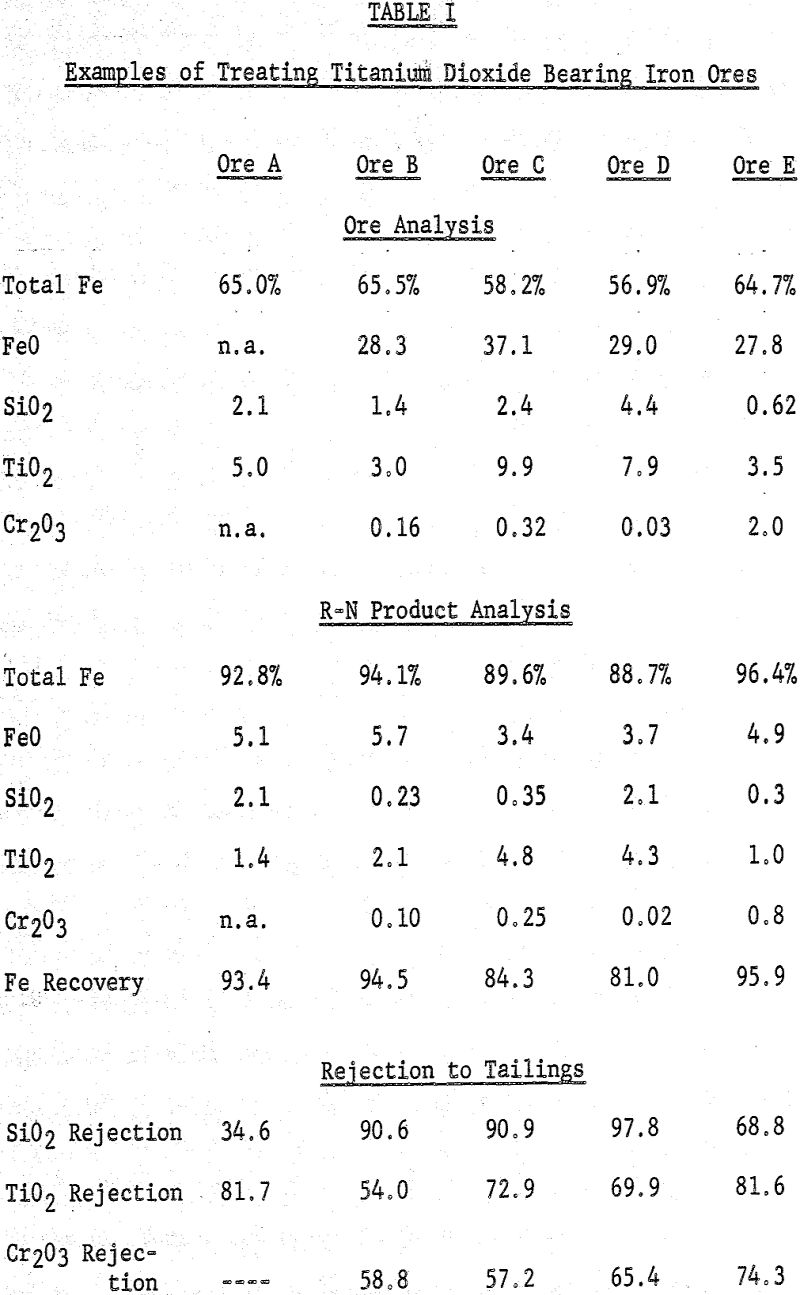
Most iron ores treated by the R-N direct reduction process yield metallic iron products considerably lower in titanium, manganese, and chromium oxides and alkaline metal sulfates/sulfides than were contained in the original ores. These metallic iron products are suitable as feed stock for the open hearth and electric steel-making furnaces. The elements which are rejected in part are acceptable in the product since the product is designed to by-pass the blast furnace.
R-N reduction conditions (PCO+H2-¹ atmosphere, 1830° to 2150° F.) are adjusted for each ore. The iron ore which is reduced to 90 to 95 percent metallic iron is then subjected to the conventional beneficiation techniques. Under these conditions, 55 to 80 percent of titanium oxides, 75 to 95 percent of manganese oxides, 60 to 90 percent of chromium oxides and up to 97 percent of alkaline metal sulfates contained in the ore are rejected with the gangue to the non-magnetic tailings.
The metallic iron products illustrated in this work contained 89 to 96 percent total iron, one to five percent TiO2, 0.2 to one percent MnO 0.3 to 1.6 percent Cr2O3 and/or up to one percent alkaline metal sulfide. Usually only one of these components was present.
About 80 to 95 percent of the iron in the ore fed to the process is recovered, mostly as metallic iron. These products are suitable as feed to the open-hearth and electric steel-making furnaces. Most of the significant quantities of titanium dioxide, manganese oxides, chromium, oxides and/or alkali or alkaline earth sulfates which may be present in the ore are rejected to the non-magnetic tailings. Metallic iron products are obtained from ores containing one to five percent TiO2, 0.2 to 1.5 percent MnO, 0.3 to 1.5 percent Cr2O3 and/or up to one percent alkaline metal sulfides.
Under R-N conditions (PCO+H2-¹ atmosphere, 1832° to 2150° F.) manganese oxides and carbonates are reduced mostly to non-magnetic MnO, while 90 to 95 percent of the iron content of the ore is reduced to the metallic state. Subsequent beneficiation yields a metallic iron product with rejection of 75 to 95 percent of the manganese and 89 to 98 percent of the silica to the non-magnetic tailings.
During reduction of ores by the R-N process, 90 to 95 percent of the iron is reduced to the metallic state. The chromium oxides are largely rejected to the non-magnetic tailings during beneficiation of the reduced ore. When the chromium is present in silicates and other complex minerals, rejection to the non-magnetic tailings is generally poor.
Iron ores containing sulfates of metals such as barium, calcium, magnesium, potassium, and sodium are amenable to treatment by the R-N process. Ore H, mentioned in the section on manganese, will be used as an example. This ore also contained 15 percent barium sulfate. Barium sulfate was the only barium mineral detected in the ore by X-ray diffraction techniques, and was observed as visible inclusions ranging from 4 to about 48 mesh per inch in size.
