Table of Contents
The recovery and recycling of metals from process wastes is a major research effort of the Bureau of Mines. The present work was undertaken by the Rolla Metallurgy Research Center to develop data that could be used in a pyrometallurgical process to recover zinc and lead from the flue dust generated in the secondary smelting of brass, and from other dusts containing large amounts of zinc oxide.
There is virtually no recovery of zinc and lead from brass smelter dusts at the present time, mainly due to economics. Zinc metal is a relatively inexpensive nonferrous metal, so the contained value may not offset the high cost of processing. However, the recovery of zinc from industrial metallurgical wastes may become a necessity as stricter environmental controls are imposed and more material is collected. In the meantime, the flue dust collected from secondary brass furnaces (averaging 2 tons/day per smelter) represents an aggravating problem to the operator. The material cannot be recycled, as is, to the furnaces, and it is ordinarily too light and fluffy for practical shipment to a user, such as a zinc smelter. Landfill disposal is not a simple problem because such sites are becoming scarce and because of environmental problems.
Zinciferous dusts can sometimes be fed into the leaching step of the electrolytic zinc extraction process. This practice is often inadvisable due to impurities in the dust that are harmful to the electrolysis and a problem to remove from solution. Although Powell showed that brass flue dust could be processed in this manner, zinc ferrite, which may be present, is not readily soluble.
Brass smelter dusts contain zinc oxide (ZnO) or zinc ferrite (ZnO·Fe2O3), the metal content of which is responsive to reduction and vaporization using CO, hydrogen, hydrocarbons, and various carbonaceous materials at a sufficiently high temperature. When hydrogen or CO is used as a reductant, the equilibrium partial pressures of H2O or CO2 offgases are very low. For example, at 1,200° C the partial pressures of H2O in hydrogen, and CO2 in CO must be on the orders of 10-¹ and 10-² (or less) atm, respectively. This means a large excess of hydrogen or CO must be fed into the reduction chamber. Use of hydrocarbons alone for reduction would be very inefficient due to carbon precipitation. For these reasons it is preferable to use an inexpensive active carbon that might allow a lower reduction temperature.
The purpose of the research described herein was threefold: (1) To reduce the zinc oxide and ferrite in brass smelter dusts and vaporize the reduced zinc away from the residue at the lowest practical temperature, (2) to determine the partition of lead between residue and collected zinc, and (3) to identify substances that may enhance or retard the reduction or vaporization of lead and zinc. The resulting techniques could be the basis for a pyrometallurgical process to recover zinc and lead from brass smelter flue dusts.
Experimental Procedures and Results
In the process proposed herein, oxides and ferrites of zinc are reduced by carbon to form CO, CO2, and zinc vapor. The latter is condensed at a cooled part of the furnace while the offgas (mainly CO) is burned to form CO2 after passing through a fume trap. The combustion of the exit gas can be used to preheat the furnace or for other purposes.
Preliminary Tests
In order to decide on the best form of carbon, preliminary reduction studies were carried out using coke breeze, activated charcoals, graphite, and a wood charcoal powder. Individual carbons (10 to 15 pct of the charge) were mixed with ZnO (99.5-pct pure), and 25 grams of the mixture was placed in a graphite boat. The boat was placed in a 1-¾-inch-ID quartz tube, in a furnace preheated to 300° C, and the air in the tube was purged with helium. The furnace temperature was raised to either 850°, 950°, or 1,050° C. The inlet end of the tube was then closed; the other end had a 1/8-inch opening connected to traps for removing moisture and solid particles in the offgas. The offgas was burned. The furnace temperature was maintained until the gas evolution ceased and was then gradually cooled to 300° C. A slight flow of helium during cooling prevented reentrance of air. Condensed zinc at the cool end of the tube was scraped out, and the boat with the residue was removed from the furnace and weighed.
The preliminary trials indicated that charcoal successfully reduced all the ZnO at 1,050° C. No other form of carbon could do so. Wood charcoal was also the least expensive among the charcoals tested, and for these reasons was chosen for the treatment of the brass dust.
Reduction of Brass Smelter Dusts
Larger scale experiments were carried out using brass smelter dusts mixed in a twin-shell blender with wood charcoal in the ratio of 9 to 1 or 6 to 1 (10 or 15 pct of the combined weight), and heated to 1,050° C, in graphite boats. Inside dimensions of the boats were 2-7/8 inches by 10 inches by 2 inches. A 500-gram to 1,500-gram charge was placed in a 4-inch-OD quartz tube, and the reduction and vaporization were carried out as before. Figure 1 shows a schematic drawing of the experimental apparatus. A slight difference from the preliminary tests was the use of a protective quartz sleeve in the reaction tube. In later tests, the feed mixtures were pelletized prior to reduction.
The reduction time was varied in a few cases in order to determine the time dependency of vaporization. Additions of NaCl or NaOH were made (in addition to the charcoal) in order to determine the effects of each on the vaporization rate of lead and zinc. One dust with a high sodium and chlorine content was leached in boiling water to remove the water-soluble compounds. The insoluble portion was filtered off, dried, mixed with 10 pet charcoal, and reduced at 1,050° C.
In all cases, the residues were weighed and analyzed chemically. Calculations were based on the original analysis of the dust and the amounts of zinc and lead remaining in the residues.
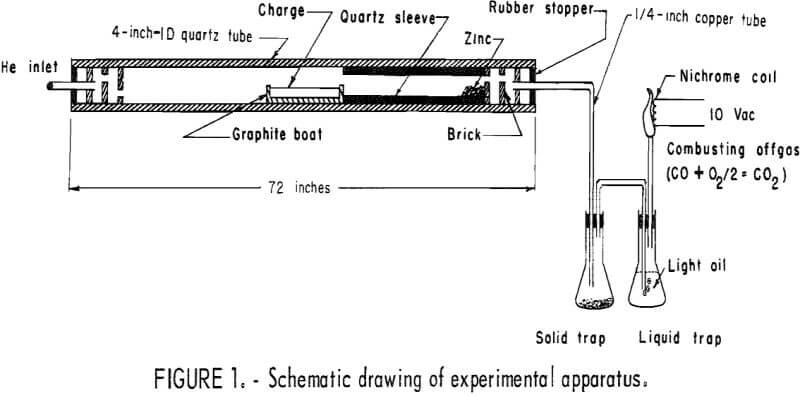
Five-hundred-gram samples from three different smelters were reduced in the 4-inch-ID tube; the reduced zinc was subsequently vaporized. Table 1 shows the chemical composition of these dusts. The charcoal had 7.8 pct volatile material and 4.6 pct ash. The total time required for the completion of reduction, as determined by a cessation of offgas burning and vaporization, was 3 to 4 hours, depending on the dust. Table 2 summarizes the results.
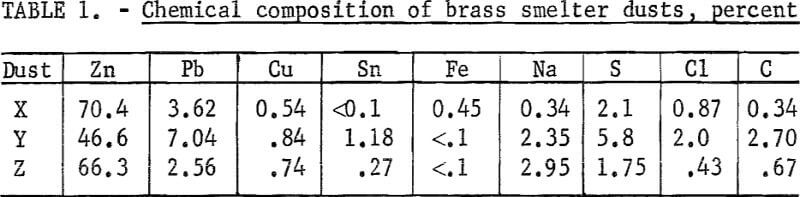
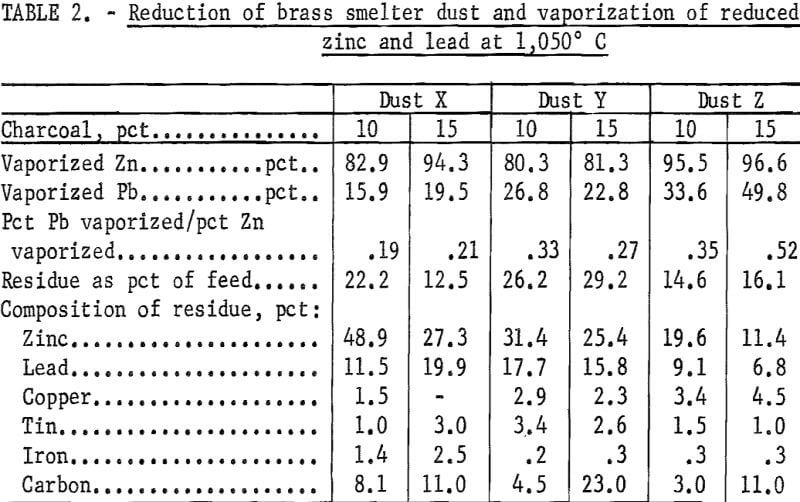
When 10 pct charcoal was added, the residues were fused; however, the residues of the 15-pct charcoal mixtures were powdery. The best volatilization of zinc was obtained from dust Z, from which the largest amount of lead was also vaporized. Slightly more than 80 pct of the zinc was vaporized from dust Y for both 10- and 15-pct charcoal additions. Substantial differences in zinc evolution with varying charcoal additions was observed with dust X. It was found that 83 pct was evolved with 10 pct charcoal, but that evolved from the 15-pct charcoal mixture was above 94 pct. The smallest ratio of the vaporized lead to zinc was observed with dust X; the largest ratio was observed with dust Z.
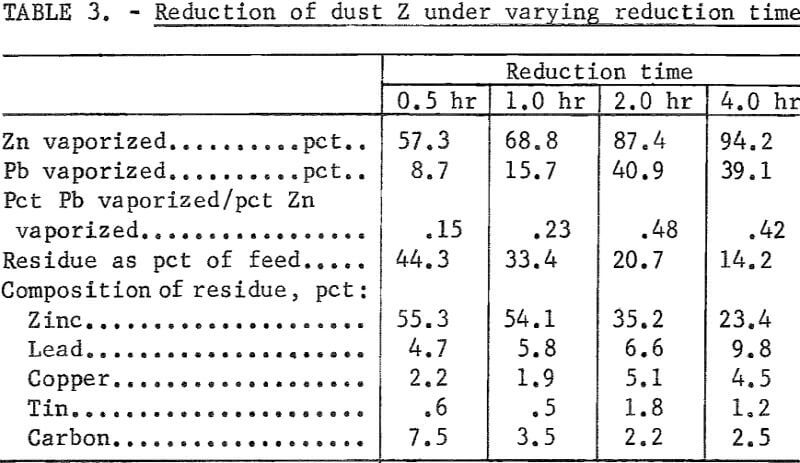
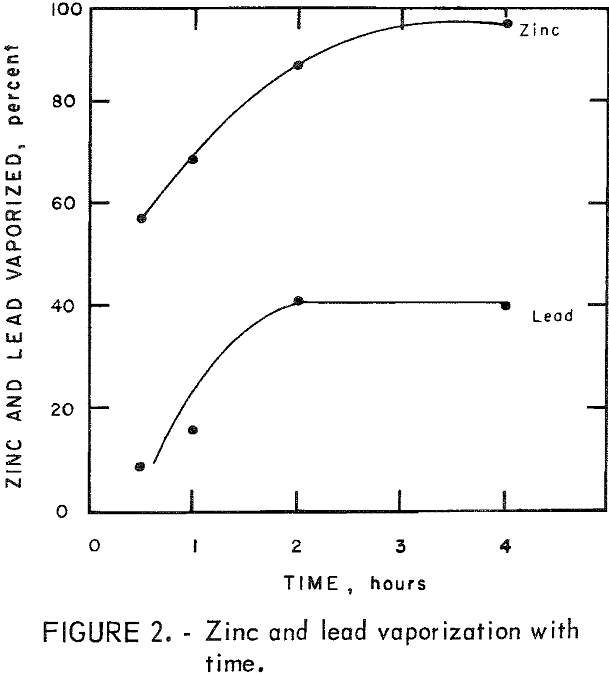
Reduction of 500-gram charges of dust Z, containing 10 pct charcoal, was carried out for various reduction times at 1,050° C to determine the time required before substantial vaporization of lead occurred. The residue of the 4-hour run was fused, but all others were powdery. The results are summarized in table 3. Figure 2 shows the cumulative percent of lead and zinc vaporization with time.
Effect of Pelletization on the Vaporization of the Zinc
When the flue dust was charged to the furnace as powder, fusion took place after a certain period of reduction, and slowed the zinc vaporization. To combat this, pellets of varying sizes were prepared from a mixture of dust Z and 10 pct charcoal. Reasonably hard green pellets were produced by addition of 15 to 17 pct water without any binder. The water content was reduced to less than 5 pct by air-drying overnight. These predried pellets were then oven dried at 125° C for a few hours. The air-drying was necessary to eliminate the cracking or spalling that occurred when they were oven dried directly after pelletizing. The oven dried pellets averaged 20.9 pounds in compression and 12.1 drops from an 18-inch height. The bulk density was more than 3 g/cm³ after oven drying, which is 10 times that of the original dust, even though low-density charcoal was present.
The reduction and vaporization of zinc was carried out at 1,050° C on pellets of three different diameters, averaging 0.2, 0.4, and 0.6 inch. The results are summarized in table 4.
The time required for the reduction and vaporization of the reduced zinc from 500-gram charges decreased with an increase in the pellet size. The reaction time was 35 pct less for the 0.6-inch-diam pellets than for the 0.2-inch-diam pellets.
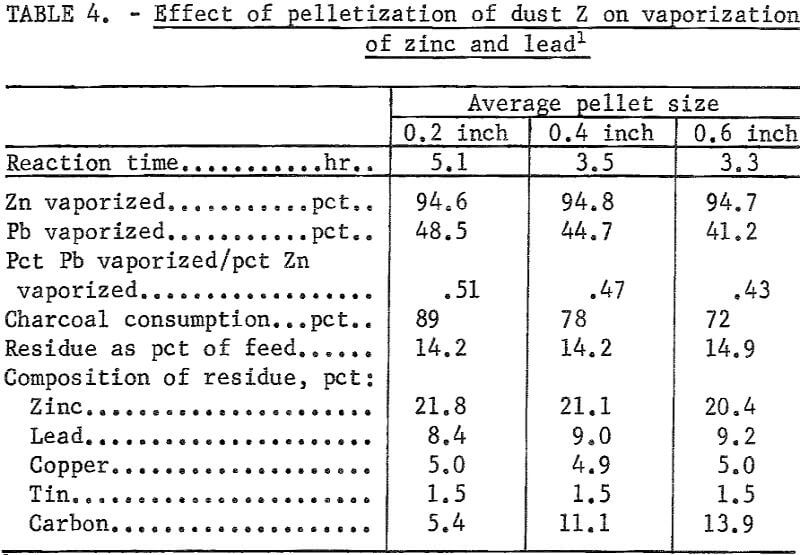
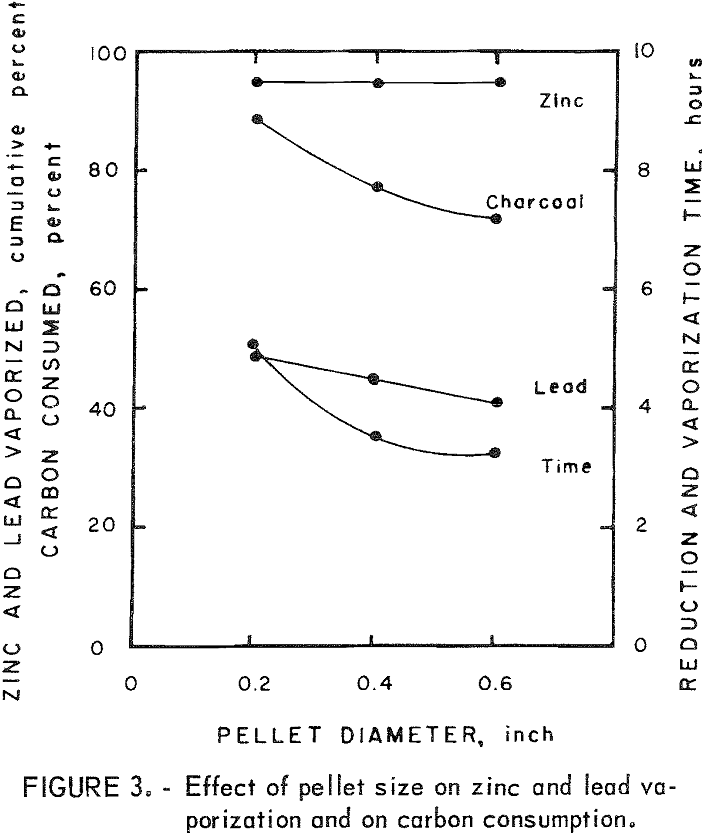
Charcoal consumption decreased with an increase in the pellet size. The residue from the 0.2-inch-diam pellets was completely fused, whereas those from the 0.4- and 0.6-inch-diam pellets retained the pellet shape even though they were partially fused.
Figure 3 shows the relationship between the pellet size and the reduction time for 500-gram charges. It also shows the reduction in charcoal consumption with increase in pellet size.
Effect of Sodium and Chloride Compounds on Vaporization of Lead and Zinc
The quantity of lead vaporized varied from dust to dust, and it was suspected that sodium or chloride compounds might be assisting the vaporization. For this reason, additions of 4 pct NaOH or NaCl were made to dust X, from which very little lead vaporized. In another experiment, water-soluble sodium and chloride compounds were removed from dust Z by leaching with water. These dusts were mixed with 10 pct charcoal and reduced at 1,050° C. Table 5 summarizes the results.
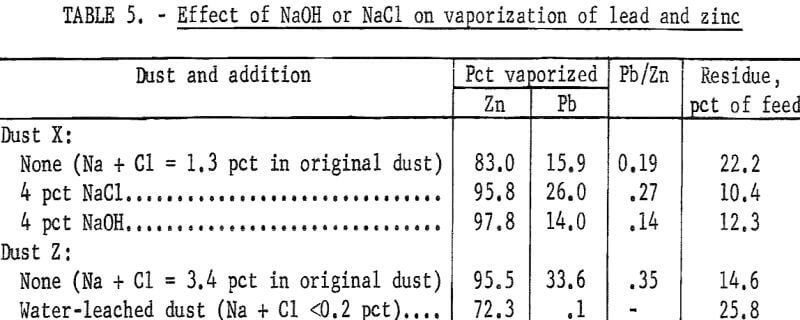
The addition of NaCl increased the lead and zinc vaporization, but the NaOH substantially increased only the zinc vaporization. The removal of sodium and chloride compounds from dust Z lessened both zinc and lead vaporization. One interesting effect of the NaOH addition was that reduction took place at a much lower temperature. Without NaOH the reduction of the dusts usually started above 850° C (as indicated by the combustible offgas), but the reaction was violent at 700° C when NaOH was added.
Discussion of Results
Maier indicates that zinc oxide can be reduced by carbonaceous materials at temperatures as low as 800° C. However, very little reduction of ZnO occurred below 900° C when coke breeze or graphite powder was added. Substantial reduction of ZnO occurred at about 850° C when powdered activated charcoal or wood charcoal was added.
The amount of zinc vaporized at 1,050° C varied from dust to dust. This may be due to various oxides in the dust, particularly those with low melting points such as PbO, SnO, and CuO. Oxides or metals with low melting points and a low vapor pressure are detrimental because they decrease the surface area available for reduction and vaporization of zinc.
When the dust-carbon mixture contained 10 pet charcoal, the zinc vaporization was around 80 pct for dusts X and Y although carbon consumption was low. When 15 pct charcoal was used, the vaporization was above 94 pct for dust X, but no large increase in the vaporization was observed with dust Y. These facts indicated that some of the charcoal became inactive due to poisoning (formation of a stable metal-carbon layer on the surface of the charcoal); however, X-ray diffraction did not reveal any metal carbides in the residue. Low zinc volatility from dust Y would be explained by the formation of a complex zinc sulfide, as indicated by the X-ray pattern, since much sulfur was present. Zinc vaporization from dust Z was above 95 pct for both 10- and 15-pct charcoal additions. This high zinc volatility is thought to be due to catalytic action caused by the large amount of sodium present.
It would appear from figure 3 that the amount of lead vaporized decreases with an increase in pellet size. This result is misleading because a comparison of tables 3 and 4 will show that lead vaporization is primarily dependent on the total time the charge is in the furnace. Since there is a substantial reduction in time required for zinc vaporization as pellet size is increased, there is a concomitant reduction in the amount of lead vaporized. In any commercial application, it would be desirable to process the charge for an optimum (probably near the minimum) length of time, and therefore it might be expected that less of the lead would be recovered with the zinc, and more with the residue.
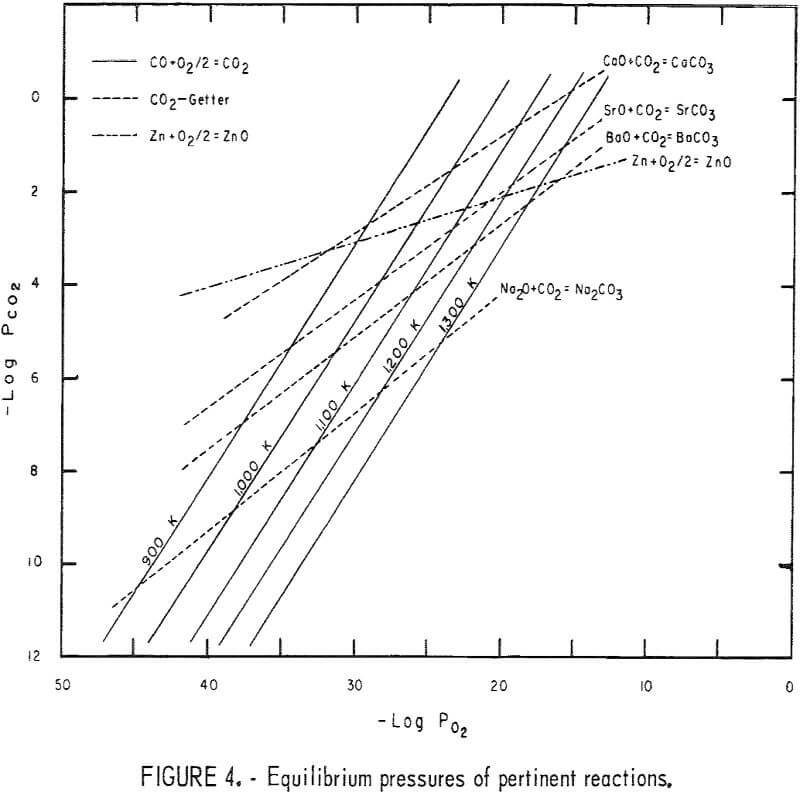
Figure 4 shows some relationships between the partial pressures of CO2 and O2, in atmospheres, for various CO2 getters. The CO2 getter can be any alkali-metal oxide, but, of those shown in figure 4, Na2O is the most effective for the ZnO reduction, and CaO is the least effective. At test temperatures , the equilibrium CO2 pressure between CaO and CaCO3 is higher than that of zinc and ZnO. The series of catalytic reactions involved in the ZnO reduction can be written as follows:
ZnO + CO = Zn + CO2……………………………………………………………(1)
CO2 + Na2O = Na2CO3…………………………………………………………(2)
Na2CO3 + C = Na2O + 2CO……………………………………………………(3)
The Na2O and CO will participate in the ZnO reduction by repeating reactions 1 and 2.
When a small amount of NaOH was added to dust X, the reduction was violent at about 700° C and over 98 pct of the Zn was vaporized, compared with 83 pct without the NaOH addition. A mechanism proposed for this reaction is the formation of Na2O by decomposition of NaOH:
2NaOH + C = Na2O + CO + H2………………………………………………(4)
The occurrence of this reaction was confirmed by offgas combustion at about 400° C. The Na2O will participate in the ZnO reduction when the temperature is sufficiently high, as shown in reactions 1 through 3. It is known that Na2O works effectively as a CO2 getter in the absence of metals or metal oxides that form complex metal-sodium oxides. Therefore, the presence of PbO, FeO, SnO2, or CuO in the dust would complicate the ZnO reduction mechanism.
Some catalytic reaction is thought to result from the presence of NaCl or other alkali chloride compounds in the dust. Addition of NaCl to dust X increased the vaporization of zinc and lead, unlike the addition of NaOH, which increased only the zinc vaporization. This indicated that the sodium from the added NaCl reacted as shown in reactions 1 through 3 after decomposing to NaaO and a metal chloride.
Pellet size appeared to be important for the efficient vaporization of the reduced zinc. Smaller pellets tended to fuse during the reduction process, while the larger pellets retained a roughly spherical shape even though some were partially fused. Retention of the maximum surface would assist the vaporization of zinc from the larger pellets. The amount of charcoal consumed in tests with the larger pellets was lower than with smaller pellets. It is difficult to explain this difference.
It will be useful to potential shippers of brass smelter dust to know that pelletization is simple and readily accomplished, and that there is a resultant tenfold decrease in volume.
Conclusions
Essentially all the zinc and a significant portion of the lead can be vaporized and removed from brass smelter flue dust during an oxygen-free roast at 1,050° C. The dust can be mixed with 10 pet or more carbon in the form of wood charcoal, the reducing agent, and readily pelletized for charging to the furnace. The charcoal contained various impurities, such as CaO and FeO, that acted as catalysts for the ZnO reduction. Furnacing time was 35 pct less for 0.6-inch-diam pellets than for 0.2-inch-diam pellets. The 0.6-inch-diam pellet was optimum from the standpoint of a decrease in both reaction time and charcoal consumption. Potential shippers of brass smelter dust should know that pelletization is a simple process and results in a tenfold decrease in volume of material.
When 4 pct NaOH was added to the flue dust-carbon charge, zinc vaporization was substantially increased. When the same amount of NaCl was added, both zinc and lead vaporization were increased. Lead vaporization decreased when sodium and chlorine compounds were removed by water-leaching. A series of catalytic reactions has been postulated as an explanation of these observations.
It was shown that the volatilization of lead was a function of time, until about 40 pct of the lead in the charge was collected, after which the rate diminished rapidly.
For effective zinc vaporization at atmospheric pressure the following variables must be controlled: reduction temperature, the type and amount of carbonaceous material used as the reducing agent, and pellet size. The information obtained in this work will be useful for the design of a suitable furnace for the treatment of not only brass smelter dusts, but also other furnace dusts and slags containing a substantial amount of zinc oxide.
