Table of Contents
This research has shown that & solid waste byproduct from secondary lead smelters can be successfully processed to recover copper and lead that is lost during smelting. The major steps in the process and the products recovered are as follows:
- Copper and lead sulfides in blast furnace matte are converted to sulfates and iron sulfide is converted to ferric oxide by roasting at 600° C.
- A countercurrent water leach of the roasted matte extracts 90 percent of the copper along with other water soluble sulfates.
- A cyclic brine leach that follows the water leach extracts 96 percent of the lead which is later recovered as a pure basic chloride.
- The residues which are primarily iron oxide can be smelted to pig iron that would need to be processed further to remove objectionable impurities.
About 58 percent of all lead produced comes from secondary sources and more than half of this arrives at the smelters in the form of scrap battery plates. A wide variety of equipment and smelting procedures is used in the secondary industry to recover this lead. The two methods that are relied upon most heavily are direct conversion into metal using a reverberatory furnace or smelting the battery plates in a blast furnace.
In one method, battery plates are fed to a reverberatory furnace and are converted directly into metal suitable for kettle refining. This procedure has the advantage of low metal losses in fume, ability to handle fine material and no danger of freezeups. However, there are disadvantages too. Pressures from air pollution abatement officials require the furnace gases to be filtered. Equipment for cooling these hot gases to the filtering temperature requires considerable capital expenditure. Even after filtering, the gases still contribute seriously to air pollution by virtue of the high concentrations of sulfur dioxide that they contain. Another disadvantage of this method of smelting is that the slags from the furnace contain antimony and lead in amounts that require retreatment in a blast furnace for their recovery.
A more efficient method, for treating battery plates appears to be smelting in a blast furnace. This method is the most popular where the desired product is antimonial lead. The charge to the blast furnace generally consists of battery plates, limestone, coke, fluorspar, and scrap iron or mill scale. Products from the furnace are lead metal, matte, and a slag that normally contains less than 1 percent lead.
The matte represents a serious loss of lead. Depending on the accuracy of adjustments in charge composition, mattes will contain from 10 to 20 percent lead and most of the copper that accompanies the charge as copper connector lugs. Larger additions of scrap iron or mill scale will free part of the metal in a high lead matte but a point will be reached when the cost of such additions is greater than the value of the metal made free. Some plants report that careful control of smelting conditions will allow operation with little or no matte formation. Other plants report in the neighborhood of 2 tons of matte discarded for every 50 tons of bullion produced.
From the standpoint of both economics and conservation it would be desirable to have a suitable process for salvaging the metals in this waste. As part of its program in Secondary Metals Research, the Bureau of Mines included this problem and a method was developed that allows recovery of both lead and copper as well as iron from blast furnace matte. This report describes the application and further development of previous Bureau of Mines research dating back to 1914.
Brine Leaching Developments
Some of the most extensive research on use of brine leaching as a hydrometallurgical method for recovering lead from ores was conducted by Lyon and Ralston, two prominent metallurgists in the early days of the Bureau of Mines. At that time it was known that saturated solutions of brine and neutral ammonium acetate solutions were good solvents for both lead chloride and lead sulfate. It was also known that lead oxide and lead carbonate could be solubilized if the brine were acidified first with either sulfuric or hydrochloric acid. Practicable electrolytic and chemical precipitation methods eventually were developed for recovering the dissolved lead as either the metal or the hydroxide. These facts formed the basis for development of successful laboratory processes for recovering lead from both low-grade and complex ores.
More recently Marsden reported on the application of brine leaching as a method for recovery of lead from zinc plant residues of the Rhodesia Broken Hill Development Co., Ltd. Without use of acid these residues could be made to yield about 80 percent of their contained lead by leaching at ambient temperatures with brine. Addition of relatively small amounts of hydrochloric acid to the brine increased lead extraction to 98 percent.
Application of these same principles to recovery of lead in blast furnace matte would require that the sulfides first be converted to a form that will allow solubilizing by brine. Development of a method for accomplishing this was necessarily the first phase of the present investigation.
Materials Tested
Samples of matte for this work were obtained from two different smelters. Chemical analyses for both are given in table 1. The sample designated matte A in some respects was quite different in physical appearance from matte B. It did not have the semimetallic luster, but instead it was dull, weathered rapidly when exposed to air, and disintegrated into a rusty colored powder. The plant that produced matte A operates its blast furnace with mixed charges of widely varying types of lead wastes. Matte B was obtained from a plant that was operating almost exclusively on battery plates. Matte B also contained a significant amount of small metallic iron particles. This together with the high lead content suggests that the furnace may have been operating above capacity with inadequate time of retention to allow additional lead to separate.
Particle size of matte A as received varied from chunks as large as 1 inch in diameter to dust that passed through a 100-mesh screen. The larger pieces were soft and friable and could easily be reduced to minus 80 mesh. Matte B was received in the form of hard chunks about 1 inch in diameter and contained only a few fines. This material was considerably more difficult to grind and pulverize than matte A.
Preliminary Experiments and Results
Since sulfides are readily oxidized to sulfates, it was decided to convert the lead and copper values in the matte to brine-soluble lead sulfate and water-soluble copper sulfate. While forming these salts, it was also desirable to minimize the solubility of the other materials. Roasting in air was the method chosen for investigations, but, before attempting to establish optimum roasting conditions, information was needed on the behavior of the matte and component materials in air over a wide range of roasting temperatures. Thermogravimetric studies provided the required information. Samples of both types of matte and samples of ferrous sulfide, copper sulfide, lead sulfide, lead sulfate, copper sulfate, and

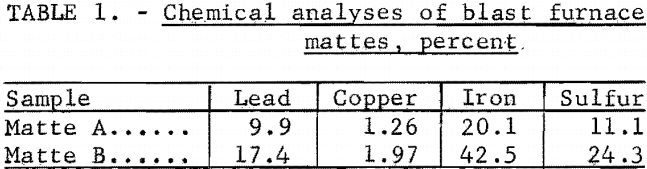
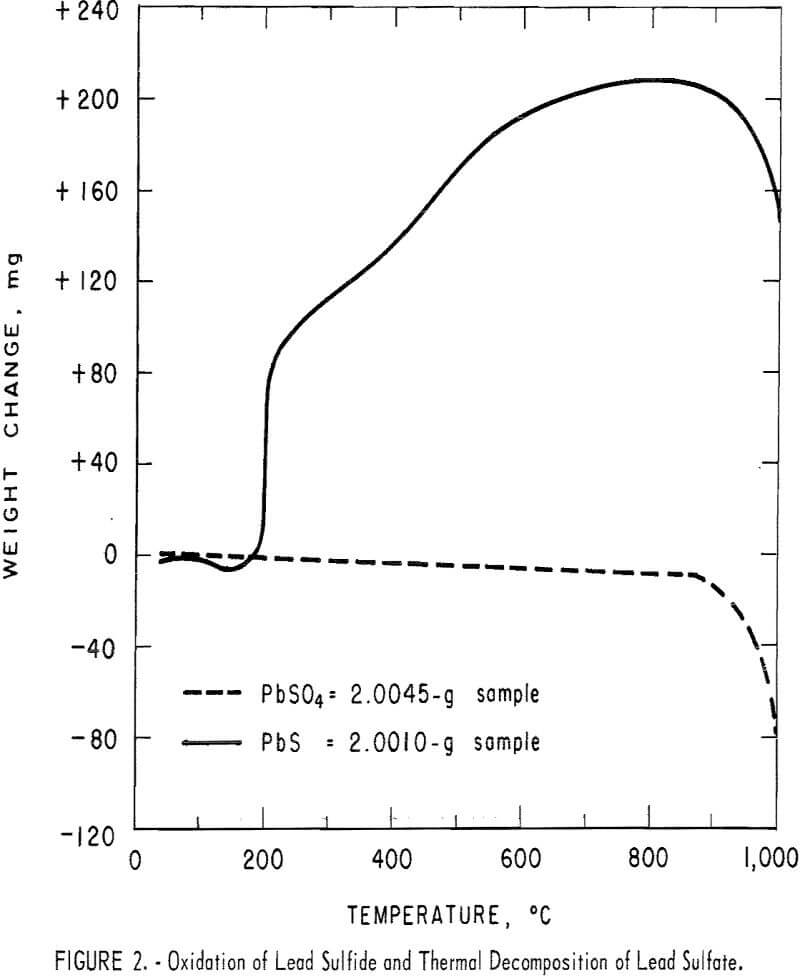
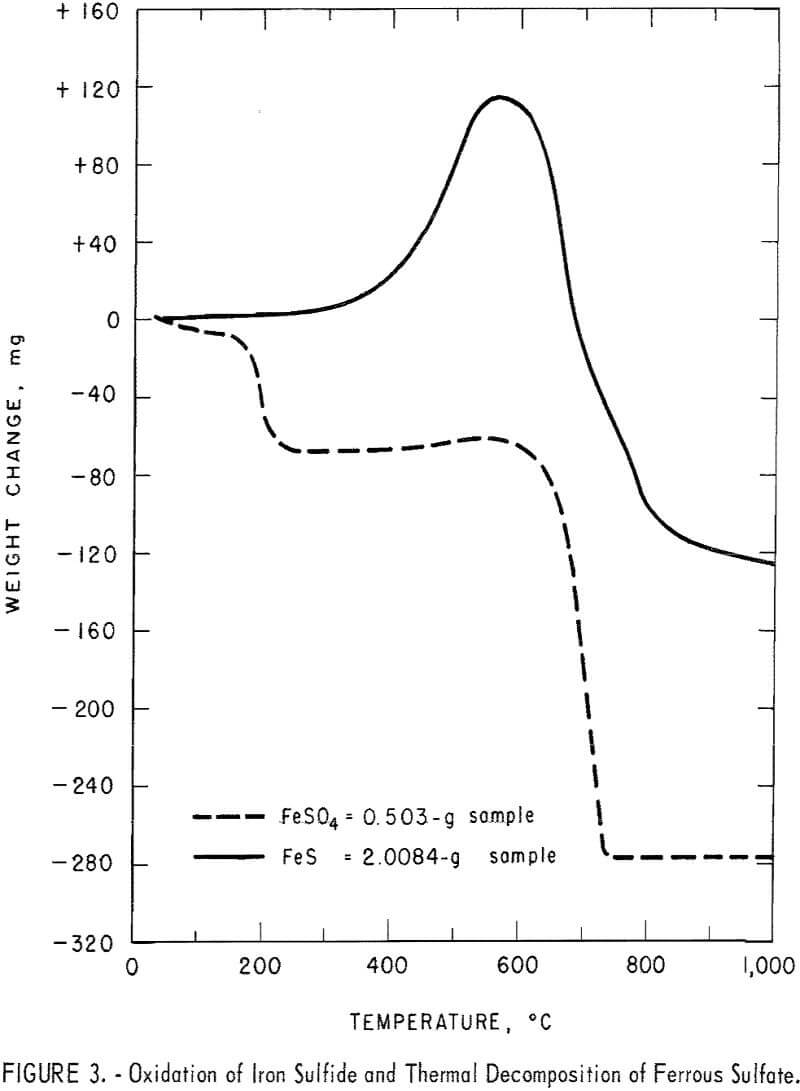
ferrous sulfate were heated in air at 5° C per minute over a temperature range of 0° to 1,000° C in the Mauer-type thermobalance described in previous Bureau of Mines research. Pyrolysis curves for each sulfide are shown with the curves for their corresponding sulfates in figures 1 through 4. The curves for the sulfides provide information concerning the thermal stabilities of the original samples, the intermediate compounds resulting from oxidation and decomposition, and the final products. Temperatures were measured in the immediate vicinity of the crucible holding the sample and they are not an accurate measure of the temperatures at which reactions occurred. However, they do provide fair estimates of ranges of thermal stability.
The curves for copper sulfide and copper sulfate, figure 1, show that soluble anhydrous copper sulfate is the stable salt from about 300° C to somewhat over 600° C. Above 600° C insoluble copper oxide begins to form. The curves for lead sulfide and lead sulfate, figure 2, show that temperatures in excess of 800° C are required to decompose the brine-soluble lead sulfate. Both curves in figure 3 show that ferrous sulfate will begin to decompose to iron oxide at temperatures slightly below 600° C.
These observations suggested that a narrow band of roasting temperatures exists in which mixtures of the three sulfides can be converted to water-soluble copper sulfate and brine-soluble lead sulfate mixed with insoluble iron oxide. This reasoning is verified by the curves of figure 4 for both samples of matte. A weight gain was not observed for matte A during the test. A possible explanation might be, as pointed out earlier, that matte A altered quickly on exposure to air. Thus considerable alteration probably
occurred during sample preparation and the subsequent period of heating to reaction temperatures.
On the basis of the information from the thermogravimetric tests it was concluded that the optimum roasting temperature would probably be found to be slightly less than 600° C. The range chosen for exploration was 450° to 600° C.
Tests to determine the optimum time and temperature for roasting were conducted in quartz boats 4 inches by 6 inches by ¾ inch in size. Boats containing matte ground to minus 80 mesh were heated in air at controlled temperatures inside a muffle furnace. Roasting conditions were evaluated by water leaching and brine leaching of the calcined products. Early in this work it became obvious that hot concentrated brine solutions were
required for extraction of significant amounts of salts from the roasted matte.
Maximum extractions of lead were obtained for samples one-half inch deep that were roasted between 500° and 520° C for periods of at least 1 hour. Temperatures indicated to be best in these tests were nearly 100° C lower than predicted by thermogravimetric studies. The reason for this was later found to be due to improper location of the indicating and controlling thermocouple in the muffle furnace. The temperature of the roasting mass of matte was probably nearer 600° C.
Data reported by Lyon and Ralston also indicated that high concentrations of sulfate ion in solution tended to reduce the solubility of lead in brine. In leaching of roasted matte addition of calcium chloride proved to be an expedient method for preventing sulfate ion from dissolving. The normally soluble sulfate ion was thus precipitated as insoluble gypsum. Chloride ion was then the only anion extracted and the maximum solubility of lead also was achieved.
As preliminary work progressed additional improvements and modifications were made in experimental procedures. Difficulty in separating clean copper and lead products from other dissolved salts led to adoption of a two-stage leach of the roasted matte. Water leaching at room temperature dissolved copper sulfate and other interfering soluble sulfates. Solids remaining were brine leached to extract lead giving a solution from which a pure lead product could be separated by crystallization of lead chloride or precipitation of pure basic salts of lead. The complete process that evolved from this investigation is shown in figure 5 which shows copper, lead, and iron contained in the matte leaving the system as separately recovered products.
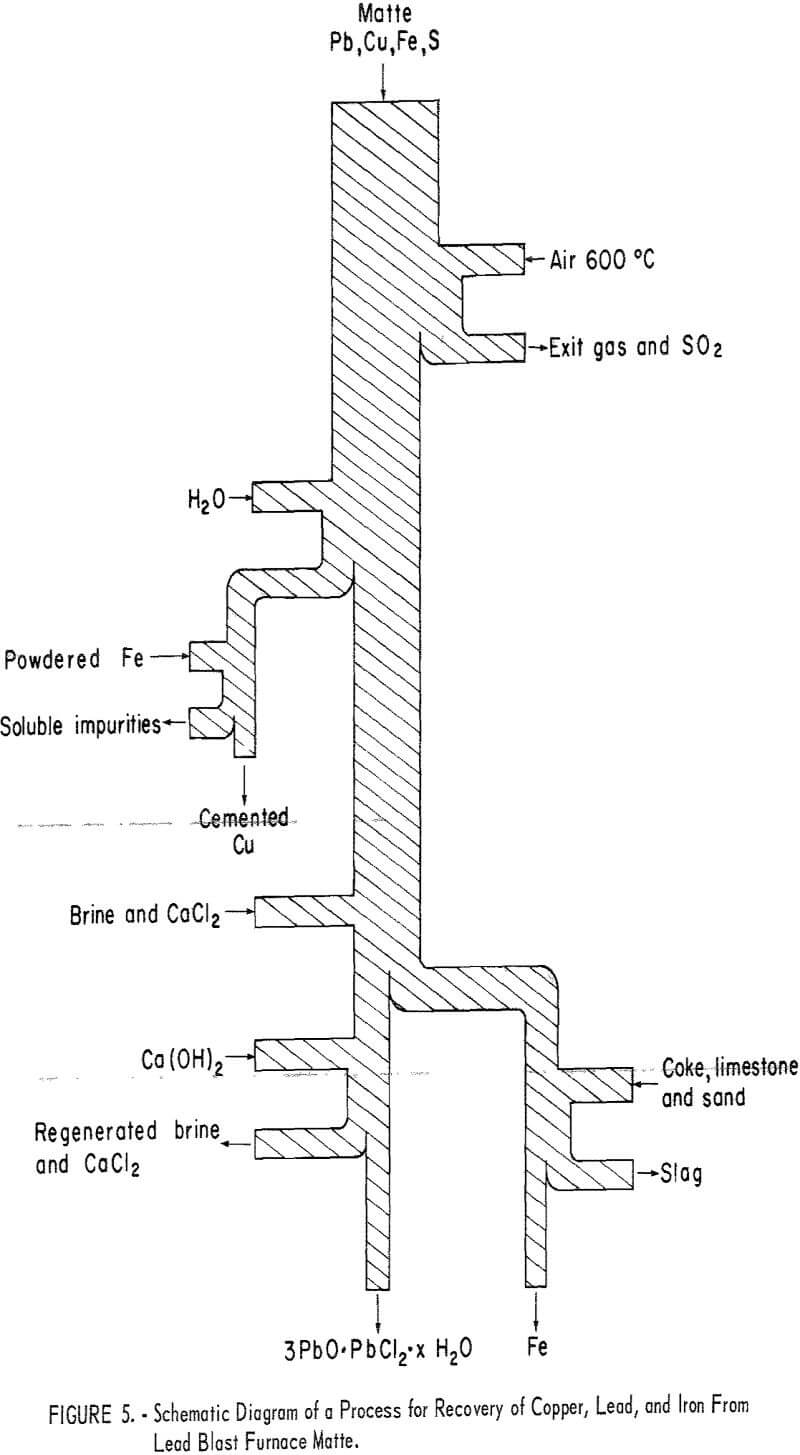
Cyclic Leach of Matte A
All of the remaining portion of matte A was used in a series of cyclic tests. The objective of these tests was to observe overall performance of the process under conditions determined to be optimum for each step. Information was desired regarding (1) efficiency of extraction of lead and copper, (2) efficiency of recovering lead and copper from leach solutions, (3) accumulation of impurities in leaching solutions, (4) recovery and regeneration of brine solutions, (5) purity of products, and (6) overall recovery of metal values.
Matte was roasted under conditions similar to those used in preliminary tests. Quartz boats filled to a depth of one-half inch of minus 80-mesh matte were heated in the muffle furnace to an indicated temperature of 520° C. Charges were allowed to oxidize for 2 hours to assure ample time for forming the desired roasted products. Roasted matte was divided into 14 equal portions of 162.6 grams. The amount chosen for each portion allowed tests to be performed in laboratory apparatus that was readily available and of a size that was convenient to handle. The roasted sample contained 1.38 percent copper and 11.1 percent lead.
Water Leach Series
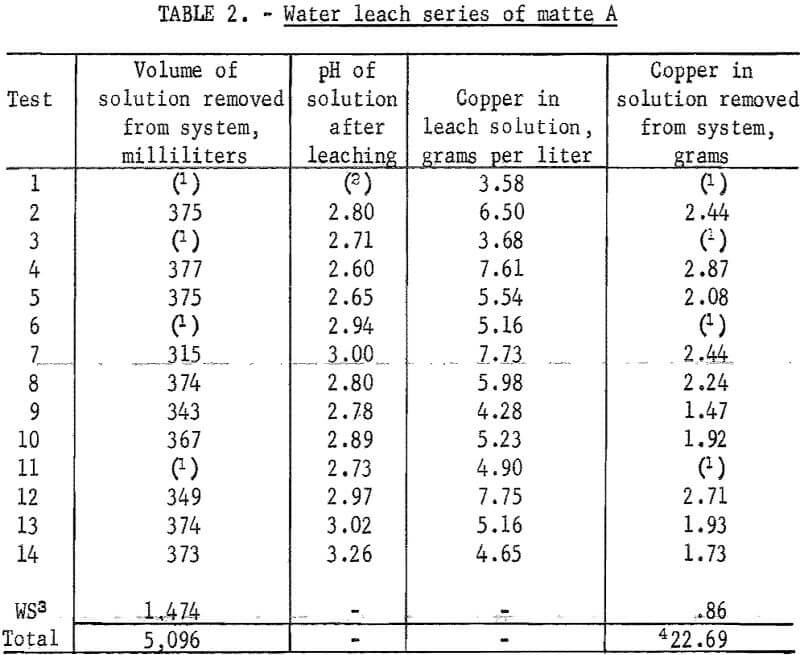
A simulated countercurrent method was used to leach copper and other soluble salts with water. The series of tests was begun by leaching the first 162.6-gram portion of matte with 500 milliliters of water at room temperature. The slurry was stirred for 30 minutes and allowed to settle. When the solution over the settled solids was clear it was decanted. Copper analysis and a specific gravity determination were made on this solution before transferring it to a new 162.6-gram portion of matte. This procedure was continued until the concentration of copper reached about 6 grams per liter at which time the solution was removed from the system.
The first portion of wet matte was washed with 500 milliliters of fresh water while stirring for 30 minutes at room temperature and was then allowed to settle. Clear solution was decanted and copper and specific gravity determinations were made. This solution was then advanced to become the first wash for the second portion of wet matte. The procedure was continued until this solution reached the point where it became the strongest solution and was removed from the system as pregnant copper solution. The wash solution then became the first leach solution for the next portion of matte entering the leaching train.
A second fresh-water wash was added to the first portion of wet matte. After stirring, settling, and decanting, this solution was moved along parallel to the first leach and first wash solutions. The number of washings given each portion of matte was determined by the copper concentration and specific gravity measurements. When no copper could be detected polarographically and when the specific gravity of the wash became less than 1.003, washing was considered to be complete. Wash solutions were advanced successively until they became first leach solutions and were eventually withdrawn from the system as pregnant leach solution. Four washes were normally required for maximum copper extraction and in a few cases five washes were necessary. While a steady-state schedule was not reached for removal of pregnant solutions with only 14 samples, this number of samples did provide the desired information on extraction efficiency. Table 2 presents results of the copper leach for all 14 portions of matte. After completion of leaching the 14 portions of matte, all pregnant solutions were combined and analyzed for copper. Based on this analysis, 72 percent of the available copper was extracted. Significant amounts of other soluble salts were also extracted from the samples. Approximately 41 grams of material was leached from each sample. Only 1.6 grams of this was copper with the balance being primarily iron sulfates. Copper precipitated readily from the combined solutions upon addition of the required amount of iron powder.
Brine Leach Series
Wet solids from copper leaching were used, after drying, in a series of brine leaching tests. The path of solutions over each portion of matte in this case was considerably more complicated than the simple countercurrent copper leach. Briefly, solutions containing the highest concentrations of lead and brine were advanced toward lead-rich solids as early as possible throughout the tests. The schematic diagram for the brine leach cycle is shown in figure 6. Additional improvements were being made in the leaching conditions so that this figure represents the final leach procedure.
Leaching was conducted using 500 milliliters of 25-percent-brine solution (300 grams per liter of NaCl) containing 9.54 grams of calcium chloride Temperature was regulated at 90° C with stirring for 2 hours. The leach solution was filtered, while still hot, through a laboratory pressure filter at 10 psi. The wet filter cake was washed with 300 milliliters of hot 25 percent brine containing calcium chloride to recover solubilized lead chloride. This was followed with 3 to 5 hot water washes to further reclaim solubilized lead and brine from the wet filter cake. Washing was considered complete when the specific gravity of the filtrate decreased to below 1.003. All wash solutions were saved for use in treating the next portion of matte. Solutions nearly saturated with lead were kept hot to avoid crystallization of lead chloride.
Hot brine filtrate rich in lead was maintained at 65° to 85° C until analysis was made for lead. Based on this analysis, calculations were made to determine the proper amount of calcium oxide required to precipitate lead
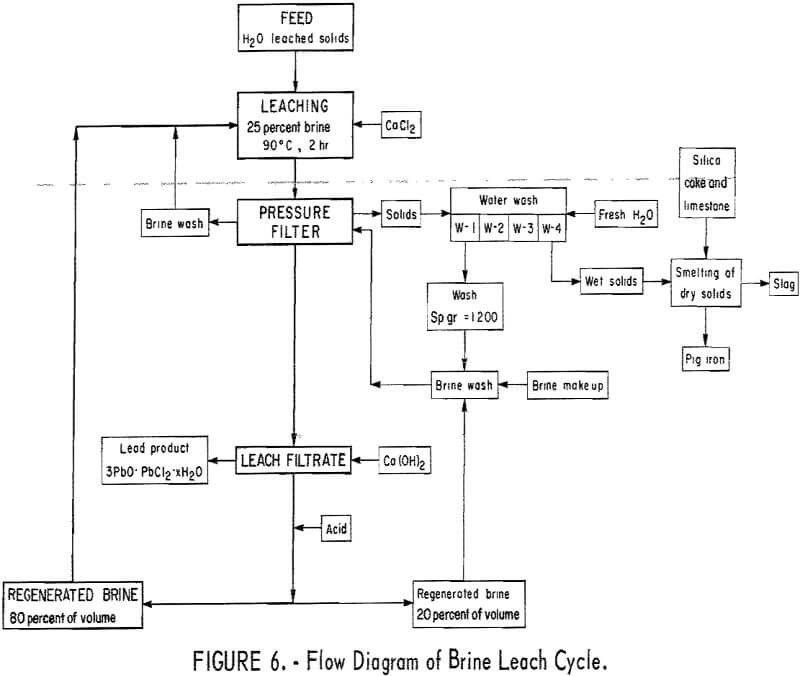
as 3PbO.PbCl2.xH2²O. This amount of oxide was slurried with a minimum amount of water and added to the hot filtrate with vigorous stirring. Progress of the precipitation reaction was followed using a pH meter. The starting pH of about 4.5 to 5.5 rose over a period of 6 to 17 minutes to a maximum pH of 8 to 9 at which point precipitation was assumed to be complete. The slurry was cooled to room temperature and filtered. The precipitated lead product was given three 200-milliliter water washes, dried, and weighed.
Clear filtrate from the precipitation step was lead-free and contained the required concentration of sodium chloride together with almost enough regenerated CaCl2² to be used in leaching the next portion of matte. However, since this solution was lead-free and the first brine wash contained lead that was washed from the leached filter cake, the brine wash was advanced ahead of the regenerated solution to assure moving all lead-rich materials to the precipitation step as early as possible. Thus, the solution used to leach the next portion of matte was made up of the filtrate from the brine wash (approximately 300 milliliters) and enough regenerated solution (approximately 200 milliliters) to bring the total volume to 500 milliliters. The balance of the regenerated brine solution was used for the next brine wash. Additional calcium chloride equivalent to the chlorine contained in the lead product was also required to bring the calcium chloride content of the leaching solution up to 9.54 grams.
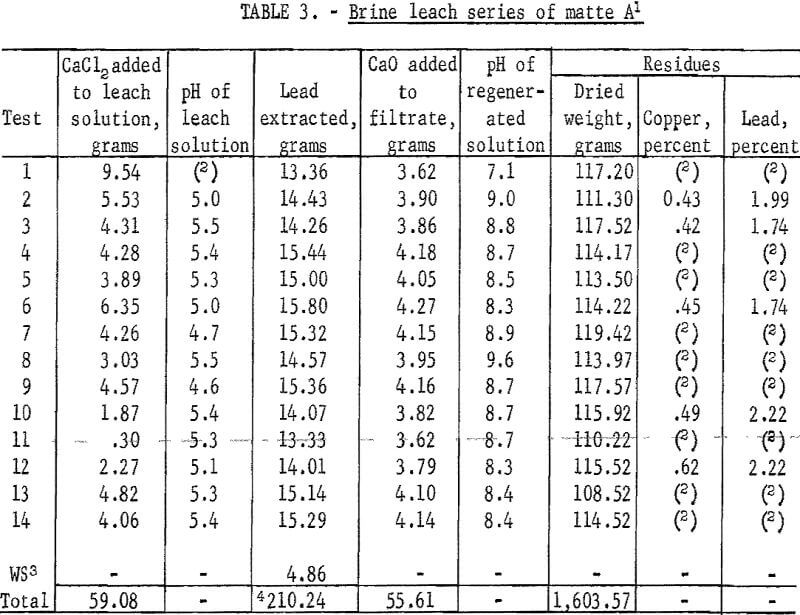
Results of this series of tests are given in table 3. Filter cakes retained from 70 to 85 milliliters of pregnant solution from each leach. The majority of the lead contained in this solution was recovered in the first brine wash and the balance was recovered in subsequent washes. The difference in the volume of filtrate from leaching and the volume of the filtrate from product precipitation was due to evaporation losses, retention of solution by lead product filter cake, and a small amount of water combined in the hydrated product.
Periodic spectrographs analyses of dissolved salts in regenerated solutions showed that no impurities were accumulating and, except for a few instances, lead product was consistently greater than 99 percent pure.
The major difficulty encountered in these tests was maintaining the proper CaCl3 balance. During the seventh leaching experiment the first water wash solution reached a specific gravity of 1.2084 which corresponds to the brine concentration of the original leach solution. It was therefore possible to move this solution (and others later) ahead as a brine wash. As a result of mixing of solutions and determining compositions by material balances rather than chemical analyses, the CaCl2 balance was disturbed. At this point the time required for precipitation of the lead product increased and a new lead compound Pb(OH)Cl appeared in the product. Additional calcium chloride added to the leach solution resulted in less Pb(OH)Cl in the product.
The explanation appeared to be that a deficiency of CaCl2 in the leaching solution caused solubilizing of some sulfate ion. This carried over to the precipitation step where regenerated CaCl2 reacted with the sulfate and contaminated the product with gypsum, leaving the regenerated solution even more deficient in CaCl2.
Total lead extracted in these tests was 210.24 grams consisting of 205.38 grams from first-leach filtrates, 3.83 grams in the final brine wash, and 1.03 grams in the final water washes. Total lead contained in the original matte was 252,68 grams. Extraction efficiency was 83.2 percent.
In summary, then, conditions chosen to be optimum for leaching both copper and lead gave satisfactory extractions and products obtained were of relatively high purity. No problems were encountered with buildup of impurities or with regeneration of brine solutions except for the difficulty of maintaining the proper CaCl2 balance.
Cyclic Leach of Matte B
The sample designated matte B was considerably different chemically from matte A as shown earlier in table 1. It also was much different in physical appearance. Attempts to roast matte B in quartz boats were unsuccessful because of a tendency toward sintering and incomplete oxidation. However, the sintering problem was eliminated and a uniformly roasted product was obtained using a rotary kiln. The kiln was comprised of a 2-inch-diameter, 4-foot-long stainless steel tube that was heated in a tube furnace 24 inches long. Speed of rotation was controlled at approximately 10 rpm. Matte (minus 80 mesh) was fed to the tube at a rate of 6 to 7 grams per minute by a vibrating feeder. Air for roasting was drawn through the tube by an aspirator. A controlling pyrometer was used to maintain a constant roasting temperature. The thermocouple for the pyrometer was positioned in the center of the tube midway through its length.
Roasting tests were made over a temperature range of 500° to 800° C to determine the optimum roasting temperature. In order to obtain complete roasting it was necessary to pass the material through the kiln four times with ball-milling after each pass. The major part of the time consumed in roasting and ball-milling was necessitated by the presence of metallic iron in the matte. Unless metallic iron is completely oxidized, copper extraction during leaching is lowered by precipitation of cement copper. The efficiency of roasting at the various temperatures was evaluated by water leaching and brine leaching the products. The optimum roasting temperature was found to be approximately 600° C as predicted from thermogravimetric studies.
Five kilograms of matte B was roasted at 600° C with a ball-milling period of 30 minutes between each pass through the kiln. The roasted product contained 1.69 percent copper and 15.2 percent lead.
Water Leach Series
The method used to extract copper from this material was the same as the simulated countercurrent leach method that was used to treat matte A. Twenty samples of 200 grams each were leached at room temperatures for 60 minutes in 800 milliliters of water. Copper analyses and specific gravity measurements were again used to determine the number of washings required and the path that the solutions would follow. Data for this series of tests are given in table 4. From the columns headed “Copper in leach solution, grams per liter” and “Copper in solution removed from system, grams,” it appears that a steady-state schedule was reached for movement of the solutions. Chemical analyses of the individual solutions and the combined solutions showed a copper recovery of 89.08 percent. This was verified by material balances on the initial samples and final residues. Copper was again precipitated from solution by addition of iron powder.
Brine Leach Series
Wet solids from water leaching were dried and a series of brine leaching tests was started using 800 milliliters of 25-percent-brine solution containing 15.84 grams of calcium chloride for each sample. In addition to searching for information similar to that obtained for matte A, considerable effort was devoted to coping with the problem of the calcium chloride balance and defining other problems that might arise.
In general the leaching conditions and procedures were the same as those described for matte A. Data and results of these tests are given in table 5.
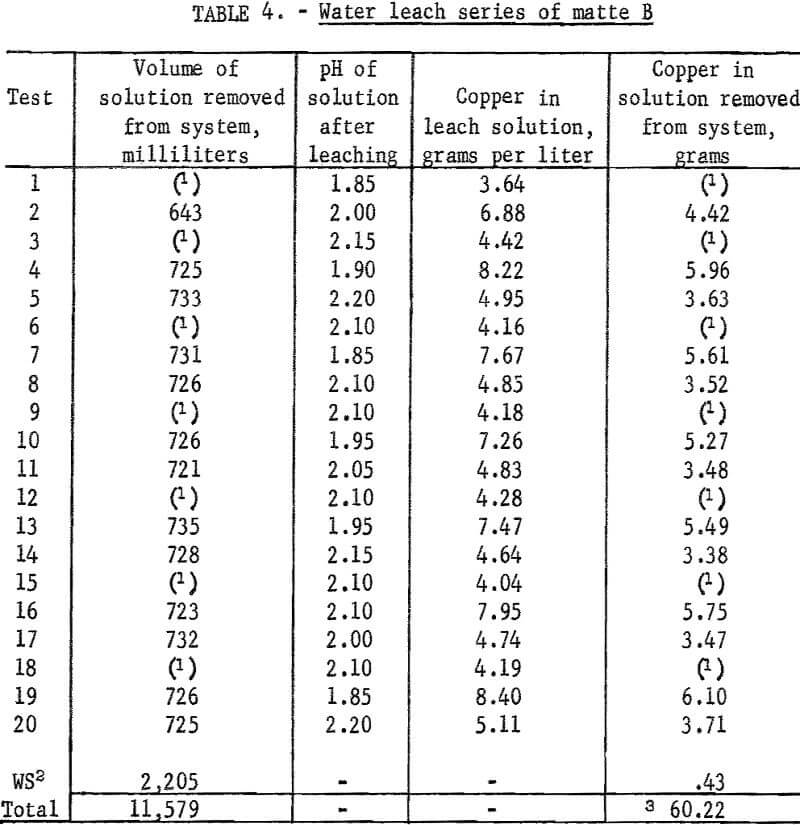
As in the previous series of tests, the calculated amount of burned limestone was slaked in water and added to the hot filtrate to precipitate the lead product, 3PbO.PbCl2.xH2O. The regenerated solution was recycled as in previous tests but lead recoveries were slightly lower than expected in the first several tests. In the absence of reliable solubility data it was suspected that the volume of solution used might be less than required for the amount of lead available for extraction. The volume of brine was increased to 900 milliliters in test 4 and this was raised further to 1,000 milliliters in test 9. No significant increase in lead extraction was obtained that could be attributed to increasing the volume of leaching solution.
During the ninth test it was also observed that the specific gravity of the regenerated solutions was decreasing. This was attributed to dilution resulting from water used to slake the line. For the next two tests lime was slaked with 25-percent-brine solution and additional sodium chloride was added to regenerated solutions on hand to bring the concentration up to 25 percent. In the 10th test the specific gravity of the first water wash solution had reached that required for advancing in the system as a brine wash.
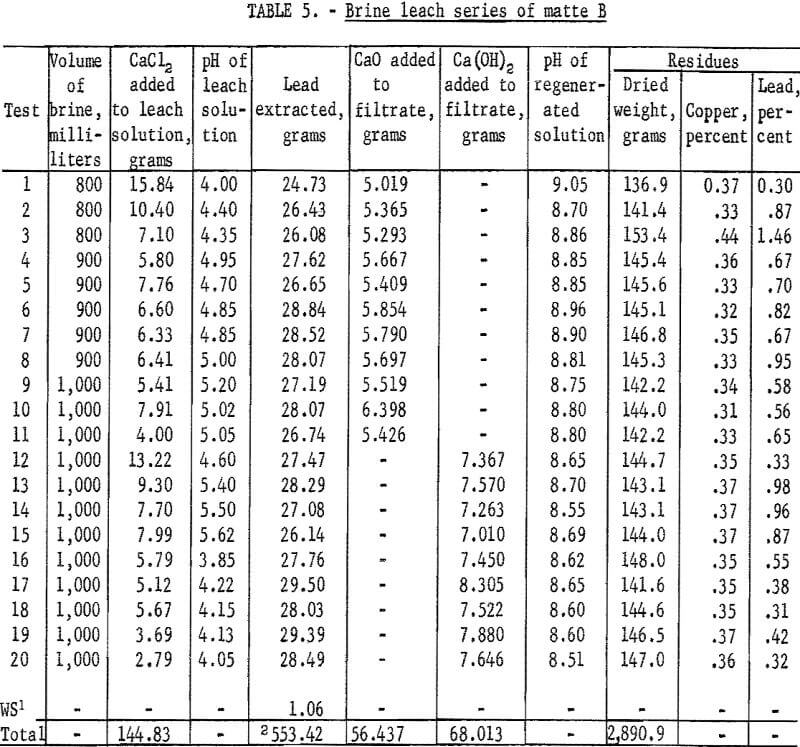
Through the first 11 tests careful material balances were relied upon for maintaining the calcium chloride balance. For all tests following test 11 this was supplemented with chemical analyses for calcium in regenerated solutions. Solutions from tests 11 and 12 were found to have from 10 to 15 percent less than the required amount of calcium chloride. Products from these tests were also found to contain gypsum. The problem now was similar to that encountered in leaching matte A. In all subsequent tests additional calcium chloride was added to regenerated solutions to provide a slight excess over that required for precipitating all sulfate as gypsum during leaching. Calcium sulfate disappeared from lead products and lead recoveries improved. Another change in procedure during test 12 was substitution of dry Ca(OH)2 for the slaked lime slurry. This was done for convenience and was not expected to improve recoveries.
Starting with test 16 the regenerated and wash solutions were adjusted to a pH of approximately 4.0 by addition of several drops of concentrated HCl. The slight alkalinity of regenerated solutions being returned for leaching was thought to cause precipitation of small amounts of basic lead salts that would remain in the residues. As a result of this adjustment, the recoveries of lead for the final four tests are slightly higher than those for most other tests.
Lead extraction for this series of tests was 91.02 percent overall. Additional information obtained, showed that simple material balances are not sufficient for maintaining the proper calcium chloride balances and control analyses for calcium in the regenerated solutions are required. In addition, a slight excess of calcium chloride is desirable. For maximum lead extraction it is also necessary to adjust the alkaline regenerated solution to a pH of approximately 4.0. It was learned too that the best procedure for precipitating the lead product was by adding brine-slaked lime or dry Ca(OH)2 to the leach filtrate.
Final Cyclic Leach of Matte B
A final series of tests was made on matte B in an attempt to improve recovery efficiency by using a modified procedure based on information gained in the previous series of tests. Four kilograms of matte was roasted in the rotary kiln at 600° C. The material was passed through the kiln four times with 30-minute ball-milling periods between passes. The roasted product contained 1.69 percent copper and 15.2 percent lead. A charge of 200 grams was used for each leach cycle.
Water Leach Series
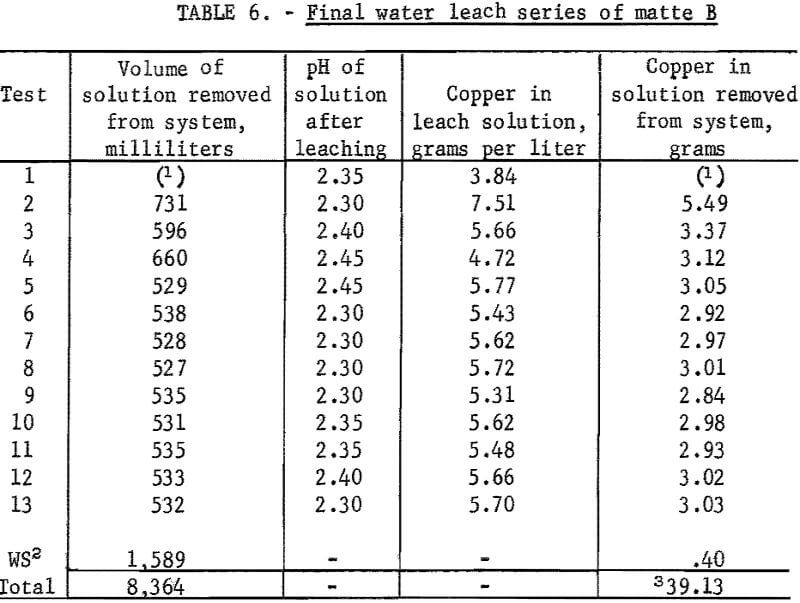
The same water leaching procedure was used in these tests as was used in the previous series except that the amount of water used for leaching was reduced from 800 to 600 milliliters after the first test. This allowed pregnant solutions containing the desired amount of copper to be removed from the system after each new sample was added to the leaching train. Results of this series of tests are given in table 6. Copper extraction based on chemical analyses of solutions was 89.08 percent. Based on head sample and residue analyses extraction was 86.35 percent.
Brine Leach Series
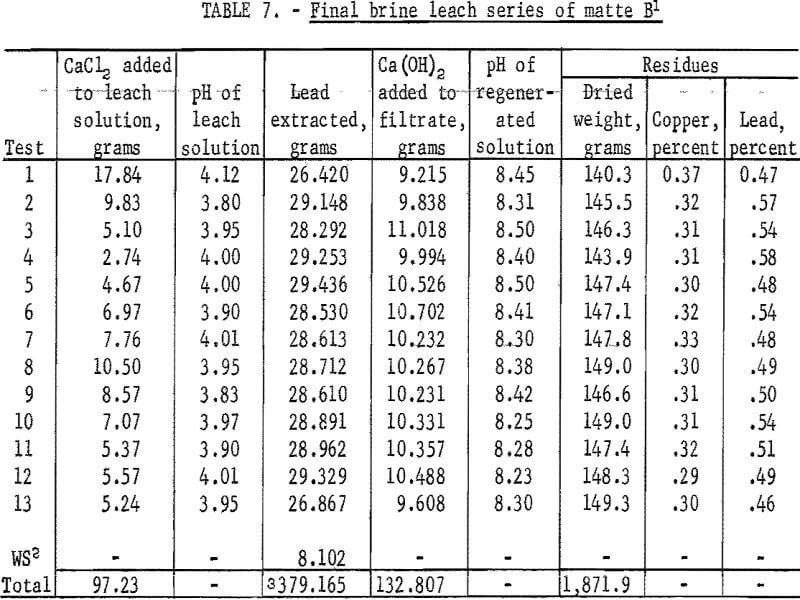
The wet solids from water leaching were dried and leached with brine in a way similar to that used in earlier tests. One liter of brine containing 17.84 grams of CaCl2 was used. Several changes were made in the procedure: (1) Brine used for leaching and washing was adjusted to pH 4.0 with HCl. (2) Water used for washing was adjusted to pH 3.8 with HCl. (3) The regenerated solutions were adjusted from a pH of about 8.5 to a pH of 4.0 with HCl. (4) A 2-gram excess of CaCl2 was maintained in all leaching cycles. (5) For lead precipitation the Ca(OH)2 was added as a smooth paste made from brine and specially prepared dry Ca(OH)2. The first water wash reached the specific gravity of 25 percent brine during the 10th test in this series. Results for all tests are given in table 7.
Products obtained following the first three tests were found to be contaminated with a compound that could not be identified by X-ray analyses. Since no significant impurities other than calcium were found by spectragraphic analyses, this compound was assumed to have been an amorphous form of Ca(OH)2 or CaSO4. Except for this development no difficulties were encountered. Lead extraction was 95.94 percent based on analyses of leach solutions. Based on head sample and residue analyses extraction was 97.53 percent.
Discussion
On a larger scale, and in a kiln providing longer retention time, it is possible that fine grinding may not be required for complete roasting. Complete roasting is essential, however, for maximum copper extraction. On a larger scale, sulfur content of most mattes should also be high enough for self-sustained roasting. Roasting temperature is critical and must be controlled closely, near 600° C.
The countercurrent water leach of the roasted matte readily extracts about 90 percent of the copper and at the same time conveniently extracts other soluble sulfates. Concentration of pregnant solutions was held at approximately 5 grams of copper per liter because the metal could be cemented readily from solutions of this strength. Unextracted copper is believed to have been present in some insoluble form other than the oxide. The pH of pregnant solutions was low enough that the simple oxide should have dissolved if it was present.
Extraction of lead from the water-leach solids improved with each series of tests. The 25-percent-brine solutions must be kept hot from the time leaching is started until the lead product is filtered. Sufficient calcium chloride must be present in the solution to prevent sulfate ion from being extracted. This also permits the lead to be extracted as the chloride, which has a considerably greater solubility in brine than the sulfate.
Dissolved lead retained in leaching solution that wets the filtered solids must be recovered by washing with hot brine. These wash solutions must be advanced to the next leach to assure that extracted lead is moved up to the product recovery step as early as possible. Following brine washing several water washes should be used to recover sodium chloride from the solids. These wash solutions will also pick up small amounts of lead not recovered by the single brine wash. In the event any solutions rich in lead are allowed to cool and become saturated, crystals of lead chloride will precipitate causing difficulty in the product recovery step.
The calcium chloride balance presents a delicate problem. This arises from mixing of solutions and attempting to adjust calcium chloride concentration as indicated to be necessary from material balances. In addition, the nature of the reactions, conditions, and techniques used in precipitating the lead causes precipitation of mixtures of 3PbO.PbCl2.xH2O and Pb(OH)Cl. The following stoichiometric conditions are required for either of these basic salts alone.
For 3PbO.PbCl2.xH2O –
Leaching: 4PbSO4 + 4CaCl2 → 4PbCl2 + 4CaSO4.
Precipitation: 4PbCl2 + 3Ca(OH)3 → 3PbO.PbCl2.xH2O + 3CaCl2 + 3H2O.
For Pb(OH)Cl
Leaching: 2PbSO4 + 2CaCl2 → 2PbCl2 + 2CaSO4 .
Precipitation: 2PbCl2 + Ca(OH)2 → 2Pb(OH)Cl + CaCl2 .
Thus, for a pure product of 3PbO.PbCl2.xH2O, 3 moles of lime should be consumed while only 1 mole of CaCl2 is consumed. In consuming these 3 moles of lime, 3 moles of CaCl2 are regenerated. For pure Pb(OH)Cl, the reagent requirement is 1 mole of lime for each mole of CaCl2 consumed.
The amount of calcium ion required overall is the amount necessary to precipitate all sulfate as gypsum during leaching. In the first series of tests on matte B the theoretical amount of calcium needed was 117.6 grams and the amount actually consumed was 116 0.grams. The amount of chlorine ion necessary for a Pb(OH)Cl product for these tests was theoretically 104.0 grams and for a 3PbO.PbCl2.xH2O product 52.0 grams of chlorine was required. The amount of chlorine actually consumed was 68.9 grams. The total product from these tests then was a mixture of both basic salts, illustrating that the problem of the calcium chloride balance is compounded by the possibility of precipitating either of the basic salts as well as mixtures of both.
A minor objective in this work was to demonstrate on a laboratory scale that the final residues remaining after extraction of copper and lead could be smelted to metallic iron. About 1 kilogram of residues from each sample of matte was smelted with coke and a low-melting flux of sodium carbonate, silica, and sodium borate. Metal recoveries were low (70 and 80 percent) because of formation a viscous slag. Residual lead apparently vaporized during smelting. Copper content of the metal was about 0.5 percent and sulfur content was about 1.0 percent in each case. Both metal products also contained objectionable amounts of antimony and tin. No attempts were made to improve either the quality or recovery of the product since the objective was merely to demonstrate that iron could be smelted from the residues.
