instances contained fiberglass spacers between the cells. Each battery contained 19 plastic or stainless steel encased cells. Three different batteries of similar construction and weight were selected at random and dismantled to determine their construction and composition. Results shown in table 1 give the range of the major components based on an average battery weight of 78.9 pounds.
Several battery cells were completely dismantled and samples were taken at random from positive and negative plates and analyzed for cadmium and nickel content. The grid material was either fine nickel screen or nickel-plated, perforated sheet steel. Analysis of the battery plate material is shown in table 2.
How to Recover Cadmium & Nickel from Scrap Batteries
Two methods for the recovery of the metal values from scrap battery plates were considered. One method involved vacuum distillation in which the metallic cadmium would be volatilized and condensed, with the plate residue being smelted for the recovery of the nickel. The other method was a hydrometallurgical leaching process in which one of the metals would be selectively leached from the battery plates.
Exploratory vacuum distillation tests showed that 99 percent of the available metallic cadmium could be recovered from the battery plates. The recovered cadmium had a purity of 99.9 percent with zinc being the only significant impurity. The cadmium was readily distilled at temperatures of 650° to 800° C at pressures of approximately 0.1 mm Hg for 60 to 120 minutes. The metallic cadmium content was 16 to 20 percent, by weight, of the negative plates, and 1.0 to 1.5 percent of the positive plates. The balance of cadmium (table 2) was present as cadmium oxide. These percentages will vary since the metal to oxide ratio is dependent upon the state of charge of the battery.
Preliminary tests using salts, acids, gases and/or a combination of these chemicals, were made to determine the dissolution rates and selectivity toward metallic cadmium and nickel. Ammonium nitrate and ammonium chloride were found to be the most promising reagents. These two solutions were tried on CdO and NiO. Ammonium nitrate was found to be the best reagent, since it readily dissolves metallic cadmium and cadmium oxide, but does not significantly attack nickel or nickel oxide.
Cadmium forms a highly soluble [Cd(NH3)4]++ complex with ammonium hydroxide and ammonium salts. Varying combinations of NH4NO3 and NH4OH were used to determine the optimum concentration of the lixiviator for the selective leaching of only cadmium from battery plates. The best cadmium extractions occurred with 4 M NH4NO3 solutions and all concentrations of NH4OH tested. However, the higher concentrations of NH4OH leached increasing amounts of nickel. The optimum leach solution was selected as 4 M NH4NO3 with 1 molar or less of NH4OH.
Leaching temperatures were investigated. Little advantage was gained by leaching at temperatures above room temperature. The cadmium extractions started to decrease at temperatures above 45° C.
Roasting
The products normally found in battery plates are Ni, Ni(OH)2, Cd, and Cd(OH)2. The main reaction of the nickel-cadmium cell on charge and discharge is
2β NiOOH + Cd° + 2 H2O = 2 Ni(OH)2 + Cd(OH)2.
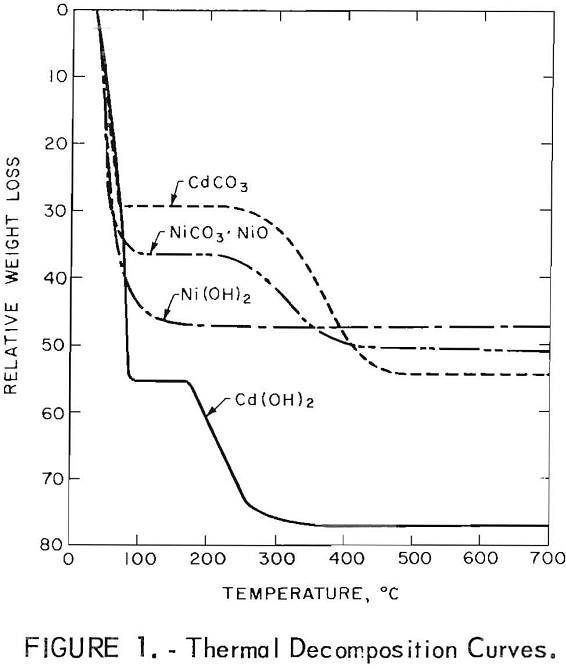
Upon exposure to the atmosphere, the battery plates and potassium hydroxide electrolyte absorb CO2 to form a basic nickel carbonate [NiCO3·NiO] and CdCO3. Duval reports the decomposition curves for Cd(OH)2, CdCO3, Ni(OH)2, and NiCO3·NiO as shown in figure 1. The curves are for wet precipitates so that the first portion of the curve is the elimination of absorbed water from the precipitate and the first plateau is the anhydrous precipitate. These curves show that all of the compounds decompose at a low temperature with the exception of CdCO3. The conversion of CdCO3 to CdO is complete at 488° C.
Battery plates were roasted over a temperature range of 140°-550° C. There was a marked decrease in the soluble nickel found in the leach solutions as the temperatures were increased. The amount of leached nickel decreased to a minimum value at 500° C but had no effect on the solubility of cadmium.
Metallic cadmium will consume the lixiviator reagent as indicated by the following reaction:
Cd° + 2 NH4NO3 = Cd++ + 2 NH3 + NO + H2O.
The result of this reaction would represent a loss of 0.71 gram of NH4NO3 for every gram of metallic cadmium dissolved. If the cadmium were oxidized prior to leaching, the reaction would be a simple solvation process according to the following reaction:
CdO + 4 NH4NO3 = Cd(NH3)4(NO3)2 + 2 HNO3 + H2O.
Therefore, roasting has a twofold advantage: It decreases the amount of nickel that is leached and it decreases the NH4NO3 consumption, so that the solution can be recycled at or near original strength. To minimize the danger of cadmium oxide fume, due to volatilization or burnoff of the cadmium metal,
a small rotary kiln with a 2-inch-diameter stainless steel tube was constructed for continuous roasting of the battery plates. In this type of furnace the plates could be pre-heated slowly to the desired roasting temperature. No detectable amount of CdO was noticed in the exhaust gas.
Product Recovery
Several techniques were investigated for recovering leached cadmium from the lixiviator. Cementation with zinc dust and electrowinning approaches did not prove satisfactory. A precipitation technique using carbon dioxide afforded a simple and clean recovery method without undue contamination of the extracting lixiviator, Carbon dioxide gas passed through the lixiviator at atmospheric pressure and room temperature produced a white precipitate, which was identified by X-ray diffraction and wet chemical analysis as pure CdCO3. Complete precipitation of cadmium could be achieved with a minimum carbon dioxide pressure of 5 to 10 psi at room temperature. Vigorous agitation was an important factor in the precipitation rate. In the first 7 minutes 99.9 percent of the cadmium was precipitated as shown in table 3.
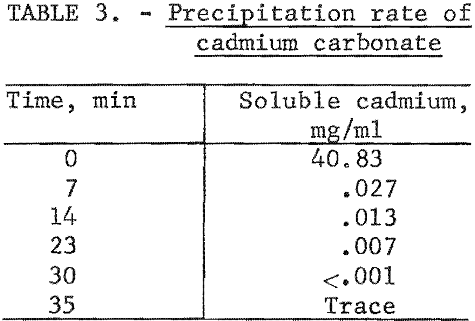
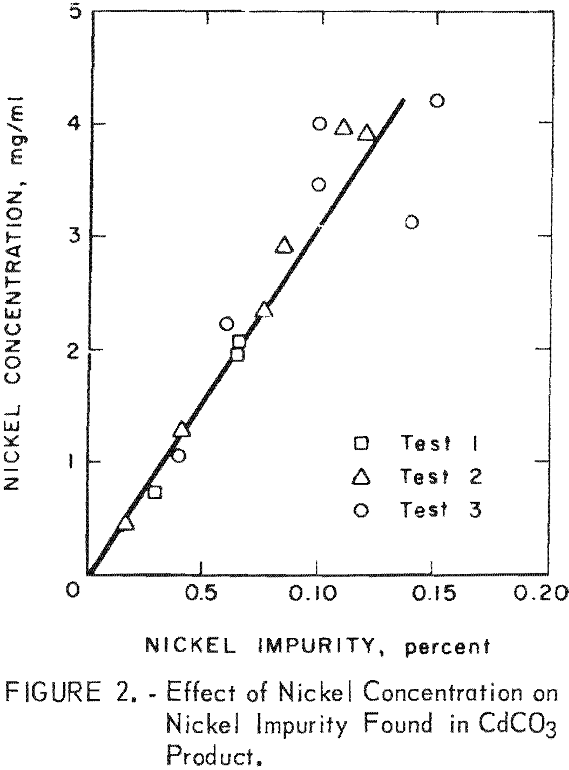
Spectrographic analysis of the cadmium carbonate revealed that the only major impurities were nickel and potassium in concentrations less than 0.2 percent. The nickel contamination in the CdCO3 product was found to vary linearly with the nickel concentration in the CdCO3 filtrate solution. Nickel analysis obtained in three larger scale cyclic leach tests is shown in figure 2.
Lixiviator Regeneration
The two following steps are required to regenerate the lixiviator for recycling: (1) the removal of CO2 to prevent precipitation of the cadmium on the plates during the leach cycle, and (2) the removal of nickel from the lixiviator to prevent nickel contamination of the CdCO3 product.
Carbon Dioxide Removal
The factors studied for the removal of CO2 were pH, temperature, and pressure. The pH was found to have the greatest effect. A 30-minute reaction time with agitation was used. By acidifying the lixiviator to pH 4.5 and heating to 50° C with a pressure of 0.66 atmosphere, the carbon dioxide was reduced to 0.09 gram per liter. This carbon dioxide concentration was found to be sufficiently low to prevent CdCO3 precipitation during the subsequent leach cycles.
Nickel Removal
Although only a small quantity of nickel is solubilized during each leach cycle, the amount is cumulative and will appear as a contaminant in the CdCO3 product unless removed. Cementation, precipitation, and electrowinning methods were tried unsuccessfully for removing the nickel from the lixiviator. An ion-exchange method had the disadvantage of requiring too much time for a complete regeneration cycle of the resin. A solvent extraction method gave the best results.
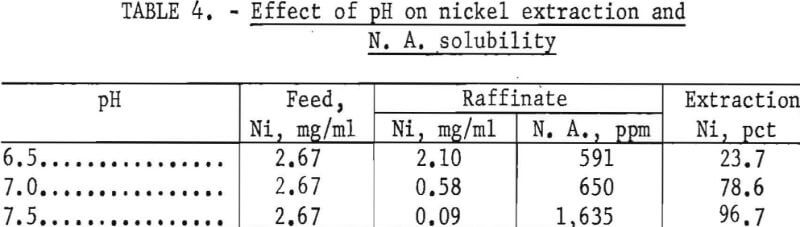
Extraction of a number of the more common metals, including nickel, from sulfate solutions using naphthenic acid (N. A.) dissolved in kerosene was described by Fletcher and Wilson. To determine the effect NH4NO3 would have on the extraction of nickel, leach solutions were prepared and adjusted to various pH levels prior to extraction with 1N-naphthenic acid-kerosene solvent. The results in table 4 show that at a pH greater than 7.0, the N. A. solubility in the aqueous phase increases rapidly. Only 78.6 percent of the nickel is extracted at pH 7.0. However, it is not necessary to remove all of the nickel during lixiviator regeneration, but only to maintain it at a low concentration. A pH 7.0 was therefore selected as the optimum level. The nickel was stripped from the naphthenic acid-kerosene solvent with 1N HNO3.
Cyclic Leaching of Roasted Battery Plates
Bench-Scale Cyclic Leaching Apparatus
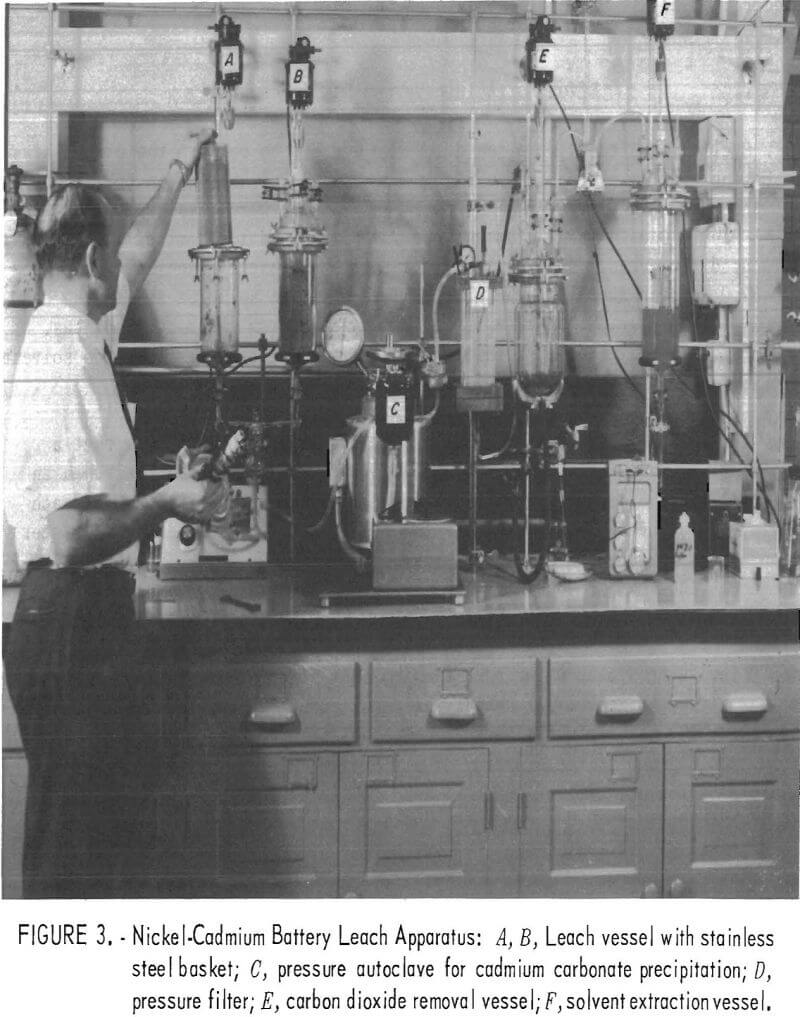
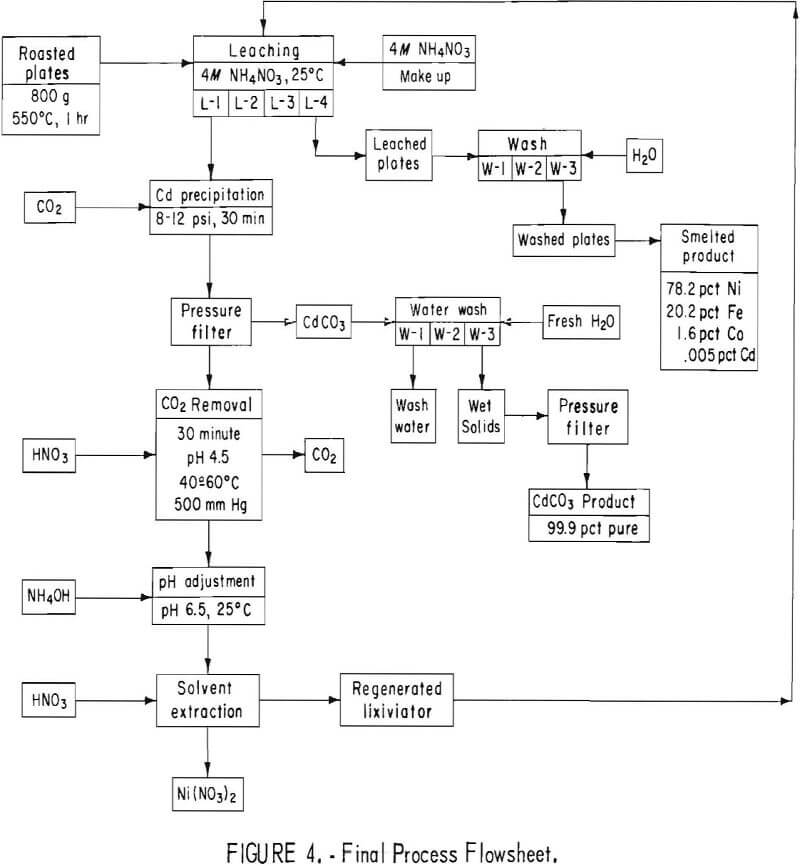
After determining the optimum conditions for leaching, cadmium precipitation and recovery, CO2 elimination, and solution regeneration, the apparatus for the complete process was assembled as is shown in figure 3.
Preliminary Leach Tests
A preliminary test was run to check the operation of the complete apparatus. No major problems were encountered until the third cycle of the solvent extraction step, when it was observed that the regenerated lixiviator turned yellow after separating from the organic phase. Spectrographic analysis of the two phases showed that the aqueous phase contained small
amounts of calcium and magnesium, and the organic phase contained a high concentration of potassium. The potassium was attributed to the incomplete removal of KOH during the washing step. Synthetic solutions containing calcium, magnesium, and potassium were also treated with the naphthenic acid-kerosene solvent. The treated synthetic solutions closely duplicated the regenerated lixiviator in that the solution turned yellow and potassium was found in the organic phase. Potassium is detrimental to the organic phase because it consumes naphthenic acid. To remove the major amount of KOH, the plates must be washed until the wash water tests approximately pH 10.5 to 11. Approximately seven wash cycles using 14 to 15 gallons of water per 100 pounds of battery plates were required. A 30-minute wash cycle was used.
The effect of KOH removal was apparent when the CdCO3 filtrate solution was acidified for CO2 removal. In previous tests, in which KOH was absent from the lixiviator, vigorous gassing of CO2 occurred during pH adjustment. No such gassing occurred in these tests, and large volumes of HNO3 were required to adjust to pH 4.5 because of the formation and neutralization of K2CO3.
Effect of Acidic Versus Basic Leaching
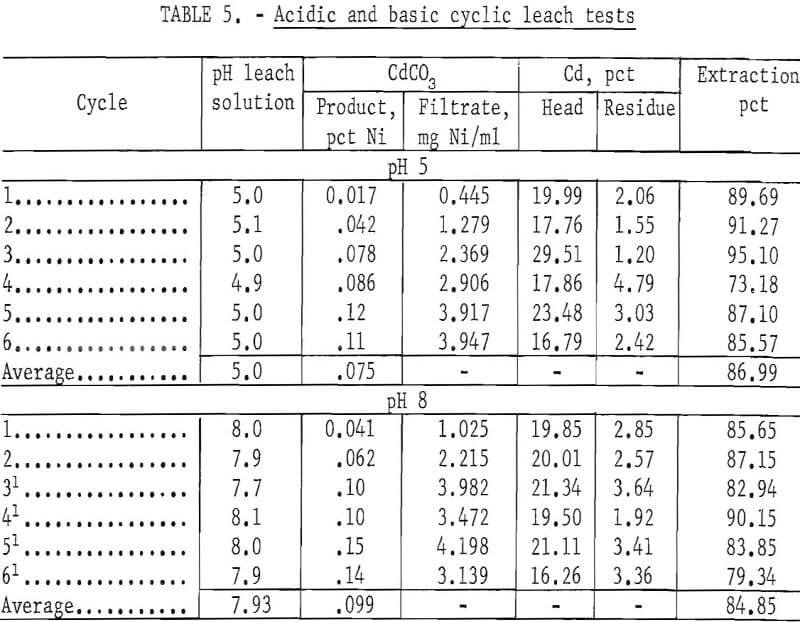
Cyclic leach tests were run at pH 5.0 and 8.0 to compare the effect of pH on the cadmium extraction and to follow the nickel buildup in the solution and the CdCO3 product. For these tests, all plates were roasted at 450° C for 1 hour. One thousand grams of roasted plates and 1,700 ml of 4 N NH4NO3 were used per cycle. The results in table 5 show that extraction of cadmium was 87.0 percent at pH 5. The nickel buildup in the solution and in the CdCO3 was low because at pH 5 little nickel was leached from the battery plates. Because the nickel concentration in the filtrate solution was low, the solvent extraction step was bypassed.
The next test was run at pH 8.0. The results (table 5) show that a basic solution (pH 8) of NH4NO3 will leach larger amounts of nickel than an acid solution. The solvent extraction step was used after the second leach cycle, because the nickel concentration in the filtrate solution was greater than 2 mg/ml. The cadmium extraction was 84.9 percent. The amount of reagents required for adjusting the pH for CO2 removal and neutralization to pH 7 for the solvent extraction step was approximately 3 to 4 times greater than the cyclic leach tests run at pH 5. Although the tests at pH 5 and 8 gave approximately the same cadmium extraction, these tests show that at the higher pH additional amounts of nickel are leached from the plates. Adjusting the pH of the lixiviator to the acidic side has several advantages. They are (1) keeping the quantity of solubilized nickel to a minimum, (2) minimizing the quantity of reagents required in a complete leach cycle, (3) requiring less pH adjustment of the lixiviator after the solvent extraction step, and (4) minimizing the probability of CO2 being absorbed by the solution causing the Cd to be precipitated on the plates during the leach cycle.
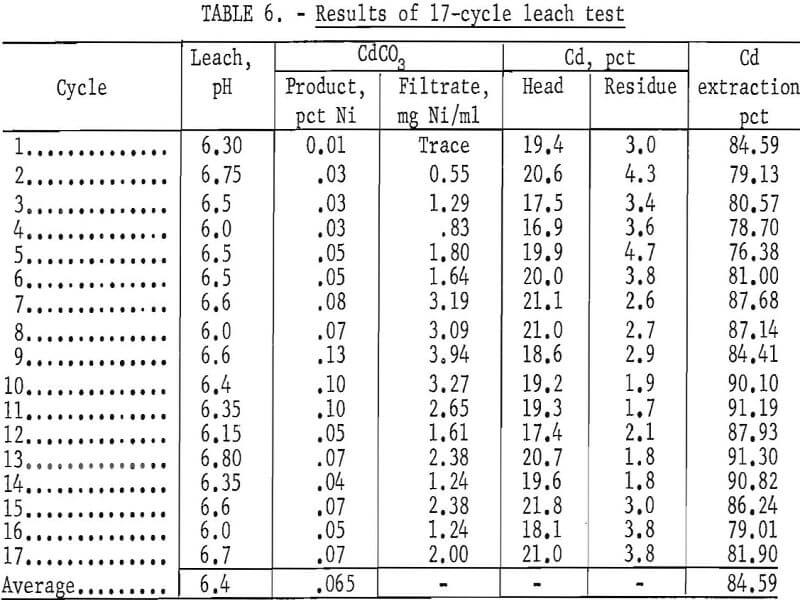
Multicycle Leach Test
A 17-cycle leach test was made in an attempt to increase the extraction efficiency of cadmium, study impurity buildup, determine the organic reagent loss, and evaluate the overall process. The procedure and conditions were modified slightly as follows: (1) a slight positive pressure of helium was maintained wherever possible to prevent CO2 from being absorbed into the system, (2) the solvent extraction step was bypassed if the nickel concentration in the CdCO3 filtrate solution was less than 2.0 mg per ml, and (3) the lixiviator was adjusted to pH 6.5 at the start of each leach cycle.
A charge of approximately 1,100 grams of plates roasted at 450° to 500° C was used for each cycle. The plates were washed many times in cold tap water to remove the KOH electrolyte before roasting. A starting volume of 1,700 ml of 4 N NH4NO3 (320 g/liter) was used. The plates were leached three times according to the schematic diagram in figure 4. After the final leach, each batch of plates was removed from the system, washed with 1,500 ml H2O + 10 ml concentrated NH4OH, and rinsed in hot water.
The results in table 6 show that 84.6 percent of the cadmium was extracted, and that the CdCO3 product contained an average of 0.065 percent nickel. The average pH of the lixiviator was 6.4. The volume of HNO3 needed during the CO2 removal was 50 percent less than the amount required in the previous test run at pH 8.0. An analysis of the naphthenic acid-kerosene solvent showed that, for the nine cycles in which the solvent extraction step was used, there was a loss of 3.9 parts per thousand per cycle of naphthenic acid. No difficulties were encountered during the 17 leaching cycles.
Final Cadmium-Nickel Leach Test
The maximum cadmium extraction for previous cyclic leach tests of five or more cycles was 87 percent. The data were reevaluated to determine which factors could increase the extraction of cadmium. To prevent the solution from absorbing CO2 and precipitating CdCO3, the NH4NO3 lixiviator was adjusted to pH 3 with HNO3. To insure adequate roasting, the roasting temperature for the final test was maintained at a minimum of 550° to 600° C. In several of the initial small leach tests it was noted that extractions of greater than 90 percent were achieved using solution-to-solid ratios of 12 to 1, or greater. In the large-scale tests, the ratio was approximately 5 to 1. Therefore, the weight of battery plates to be leached per cycle was decreased to approximately 800 grams, and a fourth leach step was added, in which a solution-to-solid ratio of approximately 8 to 1 was used. A schematic diagram of the final process is shown in figure 4. The conditions selected for the final test were (1) roasting at 550° to 600° C, (2) using 800 grams of battery plates, (3) 1,700 ml of 4 N NH4NO3 per leach, (4) adding a fourth leach per cycle, and (5) adjusting the lixiviator to pH 3 before starting each leach cycle.
The results in table 7 show that the average extraction was 94.2 percent for the complete test. For the first six cycles the lixiviator was adjusted to pH 3 before it was transferred to the first battery-plate leach vessel. The average extraction for the first six cycles was 96.3 percent. During the leach the reaction products raise the pH of the lixiviator. Starting with the seventh cycle, the lixiviator was first added to the plates and an attempt was made to maintain the pH of the lixiviator at pH 4 to 5 during the leaching period. Maintaining the pH at 4 to 5 required large amounts of acid because of the strong buffering action of the solution. The results show that the cadmium extraction started to decrease after the seventh cycle, and the nickel impurity in the CdCO3 started to increase. At a pH of less than 5, it was difficult to precipitate the cadmium.
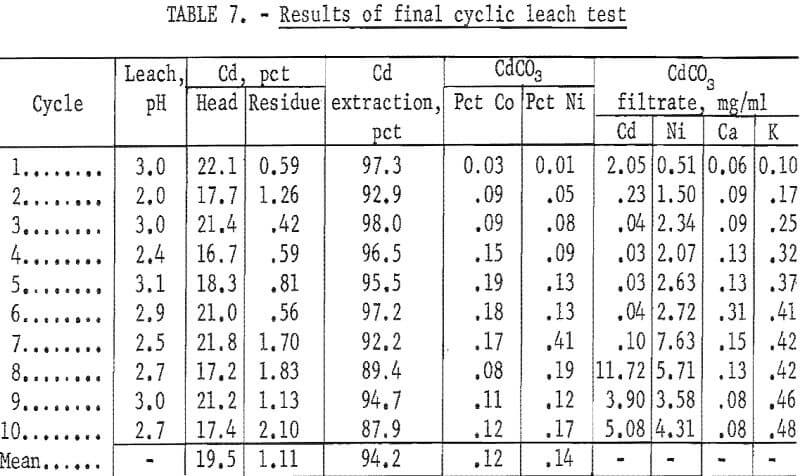
There was no significant impurity buildup in the solution. The calcium and potassium reached a maximum concentration of approximately 0.13 and 0.45 gram per liter, respectively, and did not tend to increase beyond this value. The only impurities found in the CdCO3 product were cobalt and nickel as is shown in table 7.
Analysis of the organic phase after the 10 cycles showed a total weight loss of 3.89 percent of the naphthenic acid or 0.39 percent loss per cycle. This would represent 0.55 gram of naphthenic acid per 13000 ml of aqueous solution.
Smelting of Residue
The leached residues from the first four cycles of the final test were used for smelting to metallic metal at 1,420° C. The charge consisted of 2,134 grams of leached plates and 78 grams of crushed graphite. The leached plates contained approximately 75 percent paste (82.2 percent Ni as NiO and metallic Ni, and 0.72 percent Cd) and 25 percent metallic iron as grid material. The material was smelted in a clay-graphite crucible using an induction furnace. During the initial heating, brown fumes were observed that were assumed to be CdO. A sample was taken of the molten metal and of the cast metal after cooling. The analysis is shown in table 8. Spectrographic analysis of the samples showed no impurities present other than those shown in table 8.

Conclusion
Results have shown that two marketable products can be recovered from secondary battery-waste material. The major steps in the cyclic leach process and the products recovered follow:
- The battery plates were washed to remove the KOH electrolyte. Poor washing causes the use of large amounts of acid for the neutralization step of the lixiviator after CdCO3 precipitation.
- Roasting at a minimum temperature of 550° C was required to decompose the Ni and Cd salts and to completely oxidize the metallic Cd and Ni. This step is important so that the leach will be selective and none of the reagent will be consumed.
- A strong leach solution of 4 M NH4NO3 with a starting pH of 3 for each leach cycle gave cadmium extractions of greater than 94 percent.
- Carbon dioxide at 10 psig precipitated 98 to 99 percent of the leached cadmium from solution as CdCO3 with an impurity content of 0.14 percent nickel and 0.12 percent cobalt.
- The CO2 was removed rapidly from the lixiviator by adjusting to pH 5.0 or lower, heating to 50° C, and evacuating the system to 0.66 atmosphere.
- The leached nickel in the lixiviator was extracted by adjusting to pH 6.5 and using a 1N-naphthenic acid-kerosine solvent extraction step. Complete removal of the Ni from solution was not necessary, but should be maintained at 2 g/l or less.
- The residue, which is primarily NiO and iron, can be smelted to produce a product which contains 78.2 percent Ni, 1.6 percent CO, 20.2 percent Fe, and no detectable amounts of Cd.
Analytical Determination of Naphthenic Acid
The method for the determination of naphthenic acid (N, A.) in aqueous solutions at varying pH levels as outlined by Fletcher and Wilson was tried. Constant weights on each determination were difficult to achieve and duplication of analytical results were not possible A new method was developed which consists of taking a known volume of the organic phase and transferring it to an erlenmeyer flask containing an equal volume of ethyl alcohol. The mixture is then titrated to the phenanthroline end point using a standardized alcoholic (95 percent) solution of potassium hydroxide. Blank values were determined by titrating an equal volume of untreated kerosene dissolved in an equal volume of ethyl alcohol to the phenanthroline end point.
A modified procedure for devised for determining the quantity of N. A. found in the aqueous ammonium nitrate phase. A known volume of the aqueous phase was transferred to a beaker and acidified with HCl to pH 3.5. The contents were transferred to a clean separatory funnel and shaken with 100 ml of anhydrous ether. After phase separation the ether layer was transferred to an erlenmeyer flask containing an equal volume of ethyl alcohol. The mixture was then titrated to the phenanthroline end point with the standardized alcoholic (95 percent) potassium hydroxide. Blank values were determined in a similar procedure by taking an equal volume of the untreated ammonium nitrate solution.
