Table of Contents
The earliest known specimen of lead, dating from 2000 B.C., is a figurine found near the Dardanelles at the site of the ancient city of Abydos. Since that time, the method for producing lead from its most abundant mineral, galena, has changed very little. Basically, a lead concentrate is mixed with fluxing agents, roasted to remove sulfur, and then heated to about 1,000° C with carbon to obtain an impure metallic product.
This smelting process is an efficient, low-cost operation but results in both SO2 and lead emissions.
The Federal Bureau of Mines is currently investigating several alternatives to smelting, one of which is shown in figure 1. In this method, galena is leached with hot ferric chloride solution to obtain lead chloride and elemental sulfur according to the following reaction:
PbS + 2FeCl3 → PbCl2 + 2FeCl2 + S°.
At 100° C, over 99 pct of the PbS is converted to PbCl2 in less than 15 min. The leach solution, consisting of brine (NaCl) containing slightly more than the theoretical amount of FeCl3, dissolves the PbCl2 as it is formed, leaving a residue of elemental sulfur and gangue. High-purity PbCl2 (>99.9 pct) crystallizes from the leach solution on cooling and may be electrolyzed in a fused-salt cell to obtain corroding-grade metal (>99.94 pct Pb) and chlorine gas. The chlorine gas is then used to regenerate the leach solution for further use. By products such as Ag, Cu, Zn, and S° may be recovered from either the leach solution or leach residue by further processing.
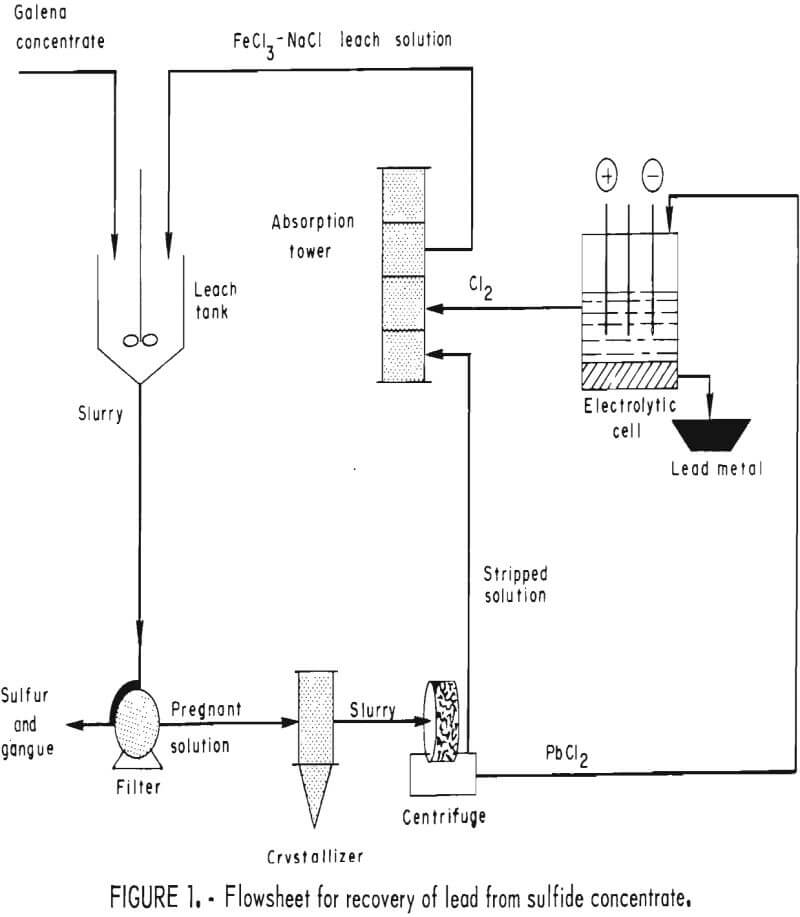
Lead is especially well suited for fused-salt electrolysis because its low melting point (327° C) makes it possible to produce metal in the liquid state and remove it from the cell without interrupting production. Lead chloride appears to be a desirable starting material because it is not hygroscopic, is safe to handle, and is an excellent conductor of electricity when molten. PbCl2 is also easily prepared in a pure form, obviating any need for refining the lead product. Because of the high specific gravities of Pb and PbCl2 and the high current density (>1,000 amp/ft²) possible with fused salts, equipment sizes are minimal. Energy requirements are low (<1.0 kwhr/lb metal), and the use of expensive metallurgical coke is avoided.
Similar methods have been proposed in the past but did not attain commercial acceptance. Because environmental problems now faced at lead smelters are more critical, new methods as alternatives to smelting have become more attractive; the process just described is considered a promising approach. The leach step (equation 1) has been fully described in a previous publication; the present paper concerns only the electrowinning of lead from lead chloride.
Investigation of Variables
Tests were made first to optimize variables involved in recovering lead from PbCl2 by fused-salt electrolysis. Variables selected for investigation were electrolyte composition, temperature, current density, and electrode spacing. Since a chief purpose of the work was to avoid lead emissions, fuming (PbCl2 volatilization) had to be eliminated. The atmosphere in the working area was therefore checked periodically with an MSA Lead-in-Air Detector, and conditions responsible for any lead emissions were noted and corrected as the tests proceeded.
Experimental Procedures
All small-scale tests were made with the apparatus shown in figure 2. The test cell consisted of a 1,000-ml, tall-form, Pyrex beaker inside a 316 stainless steel container. Heat was furnished by a Lindberg model 5600 crucible furnace equipped with an automatic temperature controller; the temperature was monitored by a Chromel-Alumel thermocouple connected to a Leeds and Northrup Speedomax recorder. A Trygon model M15-50A rectifier supplied dc current, which was measured with a Leeds and Northrup model 8690 potentiometer in conjunction with a 50-amp, 50-mv shunt. Voltage was checked with a Heathkit model 1 M-102 multimeter.
In a typical test, the beaker was placed inside the furnace, which had previously been brought to the desired temperature. A weighed amount of the salt combination being investigated was added, and the electrode assembly was lowered into place after the salt was molten. The assembly (fig. 2) consisted of a graphite disk, 1½ inches in diam by 2 inches thick, separated by glass spacers from a similar disk, 1½ inches in diam by ½ inch thick.
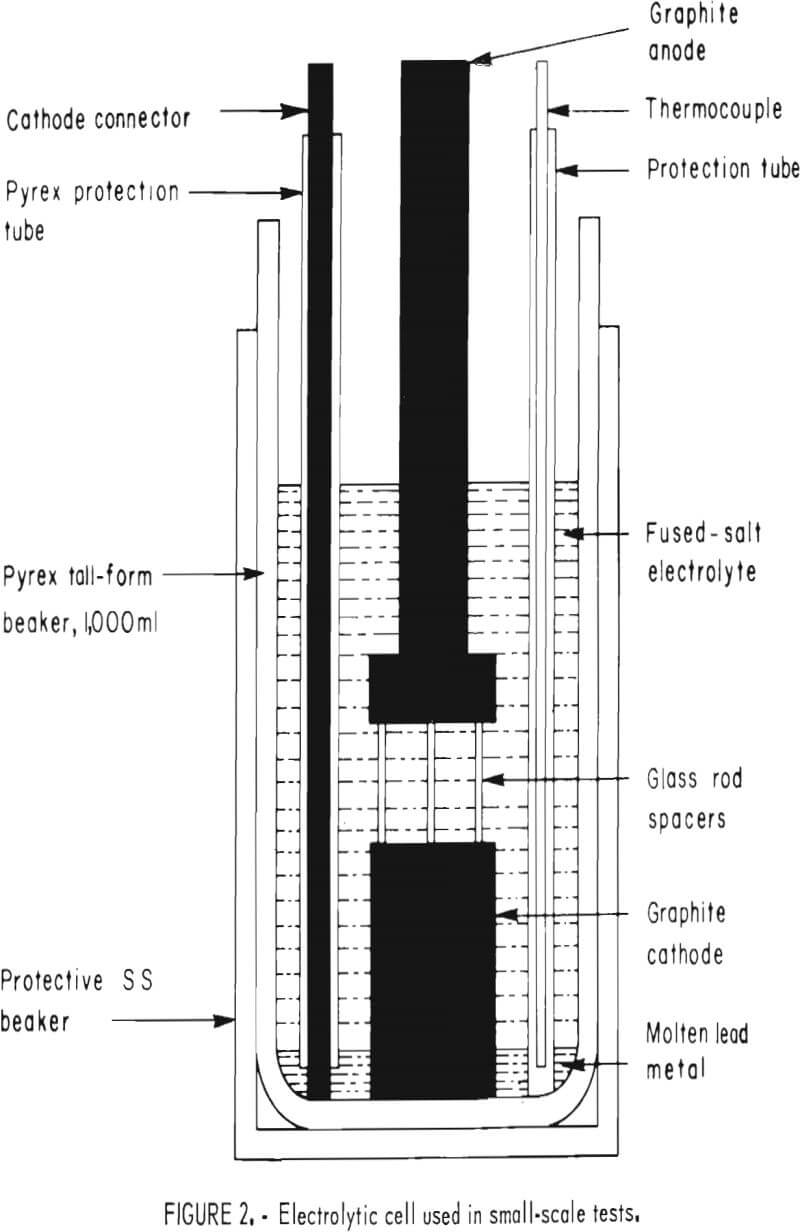
The upper disk served as the anode, being connected by a ¾-inch-diam graphite rod to the dc power supply. A ¼-inch graphite rod protected by a glass sleeve joined the metal pool at the bottom of the cell to the negative side of the rectifier. After the temperature had stabilized, 300 grams of high-purity lead metal was added and electrolysis started. The procedure was repeated in all tests with controlled variations in bath composition, temperature, current density, and electrode spacing. Bath composition was predetermined by weight but was also checked by X-ray analysis. After each run, the contents of the beaker were poured into a graphite mold. The weight of the deposit was then determined by subtracting the weight of lead added to the cell at the start of the run from the weight of the metal button.
Since lead poisoning was a potential hazard, personnel were given a periodic medical check. Every quarter, urine samples were analyzed for lead by atomic absorption. Urinary delta-aminolevulinic acid was determined at the same time by the Davis method. Once a year, blood and urine samples were submitted to a clinical laboratory for an independent evaluation. During the 3-year period in which the various phases of the work were carried out, elevated lead levels were never detected in the blood or urine samples of anyone working on the project.
Electrolyte Composition and Temperature
Molten PbCl2 by itself is not a suitable electrolyte because it dissolves metal deposited at the cathode and has a relatively high melting point (501° C) and an excessive vapor pressure (2.8 mm Hg at 550° C). It is necessary, therefore, to combine PbCl2 with alkali halide(s) to reduce the melting point of the bath and eliminate fuming (PbCl2 volatilization). Addition of such halides also increases the conductivity, although not to any great extent because PbCl2 by itself is a good conductor (1.596 ohm-¹ cm-¹ at 528° C). The improvement in conductivity is determined by the cation size of the salt added; the greatest increase is obtained with LiCl. Larger cations, however, give better bath stability by decreasing the vapor pressure of PbCl2. PbCl2 forms a very strong complex with CsCl and progressively weaker complexes with RbCl, KCl, NaCl, and LiCl. The decomposition potential of PbCl2 at its melting point is 1.25 volts. This is raised slightly by the addition of other chlorides, but the effect is minor compared with the gain in conductivity.
To produce lead in the molten state, electrolysis must be conducted above 327° C, its melting point. High temperatures are not desirable, however, because they result in increased volatility of PbCl2, greater solubility of Pb metal in PbCl2 , and an enhanced back reaction between lead and chlorine. It is necessary only to provide an adequate differential between the melting point of the deposit and the operating temperature to allow for variations in heat input. In this case, a minimum of 450° C is indicated.
Based on the foregoing data, a series of tests was made to determine the optimum electrolyte composition and temperature. Salts were confined to chlorides that gave mixtures with PbCl2 melting below 450° C and that had good electrolytic conductivity, reasonable cost, negligible vapor pressure, and a higher decomposition potential than PbCl2. All of the salts involved in electrolyte preparation were used as received, with no treatment of any kind. Eutectic compositions were employed in the tests with other variables, except temperature, fixed. Operating conditions included a current density of 5 amp/in³, an electrode spacing of 1 inch, a time of 2 hours, and temperatures ranging from 450° to 650° C. Results are shown in table 1.
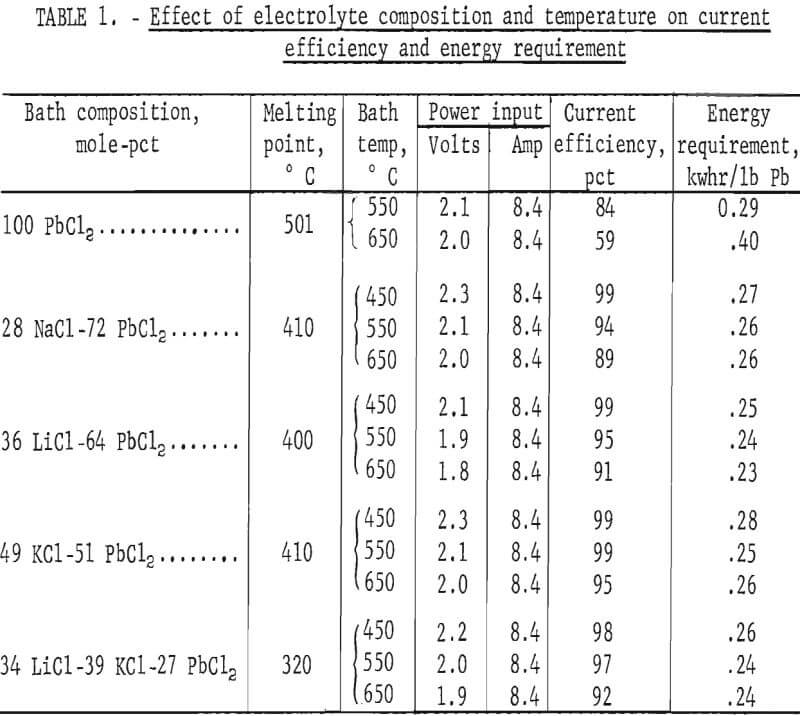
Lead chloride by itself gave very poor results. Fuming was excessive, ranging from 5 g/hr at 550° C to 15 g/hr at 650° C. Some of the metal produced also redissolved in the bath, causing a rapid drop in current efficiency as the temperature was raised. Addition of other salts prevents this. For example, the solubility of Pb in PbCl2 at 610° C goes from 0.037 mole-pct with PbCl2 alone to 0.0006 mole-pet when 40 mole-pct KCl is present. The LiCl-PbCl2 system had the best conductivity of any combination. However, both the LiCl-PbCl2 and NaCl-PbCl2 baths fumed at all temperatures used in the tests. KCl was the only halide tried that substantially reduced PbCl2 volatilization, but even the KCl-PbCl2 and LiCl-KCl-PbCl2 baths fumed to some extent above 450° C. The best overall results were obtained with the LiCl-KCl-PbCl2 system. This combination also had an advantage in that the PbCl2 content could be varied over a wide range without any possibility of freezing the bath. Energy requirements given in table 1 are comparable but do not include the energy required for heating. Based on these tests, a temperature of 450° C was selected for use in all subsequent work.
PbCl2 Concentration
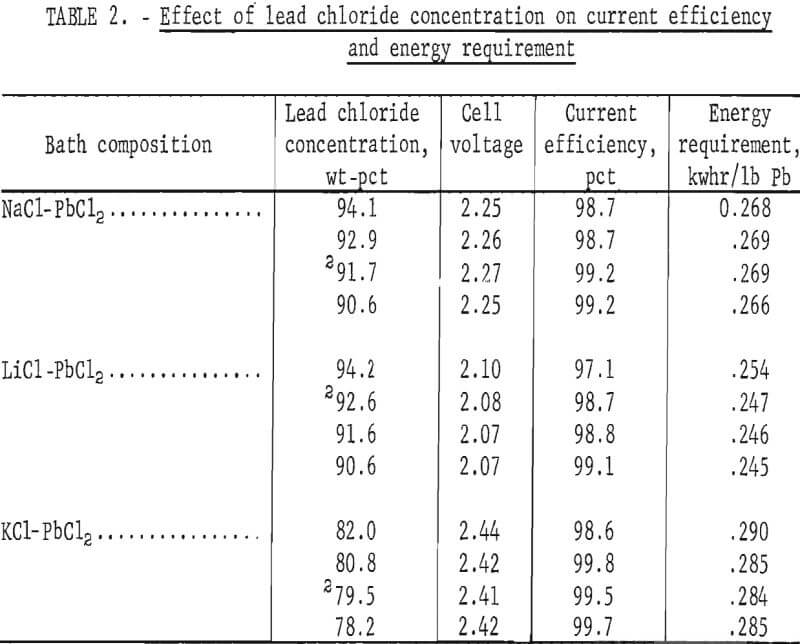
More extensive tests were made next to determine the effect of PbCl2 concentration on the voltage, current efficiency, and energy requirement. All tests were conducted at 450° C with a current density of 5 amp/in² and an electrode spacing of 1 inch. Results obtained with the NaCl-PbCl2, LiCl-PbCl2, and KCl-PbCl2 systems are shown in table 2. The data indicate the narrow range in which these mixtures are molten at 450° C. As expected, the energy requirement was highest with KCl-PbCl2. Fuming was evident at all times with the NaCl-PbCl2 and LiCl-PbCl2 baths, but none occurred with KCl-PbCl2.
As stated before, a wide range of PbCl2 concentrations can be used with the LiCl-KCl-PbCl2 system if KCl and LiCl are present in eutectic proportions (55 wt-pct and 45 wt-pct, respectively). The phase diagram for this system is shown in figure 3, and the effect of the PbCl2 concentration on electrolysis is illustrated in figure 4. The PbCl2 concentration had little effect on either the current efficiency or energy requirement. Some change in the energy requirement is apparent in figure 4, but the maximum variation over the whole range is only 5 pct. No fuming occurred until the PbCl2 concentration exceeded 86 wt-pct, and the best overall results were obtained at 40 to 70 wt-pct. In general, the LiCl-KCl eutectic appeared to be the preferred medium for electrolysis of PbCl2 owing to its low melting
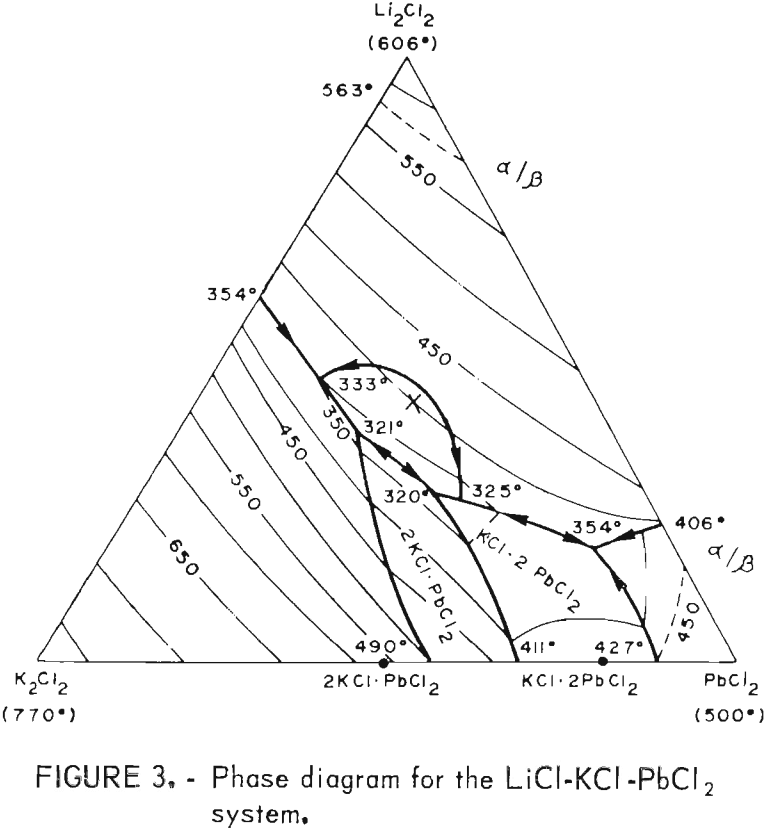
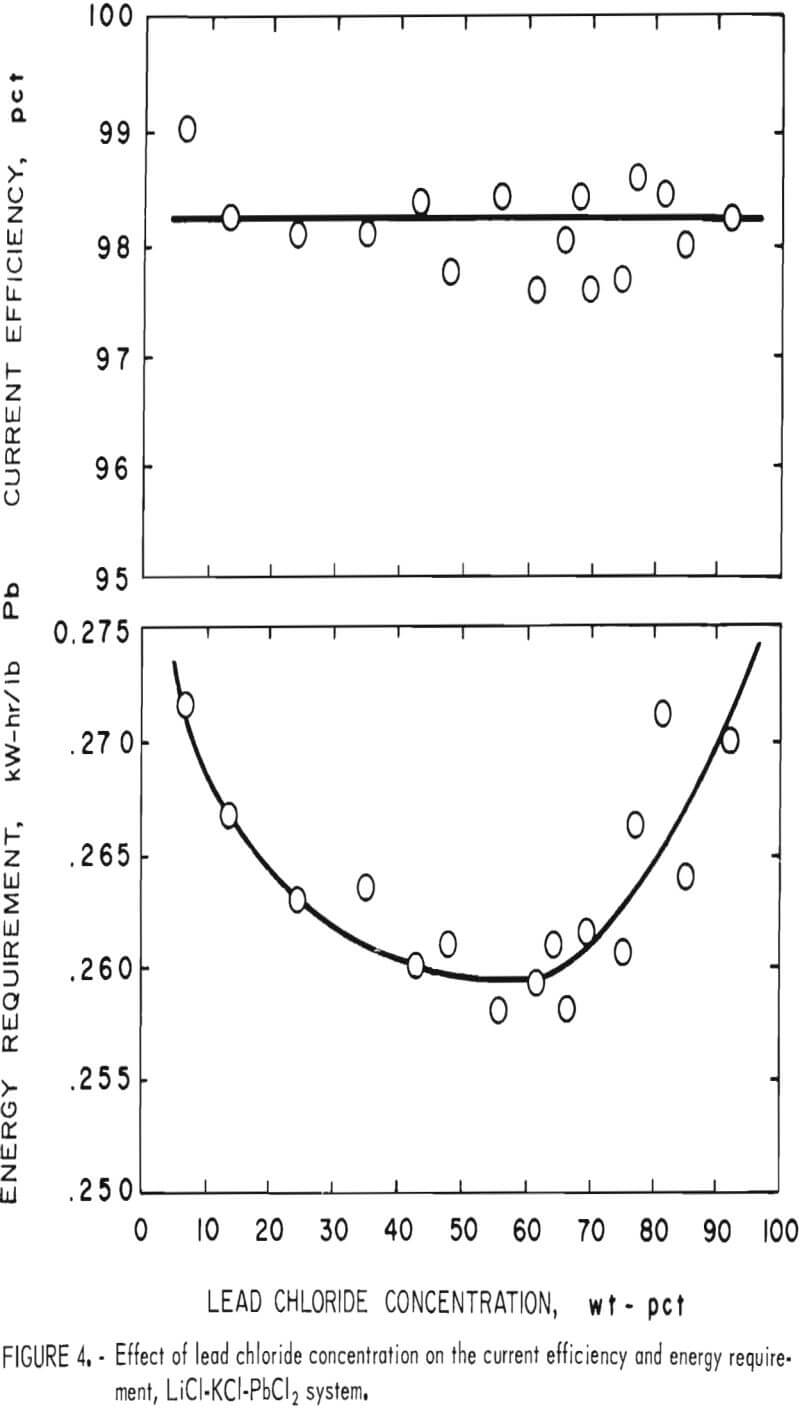
point (354° C) , high decomposition potential, good conductivity, and compatibility with Pb metal. On the basis of the above work, the LiCl-KCl-PbCl2 bath was used in all subsequent work.
Current Density and Electrode Spacing
Current density and electrode spacing are two of the most important variables, determining both the size of equipment and the energy requirement.
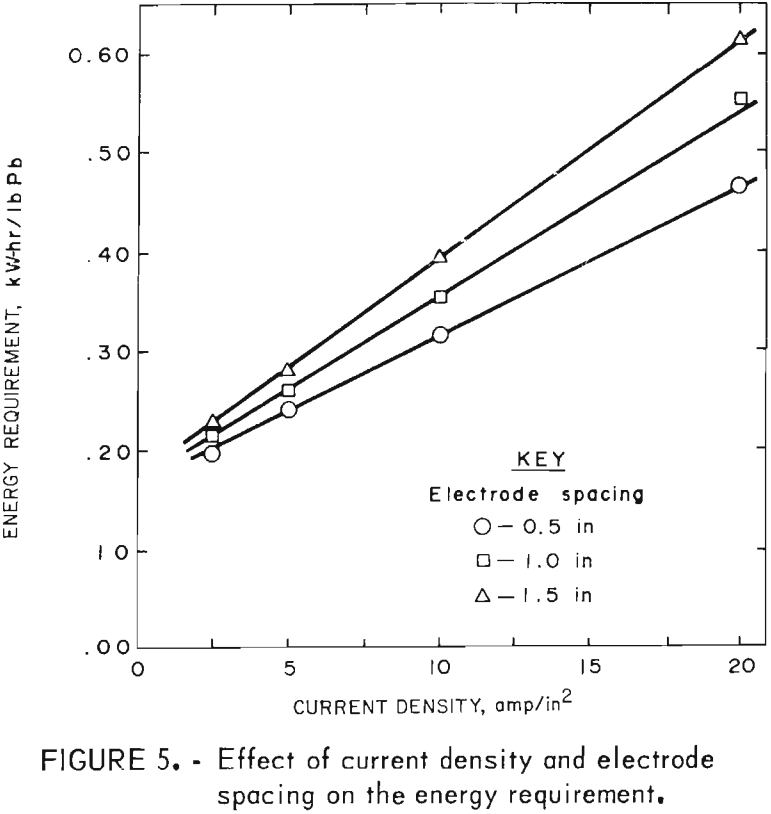
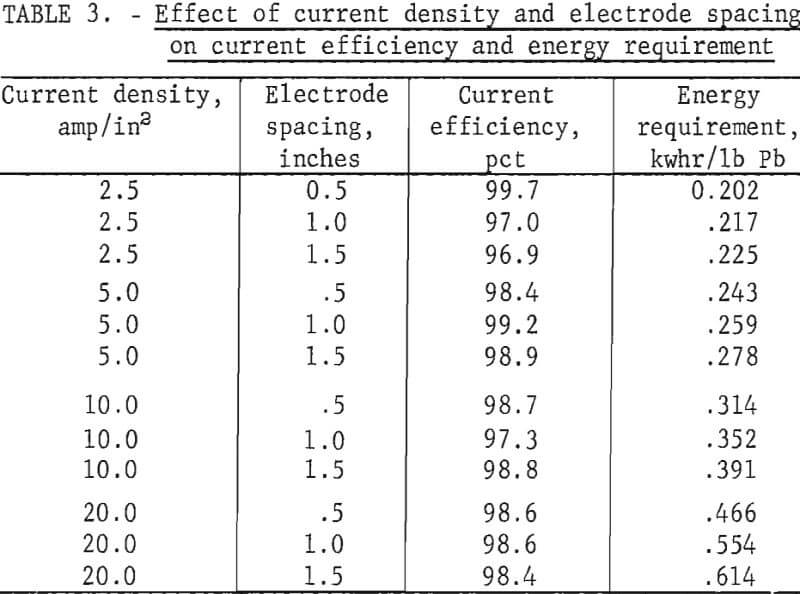
Tests to illustrate their effect were made at 450° C with a LiCl-KCl-PbCl2 bath containing 80 wt-pct PbCl2. The experimental results shown in table 3 indicate that the current efficiency is not significantly affected by electrode spacing or current density. Electrode spacing and current density did have a pronounced effect on the energy requirement, however, particularly at higher current densities (fig. 5).
Metal Purity
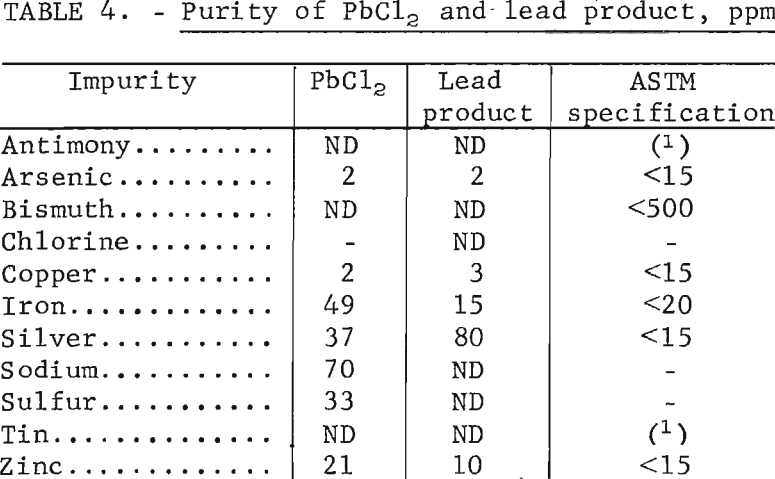
Before designing a large-scale cell, it was necessary to establish what grade of metal could be produced by fused-salt electrolysis, using PbCl2 prepared by ferric chloride leaching of galena concentrate. Hopefully, the metal would meet ASTM specifications for corroding-grade lead without further treatment. To determine this, a sample of PbCl2 made as shown in figure 1 was electrolyzed in a LiCl-KCl bath at 450° C to depletion. Results are shown in table 4 together with the ASTM specifications for corroding-grade metal. The product met all specifications except for silver, and this could have been met by agitating the pregnant solution (fig. 1) with lead shot before crystallizing out PbCl2.
Cell Design
On the basis of the preceding work, several cells were built to test materials of construction and provide data for the design of a full-sized unit. Both monopolar and bipolar electrode arrangements were tried, using either horizontal or vertical plates.
In a monopolar or multiple system, the anodes and cathodes are connected in parallel with each electrode attached to the dc source. In a bipolar or series system, only the two end electrodes are connected to the dc source; intermediate plates have no direct electrical connection and are insulated from each other. During electrolysis, the intermediate plates become bipolar, assuming a negative charge on one side and a positive charge on the other. Each pair then acts as a cell in series with every other pair, increasing the voltage and deposition rate by roughly n-1 times the number of plates, as compared with a monopolar arrangement. This is a tremendous advantage because the amperage can be greatly reduced for the same production rate, thus minimizing the size, and therefore the cost, of electrical equipment and busbar. Cell design is also simplified because only two electrode connections are required, regardless of the number of plates. It should be noted, however, that the current efficiency with a bipolar arrangement is always less than with a monopolar arrangement because of current leakage around the edges of the intermediate plates. Nevertheless, a lower energy requirement is possible with a bipolar cell simply because a heat balance can be obtained with a lower current density.
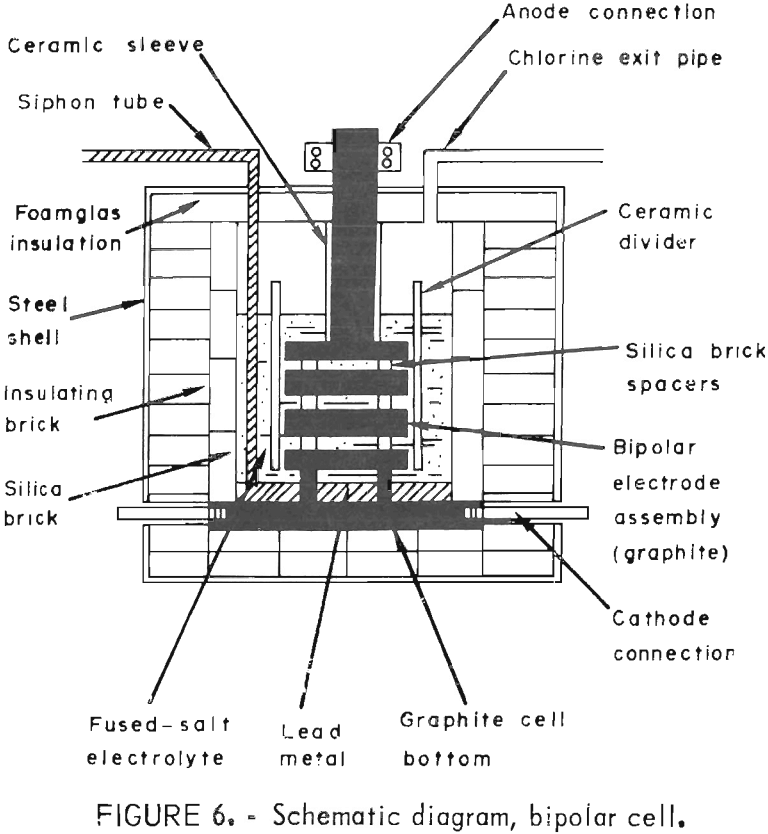
A cell with vertical electrodes was constructed first, using the monopolar arrangement. Several disadvantages were apparent with this configuration. As it was necessary to water-cool each electrode connection, there was a considerable heat loss through the top of the cell. Variations in bath level affected the current density, and it was difficult to keep the electrodes in alinement. The average current efficiency with the monopolar setup was 98 pct, and the average energy requirement was 0.68 kwhr/lb Pb. The latter was high compared with results obtained in small-scale tests. In this case, however, the dc current supplied the energy for heating as well as for electrolysis.
A bipolar arrangement (fig. 6) was tried next, using horizontal electrodes to reduce the heat loss through the top of the cell. This also offered other advantages. For example, the electrodes could be completely submerged, eliminating problems with current density variations, alinement, and air oxidation of graphite. Construction details and operating data are given in table 5. To limit current leakage around the edges of the plates, the electrodes were enclosed on two sides by the Durobrick lining of the cell and on the other two sides by ceramic dividers built into the walls. Gaps of ¼ inch were left between the dividers and the graphite plates to allow circulation of the electrolyte. The other two sides of each plate, however, came into direct contact with the lining. The plates were also tilted slightly and grooved on the top and bottom surfaces (fig. 7) to direct the flow of lead metal and chlorine gas to opposite sides of the cell. Water cooling was used on both the anode and cathode connections to avoid deterioration of the contacts and, in the case of the cathode, to prevent leakage of metal or salt through the side of the cell. A ceramic sleeve was also placed around the graphite anode lead to avoid air oxidation.


An overall view of the cell is shown in figure 8. Direct current was furnished by a Hewlett-Packard model 6466A constant-amperage rectifier (0-600 amp, 0-18 volts). For heating the cell overnight and during weekends, a 75-kva ac welding transformer was used with a Superior Electric powerstat in the primary circuit to control the output. Changing from dc to ac or vice versa was done with a 600-amp Meyers transfer switch. Temperature readings from different parts of the cell were noted with a four-channel Honeywell model C153 recorder. Voltage was checked with a Heathkit IM-102 multimeter, and the number of ampere-hours was determined with a Rapid Electric digital meter.
Operation of the cell was extremely simple. Chlorine, evolved during electrolysis, was drawn through a scrubbing tower (fig. 8), Before electrolysis, a circulating pump was turned on. The dc circuit was then activated, and enough PbCl2 added to the cell at 30-min intervals to maintain the bath level. When the run was complete, the rectifier was switched off and the metal produced was drawn by suction into a graphite mold (fig. 8). The transfer switch was then moved from the dc to the ac setting, and the ac

circuit was turned on to maintain the cell at operating temperature until the next run.
As shown in table 5, 379 amp and 11.6 volts were needed to maintain a heat balance during electrolysis. When the cell was not in service, the ac input averaged 539 amp and 5.4 volts; less energy was required because no metal was being produced. These results indicate that about two-thirds of the energy supplied to the cell during electrolysis was being used to maintain the operating temperature. The average current efficiency with the bipolar arrangement was 86 pct; the average energy requirement was 0.52 kwhr/lb Pb. This cell operated with no significant difficulties for 1 month, during which time about 600 lb of metal was made, using reagent grade PbCl2 .
Effect of Impurity Buildup
Tests were made next to determine the effect of impurity buildup in the bath. Although a very pure grade of PbCl2 can be made by the method shown in figure 1, it will inevitably contain impurities which will in time affect the grade of metal produced, interfere with the operating conditions, and/or cause the bath to be discarded. To determine how quickly difficulties would occur, 1,200 pounds of PbCl2 (equal to 17 bath changes) was prepared by ferric chloride leaching of galena concentrate as shown in figure 1. The cell was then operated for 9 weeks using this PbCl2 as feed. Results are shown in table 6 along with the ASTM specifications for corroding-grade lead. The specifications include upper limits for As, Bi, and Sn (15, 500, and 95 ppm, respectively) but these are not shown in table 6 because all three elements were below the limit of detection (1 ppm).
Some of the results were surprising. According to the standard electromotive series, Ag, As, Bi, Cu, Fe, and Sb all have lower decomposition potentials than Pb and should end up in the deposit. Na, Sn, and Zn have higher decomposition potentials than Pb and should not be found in the metal as long as there is any appreciable amount of PbCl2 left in the bath. Actually, while all of the Ag and Sb deposited with the lead, Cu and Fe did not. Also, some Zn was found in the product. Arsenic, bismuth, and tin could not be accounted for because they were not present in the PbCl2 added to the cell. None of these elements dissolved during the ferric chloride leach, although a measurable amount of each was contained in the galena concentrate. Nickel dissolved in the leach while cobalt did not. The solubility of any of these elements, however, will depend upon the form in which they are present in the concentrate. Silver and antimony could not be detected in either the PbCl2 feed or the bath but did show up in the metal. The anomalies could be due to analytical error, to complex formation changing the relative position of the elements in the electromotive series, or to the form in which the elements were present; for example, iron in the feed could have been present as an insoluble oxide rather than as a soluble chloride.
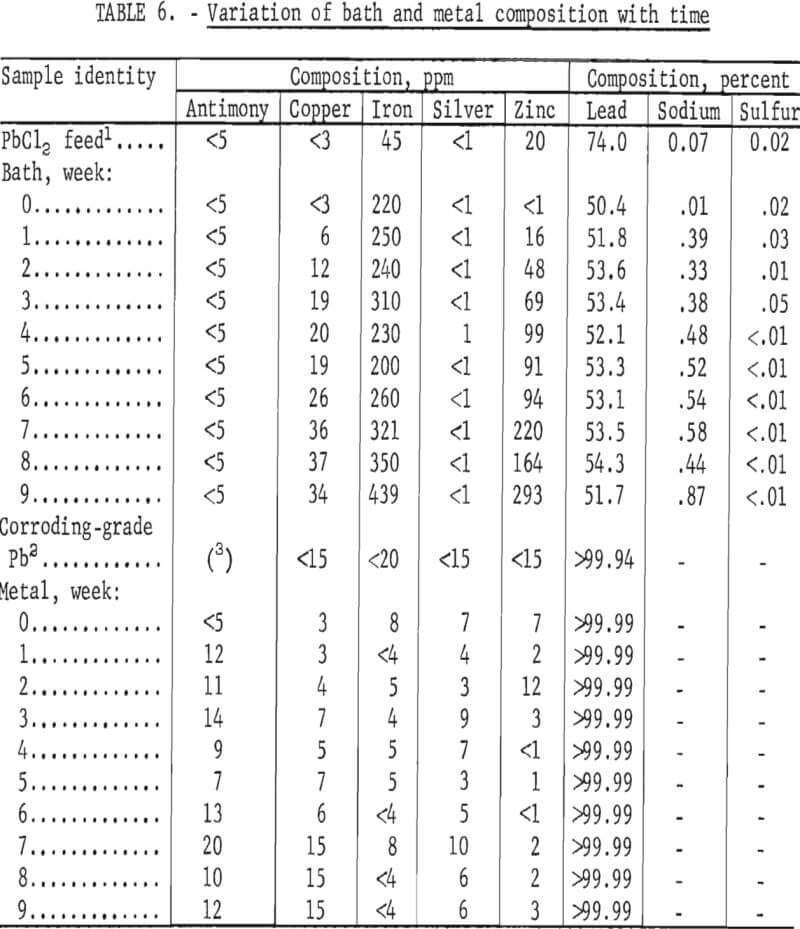
The sodium content of the bath showed a sudden jump during the first week. This was due to the addition of a batch of PbCl2 that had not been washed properly. The impurity level also rose in the seventh, eighth, and ninth weeks when another batch of contaminated PbCl2 was used. Very little effect was noted on the composition of the metal, however. The cell was finally shut down after about 4 months of operation, and the bath was removed. All components were in good condition, although there was some evidence of attack on both the electrodes and the lining. Sludge formation had not occurred, and it appeared that the cell could have been operated for at least a year without major difficulties.
One disadvantage of the LiCl-KCl-PbCl2 system is the high cost of LiCl, necessitating its recovery if the bath has to be discarded for any reason. A small amount of NaCl is usually present in PbCl2 made by the method shown in figure 1 because the PbCl2 is crystallized from a brine solution. NaCl will, therefore, build up in the electrolyte, eventually raising the melting point above 450° C. At this point, some of the bath will have to be bled off and the PbCl2 and LiCl recovered. Fortunately, the NaCl content of PbCl3 made by ferric chloride-brine leaching rarely exceeds 0.1 pct. As NaCl does not interfere with electrolysis until its concentration reaches approximately 20 wt-pct, an extended period is required for the bath to reach the point where it must be replaced.
Conclusions
Electrolysis of PbCl2 prepared by ferric chloride leaching of galena concentrate appears to be a feasible method for producing lead metal with a minimum amount of air pollution. Unlike smelting, it can be used on a small scale, requiring less capital investment. Best results were obtained with a bipolar cell having horizontal electrodes. This configuration would definitely lower the cost of electrical equipment and busbar. However, since the cost of electrolysis is so low in any case, a monopolar arrangement might be preferred because of its ease of construction and reliability. An economic evaluation showed the overall cost of producing lead to be 5.7 cents per pound with an interest rate of return on the investment after taxes of 22 pct. The purity of PbCl2 made by ferric chloride leaching is such that corroding-grade metal can be made for at least several months without any need for discarding the electrolyte or removing sludge from the cell. Materials of construction are readily available at a low cost, and lead emissions, using the LiCl-KCl-PbCl2 bath, are negligible.

