Table of Contents
- Experimental Procedure and Equipment
- Results and Discussion
- Matte Smelting
- Synthetic Matte Smelting
- Stillwater Matte Smelting
- Matte Species Identification
- Preliminary Matte-Leaching Experiments
- First-Stage Leaching
- Acid-to-Matte Ratio
- Particle Size
- Temperature and Duration of Leach
- Large-Scale First-Stage Leaching
- Preliminary Second-Stage Leaching Studies
- Second-Stage FeCl3 or Fe2(SO4)3 Leaching of Synthetic Matte
- Second-Stage FeCl3 or Fe2(SO4)3 Leaching of Stillwater Matte
- Other Leaching Options
- Summary
An important objective of Bureau of Mines research efforts is to investigate alternative technologies that may be applicable to domestic mineral resources. In addition, the Bureau seeks to avoid some of the difficulties or problem areas associated with conventional processing methods. In this regard, the Bureau has studied flotation concentration and extraction of platinum-group metals (PGM) and associated base metals from Stillwater Complex, Mont., ore. The methods described In this report are alternatives to methods that produce SO2 or that require high-pressure autoclaving.
The Republic of South Africa, the U.S.S.R., and Canada are the world’s leading producers of PGM. The United States is dependent on foreign supplies for almost all of its platinum and palladium. If the Stillwater Complex were developed, all U.S. requirements for palladium and about 25 pct of its requirements for platinum could be supplied.
The Stillwater Complex contains 225 million ounces of PGM of which 80 pct is estimated to be recoverable. The precious metals are associated with iron, nickel, and copper sulfides that occur in mineralized layers of the Banded Zone approximately 5,000 ft above the base of the complex. The ore body is continuous for about 24 miles. The horizon exposed in the Johns-Manville West Fork exploration adit has an average grade of 0.47 oz Pt-Pd per ton with a Pd-to-Pt ratio of about 3.5:1. The mineralization occurs in sulfide-rich anorthositic rocks. Accessory minerals include pyrrhotite, pentlandite, pyrite, chalcopyrite, and magnetite. Several PGM minerals have been identified: stibiopalladinite (Pd3Sb), sperrylite (PtAs2), cooperite (PtS), laurite (RuS2), moncheite (PtTe2), braggite [(Pt, Pd)S], vysotskite (PdS), kotulskite (PdTe), and ferroplatinum alloys. Concentration by froth flotation was investigated by the Bureau of Mines because the platinum-group-bearing sulfides occur as fine grains and require grinding to approximately 200 mesh for liberation. Recovery of 80 to 90 pct of the Pt-Pd values was achieved from an acidified pulp with a mercaptobenzothiazole collector.
Matte smelting followed by leaching of the metal values is a conventional metallurgical processing sequence for treating sulfide flotation concentrates containing Cu, Ni, Fe, and PGM. The processing sequence includes (1) smelting to remove gangue material and to produce an Fe-Ni-Cu-PGM green matte, (2) converting by blowing air through the green matte to oxidize iron sulfide and to transfer the oxidized iron to the slag as 2FeO·SiO2, (3) leaching the resultant Cu-Ni-PGM white matte to dissolve the copper and nickel and to obtain a PGM residue, and (4) refining the residue for recovery of PGM. Converting the green matte to white matte is accompanied by evolution of large quantities of SO2 gas. Since prevention of atmospheric pollution by SO2 is a costly processing step, direct leaching of the green matte to extract metal values without SO2 evolution is a desirable alternative.
Impala Platinum, Ltd., the Western World’s second largest platinum producer, is located on the Merensky Reef in the Republic of South Africa and beneficiates ores by flotation. The concentrate is smelted to yield a Cu-Ni-Fe-PGM matte. To remove the base metals, the matte is pressure leached in three stages with H2SO4 in an oxygen atmosphere. The final residue, which contains a high concentration of PGM, is treated by standard refining techniques to selectively recover the precious metals.
Sulfuric acid-oxygen pressure leaching for solubilizing Cu, Ni, and Fe from granulated matte has also been investigated by Johns-Manville Corp. The base metals are selectively recovered from the pregnant solution by precipitative techniques. The PGM residue is upgraded by a series of steps: (1) sulfur removal, (2) sulfation roasting at 450° to 525° C to convert the residual copper, nickel, and iron sulfides to sulfates, and (3) H2SO4 leaching to remove the soluble sulfates. The final PGM residue contains more than 80 pet of the palladium and platinum in the ore.
Another method for treating mattes is NH3-O2 pressure leaching, employed by Sherritt Gordon. The leaching procedure involves reacting the sulfide minerals with dissolved oxygen, NH3, and water to convert Ni, Co, and Cu to soluble ammines, oxidize sulfur to soluble sulfur-oxygen ions, and convert iron to insoluble hydrated ferric oxide. The primary reaction is
MS + 2 O2 + 6NH3 → M(NH3)6SO4…………………………………………………..(1)
where M is Ni, Cu, or Co.
These processes alleviate the SO2 pollution problem but require high-pressure autoclaves. The Falconbridge Matte Leach Process involves selective leaching of nickel from a Cu-Ni-PGM matte with strong HCl at atmospheric pressure. The nickel is solubilized, while the copper and PGM remain as a sulfide residue. Nickel is precipitated as NiCl2·2H2O, which is converted to NiO by high-temperature hydrolysis. The NiO is hydrogen reduced to nickel metal. The residue from HCl leaching is roasted at 800° C to oxidize the copper sulfide to CuO. Because of the high roasting temperature, SO2 is emitted. The roasted material is leached with dilute H2SO4 to remove copper and to produce a PGM concentrate. The copper is recovered electrolytically from the leaching solution.
A study of atmospheric matte leaching by Martin describes two processes in which PGM are separated from a matte containing Cu, Fe, and Ni. In one process, the matte is leached with Fe2(SO4)3, followed by electrowinning of ferronickel alloy from the pregnant liquor. The second method consists of HCl leaching of the matte, followed by a pyrohydrolysis step to regenerate the acid and produce a mixture of iron and nickel oxides. In either case, the PGM are retained in a copper sulfide component, and the nickel and iron are not completely separated but are recovered as ferronickel alloy.
The objective of the Bureau’s research was to devise a method for selectively extracting the base metals from matte prepared from Stillwater flotation concentrates and to recover the PGM in a high-grade residue. Because of a limited supply of Stillwater flotation concentrate, synthetic matte was used to develop the extraction sequences. The procedures developed were subsequently applied to the extraction of base metals and recovery of PGM from Stillwater matte.
Experimental Procedure and Equipment
Induction Smelting
Mattes were smelted in graphite crucibles inserted in a 15-kw laboratory induction furnace. An argon blanket was maintained over the charge to partially exclude oxygen and, consequently, prolong crucible life and minimize oxidation of the sulfidic charge. After melting, the charge was maintained between 1,400° and 1,600° C for approximately ½ hr. The molten charge was poured into a graphite mold. After cooling, the matte and slag were separated and weighed.
Grinding
All mattes were broken into fragments with a steel mortar and pestle. Synthetic mattes were crushed and ground in three stages: jaw crushing, roll crushing, and dry ballmilling. The Stillwater mattes were dry ground in a small tungsten carbide mixer mill.
Leaching
Atmospheric leaching was carried out in heated glass resin kettles. Each reactor was fitted with a sealed shaft stirrer, a thermometer or thermocouple well, a reflux condenser to minimize vapor losses, and a port for material addition and sample removal. The temperature was maintained with a controller and a power-stat. Pressure leaching experiments were performed in stainless steel or titanium autoclaves. After leaching, the solids were recovered by filtration, washed, and then dried at about 110° C.
Roasting
First-stage residues were loaded into assay crucibles and roasted in a globar pot furnace. A thermocouple was embedded in the sample, and the temperature was continuously monitored on a strip chart recorder. Samples were stirred several times during the roasting period.
Sample Analysis
Base metals were analyzed by atomic absorption analysis. Platinum, palladium, and gold in solid samples were determined by fire assaying. The assay beads were dissolved and analyzed by inductively coupled plasma spectroscopy (ICP). Pregnant liquors were assayed for PGM and gold by cementing aliquots with iron powder. The cementation products containing the precious metals were collected on glass-fiber filter paper. The residue and paper were fire assayed after inquarting with silver, and the dore beads were analyzed by ICP. Sulfur was determined by a standard combustion technique. Solid compounds were characterized by X-ray diffraction. A scanning electron microprobe (SEM) was used to identify elements and compounds in solid samples.
Results and Discussion
Matte Smelting
Synthetic Matte Smelting
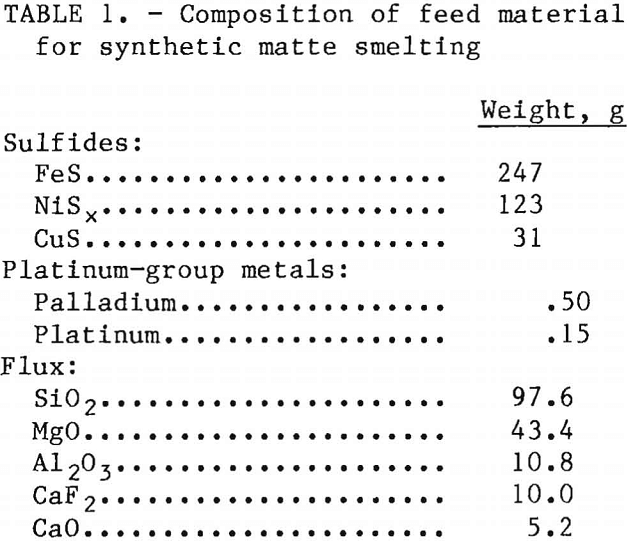
Iron, copper, and nickel sulfides and platinum and palladium powders were combined in appropriate proportions to produce matte with a composition similar to the Stillwater matte. Although Stillwater concentrate contains lead and cobalt, these metals were not added to the synthetic charge. The sulfides and precious metals were mixed with flux materials that were selected to simulate typical Stillwater gangue minerals. Table 1 lists the composition of a typical feed mixture, and table 2 lists the analyses of the matte and slag obtained from smelting. Approximately 90 pct of the copper and nickel and 94 pct of the platinum and palladium reported in the matte.
Stillwater Matte Smelting
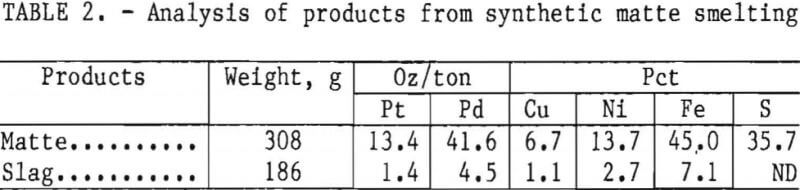
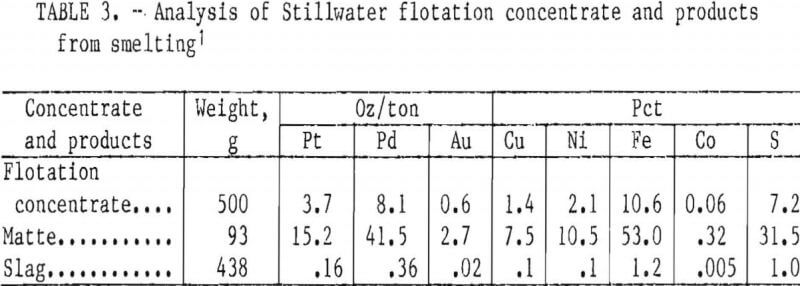
Five-hundred-graui charges of Stillwater flotation concentrate were smelted with a flux consisting of 37 g CaO, 13.3 g CaF2, and 13.3 g SiO2. The fluorspar and lime decreased the melting point of the concentrate from about 1,600° to 1,300° C. Silica was added to remove the iron oxide into the slag as fayalite (2FeO·SiO2). More than 94 pct of the copper, nickel, and precious metals reported to the matte. Typical analyses of Stillwater flotation concentrate, the matte, and the slag obtained from smelting are presented in table 3. Volatiles and material remaining in the crucible account for the 42-g weight loss during smelting. Trace elements and impurities in the matte were, in ounce per ton, Ir, 0.08; Rh, 0.22; and Ru, 0.02; and, in percent, Mg, 0.1; Ca, 0.4; and Pb, 0.3.
Matte Species Identification
Synthetic and Stillwater mattes exhibited similar X-ray diffraction patterns. The principal phases in the mattes were troilite (FeS) and a species that could not be identified. Microprobe examination of Stillwater matte (fig. 1) showed two main phases: (1) iron-sulfur-rich grains containing small amounts of nickel and copper (S-Fe>Ni>Cu) and (2) an iron-nickel phase with a trace of copper but no sulfur (Fe>Ni, tr Cu). The PGM were scattered in bright spots, not in the main grains, and were associated with lead, iron, and nickel (Pb>Ni>Fe=Pd>Pt). Composition was variable. Some of the bright spots contained lead and varying small amounts of iron and nickel, but no PGM. Positive identification of sulfur in these bright grains could not be made because the SEM sulfur peak coincided with the lead peak.
SEM examination of synthetic matte (fig. 2) showed three main phases containing iron and sulfur and small amounts of copper and nickel. The average scan was similar to that of the Stillwater matte. The PGM were scattered in bright grains of variable composition. Because lead was not present in the synthetic matte, sulfur was detected in the bright grains, but only in minor amounts (Fe=Ni>Pd=Pt>S>Cu). Bright grains containing trace amounts of PGM were also observed (Ni>Fe>>Cu, tr Pd). Since the X-ray diffraction patterns and microprobe

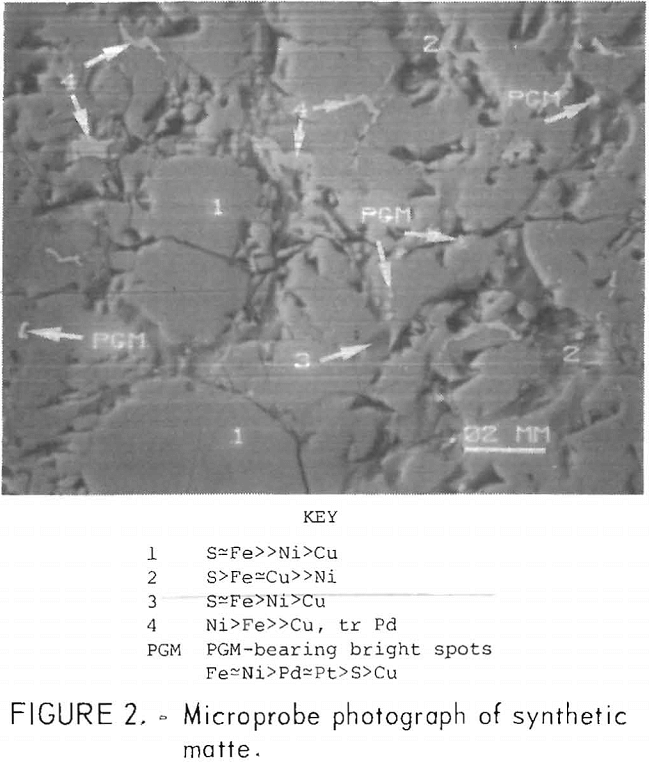
fluorescence scans were similar, the Stillwater and synthetic mattes should exhibit similar leaching characteristics. The relative proportions of iron, nickel, and PGM in the bright grains of the two mattes were also comparable.
Preliminary Matte-Leaching Experiments
Several mineral acids and oxidizing agents were used to leach synthetic matte under atmospheric pressure conditions. Solubilization of the base metals was nonselective except for H2SO4, which dissolved the iron and nickel, but not the copper and PGM.
Since pressure leaching is common industrial practice, H2SO4-O2, NH3-O2, and HCl-O2 leaching systems were investigated to evaluate extraction characteristics. Sulfuric acid pressure leaching completely solubilized the Fe, Ni, and Cu, but partial PGM extraction also occurred. Ammonia pressure leaching re-removed copper, nickel and some PGM, primarily palladium. Iron remained in the residue. Iron and PGM also remained in the residue when the matte was pressure leached with HCl. More than 90 pct of the nickel and copper were extracted.
Because pressure leaching requires expensive autoclaves and results in partial PGM extraction, efforts were directed toward the development of a two-stage leaching process conducted at atmospheric pressure. A H2SO4 leach selectively removed the iron and nickel from the matte. The first-stage residue was subsequently leached or roasted and then leached to extract the copper and to yield high-grade PGM products.
First-Stage Leaching
Because of the limited availability of Stillwater matte, most experiments were performed with synthetic mattes. The best conditions were applied to Stillwater mattes.
Sulfuric acid was added gradually to the ground matte at room temperature so that the rate of hydrogen sulfide (H2S) evolution could be controlled. Concentrated solutions (35 to 40 pct H2SO4) produced excessive frothing. An acid concentration of 18.9 pct (2.1M) gave good extraction with moderate frothing. After the addition of acid was completed, the reactor was heated slowly to the desired temperature and the leaching conducted for the appropriate period of time.
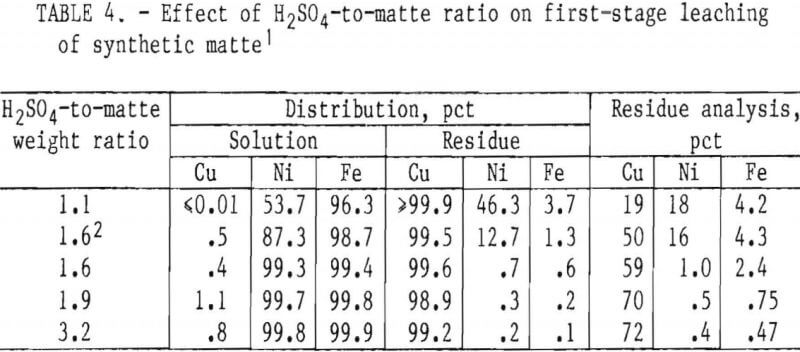
Acid-to-Matte Ratio
The effect of the H2SO4-to-matte weight ratio is presented in table 4. Ratios greater than 1.9 were necessary to achieve 99 pct extraction of the iron and nickel and to upgrade the residue to at least 70 pct Cu.
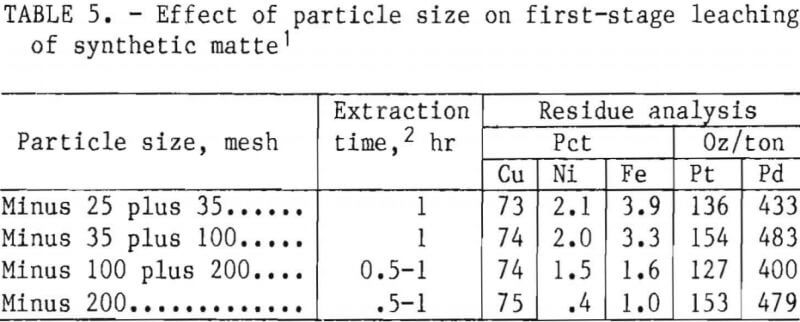
Particle Size
Table 5 reports the chemical analyses of residues from leaching different size fractions of synthetic matte. Retention of iron and nickel in the solids increased with increasing particle size. Liquid samples were removed from the pregnant liquors at ½- to 1-hr intervals during the leaching period. Nickel and iron extractions of 98 pct were obtained in 1 hr, regardless of particle size. Residue copper grade improved when the matte was ground to minus 200 mesh because the removal of iron and nickel was more complete.
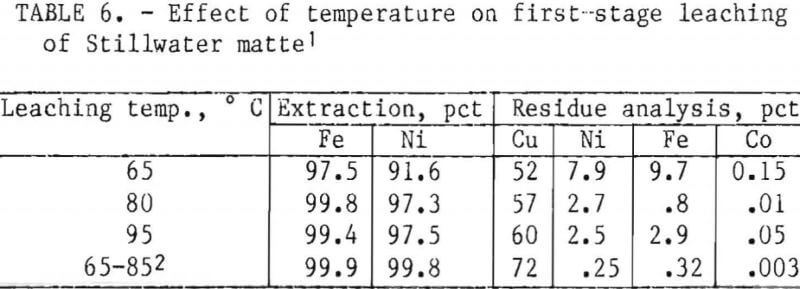
Temperature and Duration of Leach
Table 6 shows the chemical analyses of residues from leaching Stillwater matte at different temperatures. Iron and nickel extractions were better at temperatures greater than 80° C. At each temperature evaluated, iron was extracted in ½ hr, but nickel dissolved more slowly. Ninety-seven-percent nickel extraction was achieved in 1½ hr at temperatures exceeding 80° C. Longer leaching periods gave higher grade copper residues because of the more thorough removal of iron and nickel from the matte. When the residue from leaching at 65° C was releached at 85° C, the copper content increased from 52 to 72 pct, and the combined nickel and iron content decreased from 17.6 to 0.6 pct.
The following conditions gave the best results in the first-stage leaching:
- Particle size: minus 200 mesh.
- H2SO4 concentration: 18.9 pct.
- H2SO4-to-matte ratio: 1.9.
- Leaching temperature: 80° to 95° C.
- Leaching cycle: 4 hr.
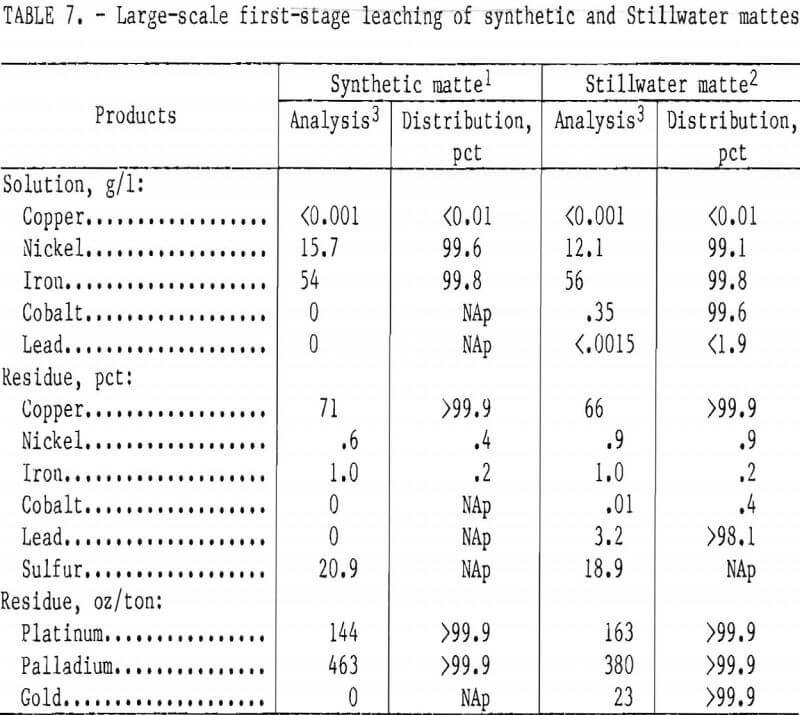
Large-Scale First-Stage Leaching
Typical results from larger bench-scale leaching of synthetic and Stillwater mattes are reported in table 7. Distribution of values and residue analyses for leaching of Stillwater matte are similar to those obtained with synthetic matte. When Stillwater matte was leached, cobalt was extracted with the nickel and iron, while gold and lead remained in the residue with the copper and PGM.
The major mineral present in the first-stage residues from synthetic and Stillwater matte leaching was djurleite (Cu1.93S). SEM studies of the Stillwater residue indicated that PGM were disseminated throughout the matrix in small grains that contained mainly copper and sulfur and some iron. PGM were also found in scattered bright spots containing lead and small amounts of nickel and copper.
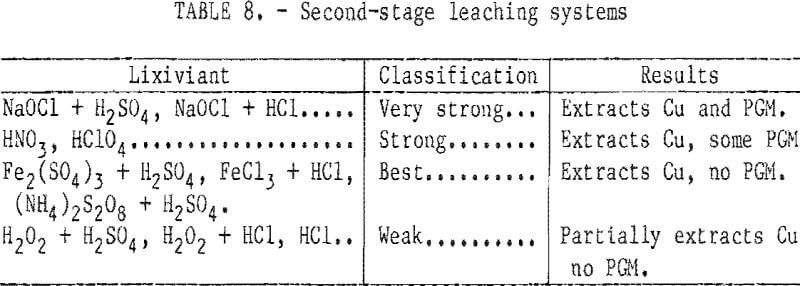
Preliminary Second-Stage Leaching Studies
Several oxidizing agents in acidic solution were screened to determine their ability to dissolve only the copper from the first-stage residues (table 8). Very strong extractants solubilized both copper and PGM and would necessitate subsequent separation and recovery of the values from the pregnant liquor. Several lixiviants, notably ferric salts, extracted only the copper and yielded high-grade precious metals products that can be refined to recover the PGM and gold.
Second-Stage FeCl3 or Fe2(SO4)3 Leaching of Synthetic Matte
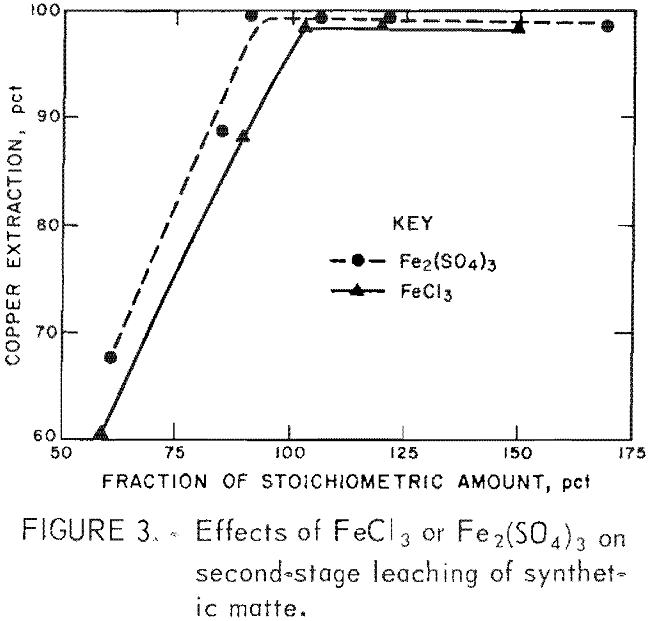
Ferric salts were selected as second-stage lixiviants because of their low cost and capacity for extracting copper from first-stage residues.
Figure 3 shows the relationship between ferric salt addition and the extraction of copper from the first-stage residue. The stoichiometric ferric ion requirement was calculated from the equation:
Cu2S + 4Fe³+ → 2Cu²+ + 4Fe²+ + S°…………………………………………….(2)
Ninety-five to one hundred percent of the stoichiometric quantity was required for 98 pct copper extraction. PGM remained in the second-stage residue.
In the initial experiments, acid was added to the leaching liquor to prevent hydrolysis of the iron in the first-stage residue. Subsequent tests indicated that copper extraction was also acid dependent. In the chloride system, a pH of about 1.1 (≅0.15M HCl) was required.
At higher pH, copper extraction was incomplete. When the pH was decreased to below 0.9, small amounts of platinum and palladium co-extracted. The sulfate system required a pH of less than 1.4 (≅0.06M H2SO4), and coextraction of PGM was not observed at greater acidities
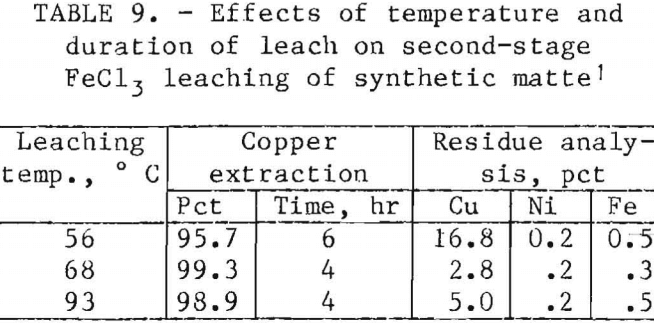
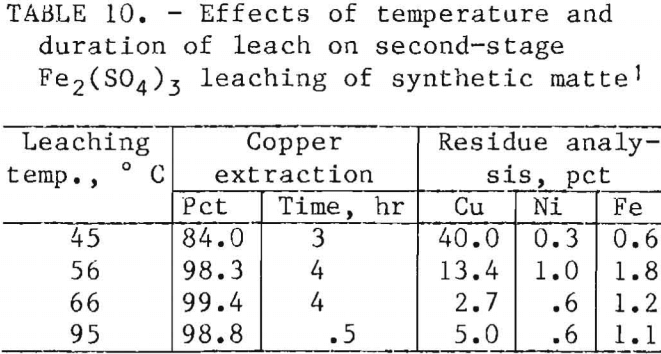
After the quantities of reagents and the pH required for copper extraction had been determined, the effects of leaching cycle and temperature were investigated. Tables 9 and 10 report the chemical analyses of the solids recovered after 2.5-g samples of first-stage residue prepared from synthetic matte were leached with stoichiometric quantities of FeCl3 and Fe2(SO4)3. In both systems, a temperature of at least 65° C was necessary to decrease the copper content of the final residues to less than 5 pct. Increasing the temperature from 66° to 95° C decreased the time for Fe2(SO4)3 leaching from 4 to 0.5 hr. However, increasing the temperature of the FeCl3 leaching system from 68° to 93° C did not improve the rate of extraction.
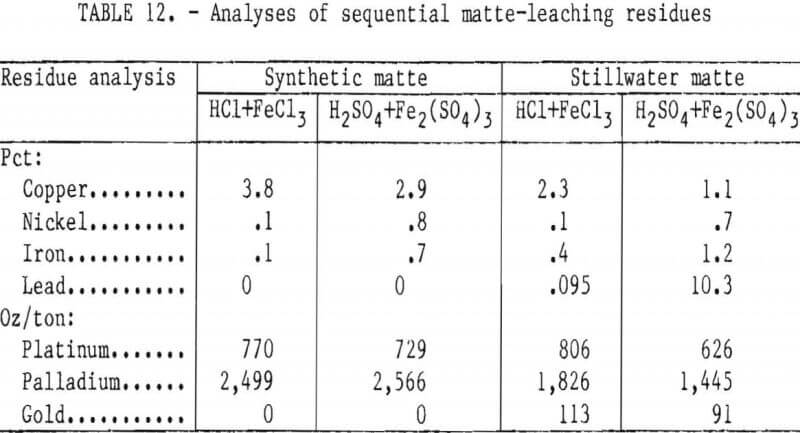
Second-Stage FeCl3 or Fe2(SO4)3 Leaching of Stillwater Matte
Leaching Stillwater first-stage residue with stoichiometric amounts of FeCl3 or Fe2(SO4)3 (table 11) confirmed the validity of using synthetic matte to develop the two-stage leaching method. Copper extractions were more than 98 pct and the final residues contained 2 to 5 pct Cu and 2,250 to 2,600 oz PGM per ton. At 70° C, leaching with FeCl3 took about 4 hr or the same time as for the synthetic material. Ferric sulfate leached the Stillwater sample in 1 hr, which was considerably faster than the 4-hr cycle exhibited by the synthetic material at 66° C. It is feasible that there are differences between the synthetic and natural mattes, arising from their dissimilar origins, that make the Stillwater material more amenable to leaching.
After the second-stage leaching with Fe2(SO4)3, X-ray diffraction identified major amounts of elemental sulfur and lead sulfate (PbSO4) in the residue. SEM examination showed that the material was nonhomogeneous. PGM were associated primarily with sulfur and copper in grains that sometimes contained lead. Silicates and alumina-bearing grains were also observed and were probably matte- occluded slag particles. The residue was upgraded twofold by dissolving the elemental sulfur in carbon disulfide. Lead sulfate would be removed during refining.
The second-stage residue from FeCl3 leaching contained sulfur and a non-crystalline, unidentifiable phase. The lead content was 0.09 pct compared with 10.5 pct for the sulfate residue. Ferric chloride solubilized the lead and copper. SEM examination showed that the PGM values in the residue were associated primarily with sulfur and copper in main grains and in bright spots.
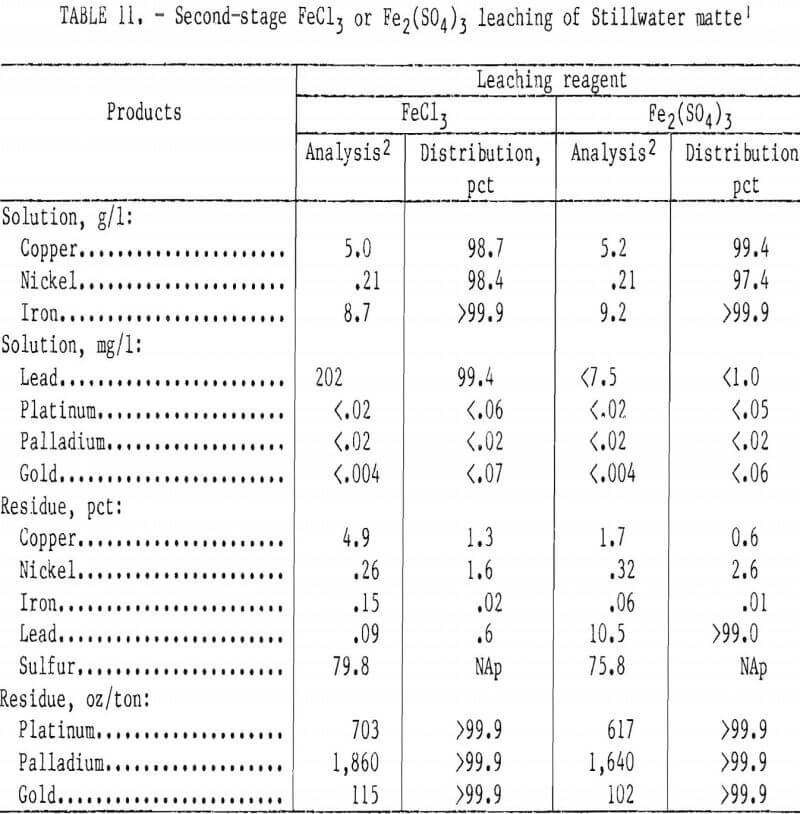
Figure 4 shows a flowsheet summarizing the two-stage procedure developed for leaching Stillwater matte. The PGM content was upgraded almost 500-fold from approximately 12 oz/ton of flotation concentrate to 5,900 oz/ton of residue. The final residue contained more than 98 pct of the PGM in the matte.
Advantages of the sulfate system over the chloride system are (1) the second-stage extraction time is shorter, 1 hr at 70° C for Fe2(SO4)3 compared with 4 hr for FeCl3 leaching; (2) Fe2(SO4)3 and H2SO4 are less expensive than FeCl3 and HCl; (3) sulfate is less corrosive and less volatile than chloride; (4) sulfate solution is more suitable for electrolytic deposition of copper; and (5) PbSO4 in the residue can be easily removed during refining, whereas lead in the second-stage pregnant liquor requires additional processing.
Other Leaching Options
Sequential Matte Leaching
Instead of leaching matte in two stages, the acid and oxidizing lixiviants were added sequentially to the matte without an intervening filtration. The matte was leached first with H2SO4 or HCl under conditions similar to those of the first leaching stage previously described (2 hr at 95° C). After nickel and iron
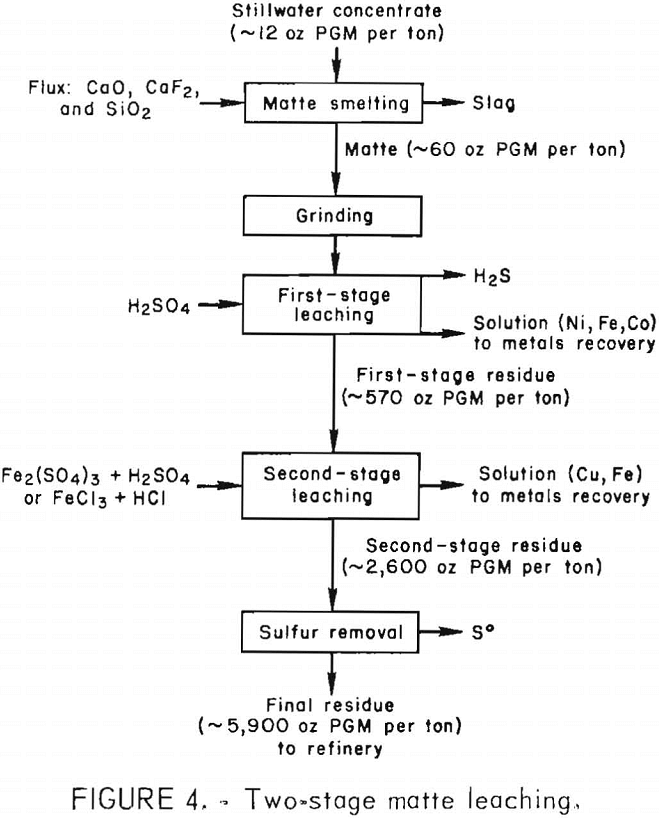
dissolution was completed and most of the H2S had been evolved, Fe2(SO4)3 or FeCl3 was added to the liquor and the leaching continued until the copper was extracted (≈4 hr). The pregnant solution contained more than 99 pct of the Fe, Ni, and Cu,
while the PGM remained in the residue. Residue grade was similar to that obtained from two-stage leaching (table 12). Sequential leaching could be used to produce a high-grade residue in one leaching step. Because all the base metals were extracted into the pregnant liquor, selective metal recovery from solution would be more complex than for the two-stage leaching solutions. Figure 5 shows the sequential matte leaching flow diagram.
Roasting-Leaching of First-Stage Residue
The purpose of the second-stage leaching is to make the copper extractable by oxidizing the djurleite (Cu1.93S), which is the major species present in the first-stage residue. Oxidation by roasting forms acid-soluble copper oxide and sulfate compounds and is an alternative to oxidative leaching. Table 13 lists the chemical species present after roasting first-stage residues at selected temperatures. Oxidation of the djurleite was exothermic and made temperature control difficult. Therefore, temperature ranges are reported in the table. No oxidation occurred below 250° C. The major species present in the roasted material were CuSO4 and Cu2O when maximum roasting temperatures were between 320° and 390° C. At 500° C, a mixture of CuO, Cu2(SO4)O, and CuSO4 resulted. At 800° C, complete oxidation to CuO occurred. Dilute H2SO4 (1M) solubilized more than 98 pct of the copper from first-stage residues that were roasted at temperatures above 360° C. PGM remained in the solids. Roasting below 320° C resulted in decreased copper extraction. Since PGM values were lost to the leaching liquor when roasting was conducted at 500° C or above, roasting between 360° and 500° C would permit the most thorough separation of copper and PGM.
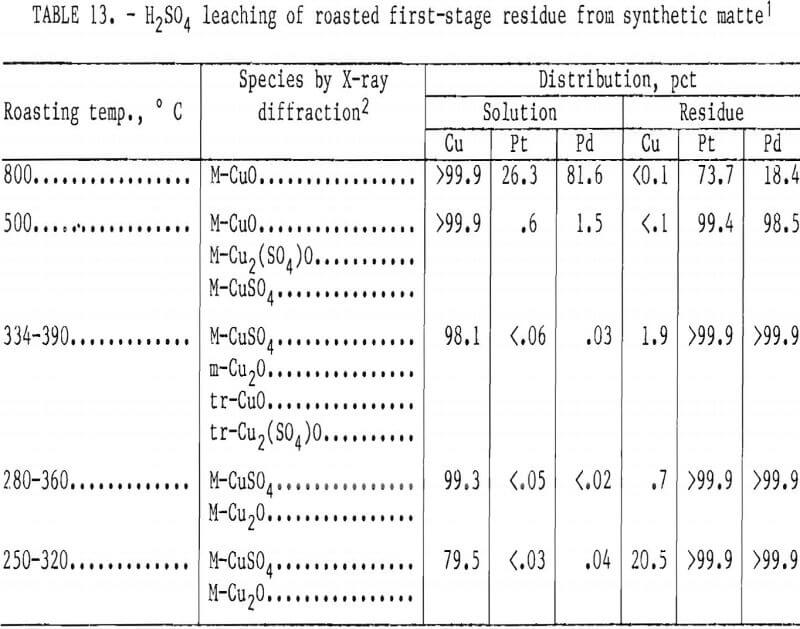
The effect of H2SO4 on the leaching of first-stage residue that had been roasted at 350° to 400° C was investigated. Very-dilute acid solutions were used. Leaching with hot water extracted 31 pct of the copper. Basic copper sulfate salts such as anterlerite (Cu3SO4(OH)4) and bronchantite (Cu4SO4(OH)6) remained in the residue. One mole of H2SO4 per mole of copper solubilized 97 pct of the copper without coextracting PGM values. In practice, the copper would be recovered by electrowinning, and an acid concentration compatible with the electrowinning operation would be used.
Analyses of the products of the roasting-leaching procedure are reported in table 14. Copper extraction was complete in ½ hr at 25° C. There was no advantage to leaching at higher temperatures. The copper content of the pregnant liquor was increased from 11 to 48 g/l by increasing the slurry solids in leaching from 23 to 100 g/l. Copper concentrations of 40 to 50 g/l are satisfactory for copper electrowinning. The final residue from Stillwater matte contained 8,715 oz of precious metals per ton.
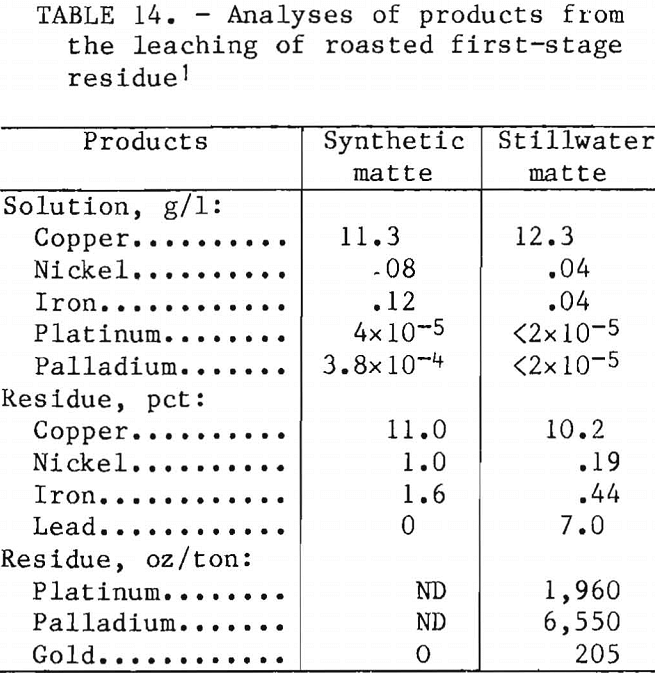
X-ray analysis of the residue from synthetic matte indicated a major CuO species and an unidentified phase for which the X-ray pattern was similar to those of ferroplatinum compounds. This unidentified phase is probably a Pt-Pd-Fe or Pt-Pd-Cu species. Trace to minor amounts of analite (Cu7S4) and quartz were also identified. SEM showed that the PGM were associated with sulfur and small amounts
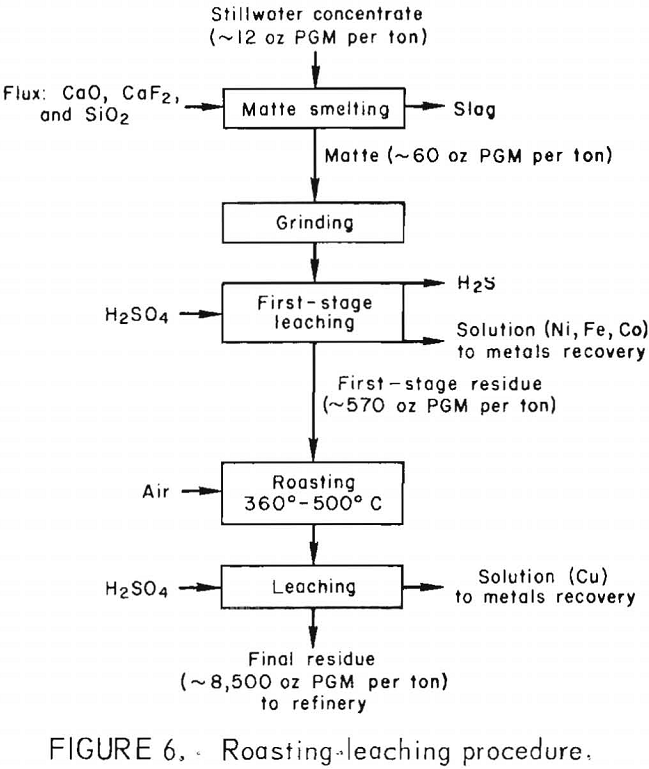
of copper and iron. Bits of material identified as Ca-Al-Mg-Fe silicate were observed in the residue and were probably pieces of occluded slag. The small quantities of residue recovered after Stillwater matte had undergone the roasting-leaching procedure were consumed during analysis and could not be characterized by X-ray diffraction or SEM. A flow diagram for the roasting-leaching procedure is shown in figure 6.
Summary
A matte-smelting, two-stage leaching sequence was developed for the extraction of PGM, Cu, Ni, and Fe from Stillwater flotation concentrates. Smelting the concentrates with a lime, fluorspar, and silica flux resulted in the recovery of 94 pct of the metal values in the matte. Leaching the ground matte with dilute H2SO4 selectively extracted more than 99 pct of the nickel and iron. The first-stage residue contained 99.9 pct of the copper and PGM values. Leaching the residue with FeCl3 or Fe2(SO4)3 lixiviants extracted 98 pct of the copper. After sulfur removal, the second-stage residue contained about 6,000 oz PGM per ton or almost 100 pct of the PGM values in the matte. The residue is suitable feed for a precious metals refinery.
Two alternative methods, sequential matte leaching and a roasting-leaching procedure, also were investigated. Sequential matte leaching used the same reagents and conditions as the two-stage leaching sequence, but eliminated the intermediate filtration. In the roasting-leaching procedure, the first-stage residue was roasted at 360° to 500° C before the copper was leached with dilute H2SO4. Both methods extracted more than 98 pct of the base metals and yielded final residues containing 5,500 to 8,500 oz PGM per ton.

