Table of Contents
In accordance with its objective to maximize minerals recovery from secondary domestic resources, Bureau of Mines conducted research on recycling chrome refractory wastes. Since 20 pct of the U.S. demand for imported chromite is used in the production of refractories, primarily for the steel, copper, and glass industries, samples of used chrome-containing refractories from copper converter and reverberatory furnaces were investigated to determine their potential for recycling. The samples were beneficiated using a combination of wet magnetic separation, flotation, and leaching techniques, and the concentrates were reformed into briquets for refractory evaluation.
Small refractory test specimens produced from the beneficiated concentrates gave modulus of rupture values at 1,350° C comparable to those of commercial mag-chrome brick of similar composition; however, grinding the waste refractories to at least minus 65-mesh was required in order to liberate the metallic copper. Most of the copper was easily recovered using conventional beneficiation techniques, but the finely ground chrome concentrate would require briquetting, calcining, and crushing to produce a coarse grain suitable for recycling into secondary mag-chrome refractories.
North America has no significant reserves of chromite ore. The existing deposits in the United States are low grade and would only be used if outside sources became unavailable. Approximately 97 pct of the chromite consumed in the United States is imported from the Eastern Hemisphere; the main suppliers are the Soviet Union, Republic of South Africa, the Philippines, and Turkey.
Chromite ore in combination with various quantities of magnesia is the primary constituent in basic refractories. In 1975, about 300,000 tons of chrome refractories were consumed in this country. The refractories are used as linings in steelmaking and copper smelting furnaces, rotary cement calciners and glassmaking tanks. However, most of the consumption is by the copper and steel industries. Approximately 20 pct of the chromite consumed in the United States is for refractory purposes. In 1979, domestic copper furnaces consumed magnesite-chrome (mag-chrome) refractories at an average rate of 7.5 pounds per ton of copper produced. As a result, copper smelters are accumulating significant stockpiles of waste refractories.
As part of the Bureau of Mines objective to maximize minerals recovery from secondary domestic resources and to expand the Nation’s mineral base, the Bureau’s Tuscaloosa Research Center investigated techniques for reclaiming chrome-containing waste refractories from copper smelting furnaces and reforming them into new refractories. Samples taken from copper converter and reverberatory furnaces were obtained from three major copper producers. Analyses were made on each head sample, and studies were conducted to determine grinding required to liberate the metallic copper and copper matte. A flowsheet was developed to optimize the beneficiation of the waste in order to recover the metallic copper and produce a chrome concentrate containing minimum copper impurity. Test briquets were pressed from chrome concentrates containing 0.2 to 1.0 pct Cu and fired to 1,500° and 1,700° C. Modulus of rupture values at 1,350° C were used to evaluate the refractory properties of recylced refractories and compare them with properties of commercial mag-chrome refractories of similar composition. Techniques for the reclamation of waste refractories from steelmaking furnaces are presented elsewhere.
Description of Samples
Three copper smelters in Arizona each supplied 1,000-pound samples of used magnesite-chrome refractories from converter and reverberatory furnaces for batch testing. One of the smelters also provided a 10-ton sample for use during the continuous processing tests.
The as-received samples consisted of whole and partial bricks. Metallic copper was visible in the refractories. Apparently liquid copper penetrated cracks and voids that developed in the brick during the furnace campaign and subsequently solidified into large pieces of metallic copper after furnace shutdown. Copper and copper matte were also visible at the spalled area of the bricks. Removal of the low-melting-point materials to yield a clean refractory grain was the objective of beneficiation studies.
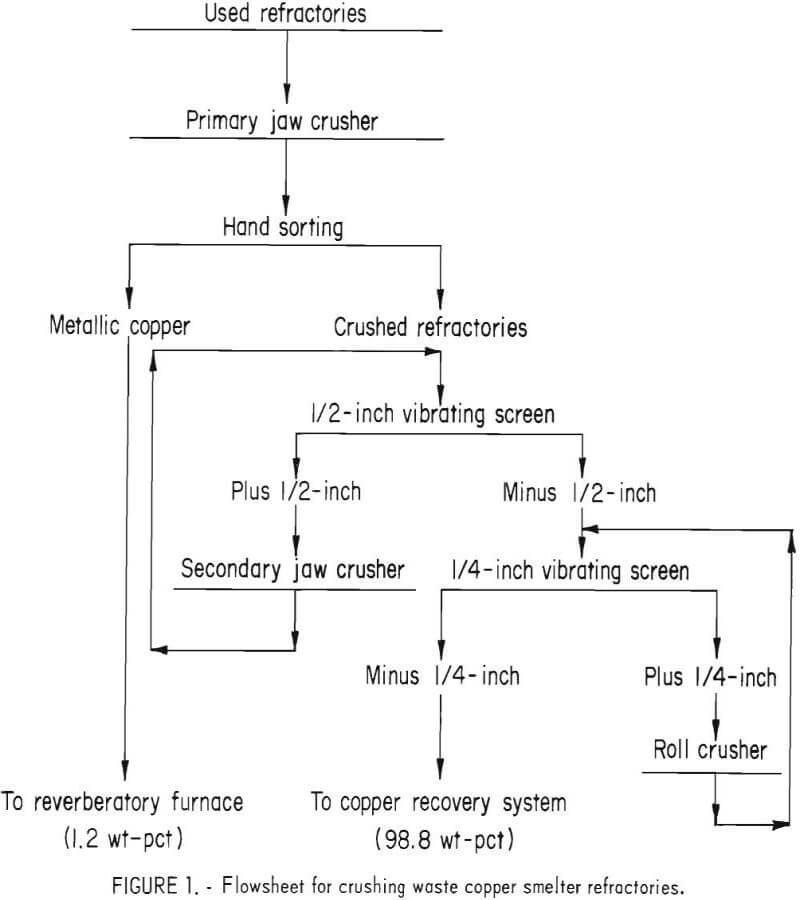
The waste refractories were crushed to pass a ¼-inch screen using the flowsheet in figure 1. The product recovered by handsorting was essentially metallic copper that could be recycled directly to the copper smelting furnace. This product accounted for 0.5, 0.4, and 1.2 pct of the total weight received for samples A, B, and C, respectively.
Representative samples of the minus ¼-inch material were further crushed to pass minus 6-mesh. Representative samples were cut from the minus 6-mesh material for laboratory batch testing, chemical, semiquantitative spectrographic, X-ray diffraction, and screen analyses.
Chemical Analyses
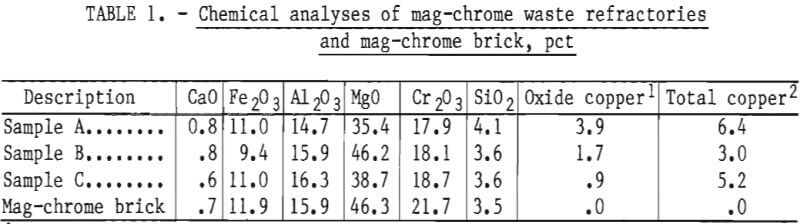
Commercial brick of the same type as those used by the copper smelters were chemically analyzed for comparative purposes and to serve as a standard for beneficiation studies. Chemical analyses of the commercial brick and those of each head sample are presented in table 1.
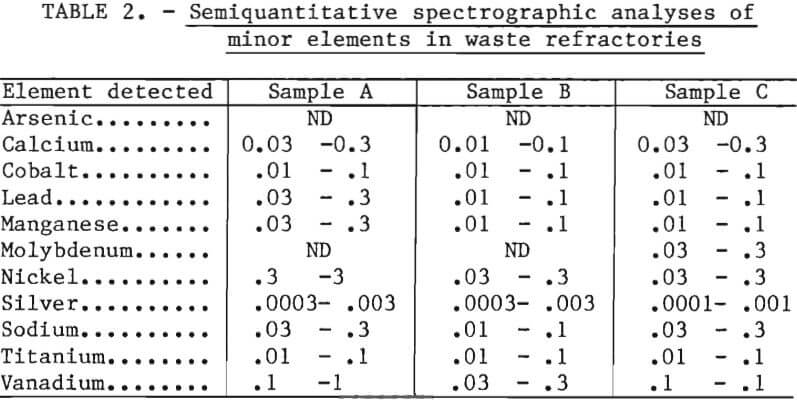
Semiquantitative spectrographic analyses of the waste refractories were made for minor elements to aid in identifying minerals not detected by chemical analyses or X-ray diffraction techniques. The analyses are presented in table 2.
Mineralogical Analyses
X-ray diffraction analyses were made of each sample for mineralogical determinations. The diffraction patterns were also valuable for determining whether significant phase changes had occurred in the refractory grain during furnace operations that might alter the refractory properties of the beneficiated grain.
Chrome spinel (Fe2+, Mg2+)0·(Al3+, Cr3+, Fe3)2O3, periclase (MgO), and minor amounts of forsterite (Mg2SiO4) were identified in all the samples. Traces of cuprite (Cu2O) were also detected in samples A and B.
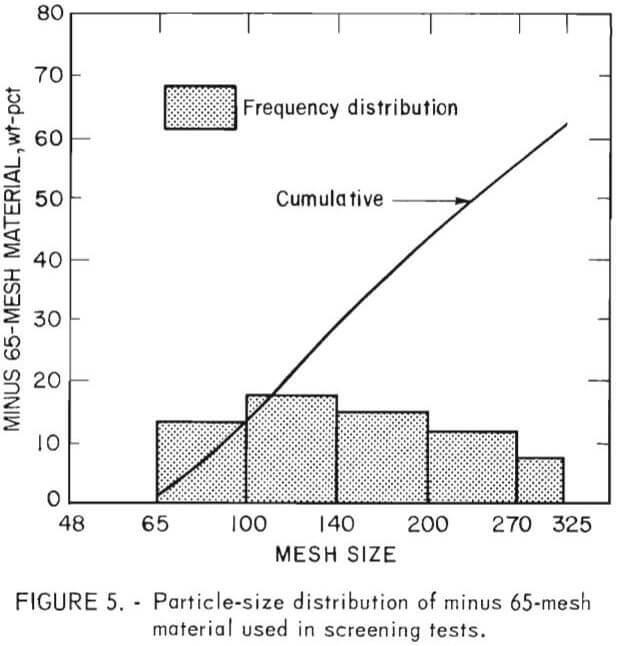
Particle-Size Distribution
The particle-size distribution was determined by screen analyses of minus 6-mesh head samples. Chemical analyses of each size fraction for chromite, oxide copper, and total copper indicated these elements and minerals to be uniformly distributed. From microscopic examinations of the size fractions it was ascertained that most of the contaminants were liberated from the refractory grain between 65 and 100 mesh.
Beneficiation
The presence of metallic copper in the wastes suggested the use of gravity separation processes. However, laboratory batch tabling and spiral concentration studies on minus 6-mesh and minus 65-mesh material showed that gravity techniques alone were not effective for copper removal. The reason for this is a combination of the fine particle size of the copper and the flattening of small particles of copper during crushing and grinding because of its malleability.
Magnetic Separation
The waste refractories contained copper matte, which is comprised predominantly of copper and iron sulfides in solid solution and has a high magnetic susceptibility. Therefore, magnetic separation was used to remove the matte in conjunction with each of the processes evaluated for copper removal.
At high magnetic field intensities, the chromite also reported with the matte in the magnetic fraction. As a result, all magnetic separations were made with a low intensity magnetic field to remove the matte without attracting the chromite. A Davis tube low intensity wet magnetic separator was selected for this purpose.
Flotation
The sulfide copper minerals are readily concentrated by flotation. The flotation of metallic copper from various gangue and waste materials has also been investigated. In most investigations the copper minerals, gangue associations, and reagent selection were the primary factors affecting flotation results.
Several reagent suites were evaluated for floating copper away from the waste refractories. The best results were obtained using sodium isopropyl xanthates as a collector, sodium silicate as a dispersant, and pine oil as a frother. The collector was varied from 0.05 to 0.8 pound per ton of refractory feed. The minimum quantity of collector for maximum copper removal was found to be 0.1 pound per ton. Increases in pine oil and sodium silicate beyond 3.0 and 0.16 pounds per ton, respectively, failed to yield increased copper removals. Thus, 0.1, 0.16, and 3.0 pounds of collector, frother, and dispersant, respectively, were the quantities of reagents per ton of refractory feed for optimum copper removal.
The natural pH of the as-received samples ranged from 9.5 to 10.2 when slurried in distilled water at 50 pct solids. Variations in pH, using lime and sulfuric acid as pH regulators, failed to provide increases in copper removal.
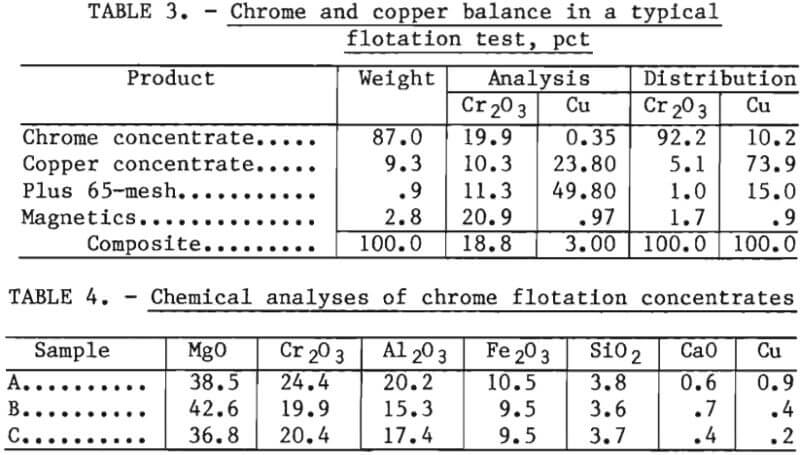
The simplified flowsheet for copper contaminant removal by flotation is shown in figure 2. Typically, the waste refractory was stage ground to pass 65 mesh using a rodmill. A minor amount of persistent plus 65-mesh material was found to be principally metallic copper. The minus 65-mesh material was fed to flotation tests. The copper concentrate had a high copper content and could also be recycled to the copper smelting furnace. The flotation tailings were treated by low intensity wet magnetic separation to remove the matte in a magnetic concentrate. The nonmagnetic product was a chrome concentrate or clean refractory grain. Table 3 presents a material balance for chrome and copper for a typical test. Table 4 presents a complete chemical analysis of the chrome concentrate for each sample studied.
Continuous Flotation
To determine if the flotation scheme could be adopted on a continuous scale, a 250-pound per hour continuous process research unit (CPRU) was assembled. A series of 16-hour continuous tests was made. Flotation reagents were the same as in laboratory batch testing.
Figure 3 presents the flowsheet for the continuous flotation testing. To provide adequate grinding a ballmill was used instead of a rodmill. The mill was operated in closed circuit with a 10-mesh screen to remove coarse copper, a 60-mesh screen separated a liberated feed for flotation, and a shaking table removed the copper from the minus 10- plus 60-mesh fraction. The plus 10-mesh fraction and the table concentrate were of high copper content and were retained as final beneficiation products. The minus 10- plus 60–mesh table tailings were recirculated to the ballmill.
The flotation circuit consisted of a scavenger and rougher float. The same quantity of collector and frother were used in each float, thus doubling their consumption over laboratory usage. The doubled consumption was required to insure maximum copper removal. The scavenger flotation tailings were magnetically separated using a drum-type wet magnetic separator.
The products were sampled at 15-minute time intervals during testing. A composite material balance for copper and chromite for a typical continuous test of 16-hour duration is shown in table 5. Table 6 presents the chemical analysis of the chrome concentrates from five tests.
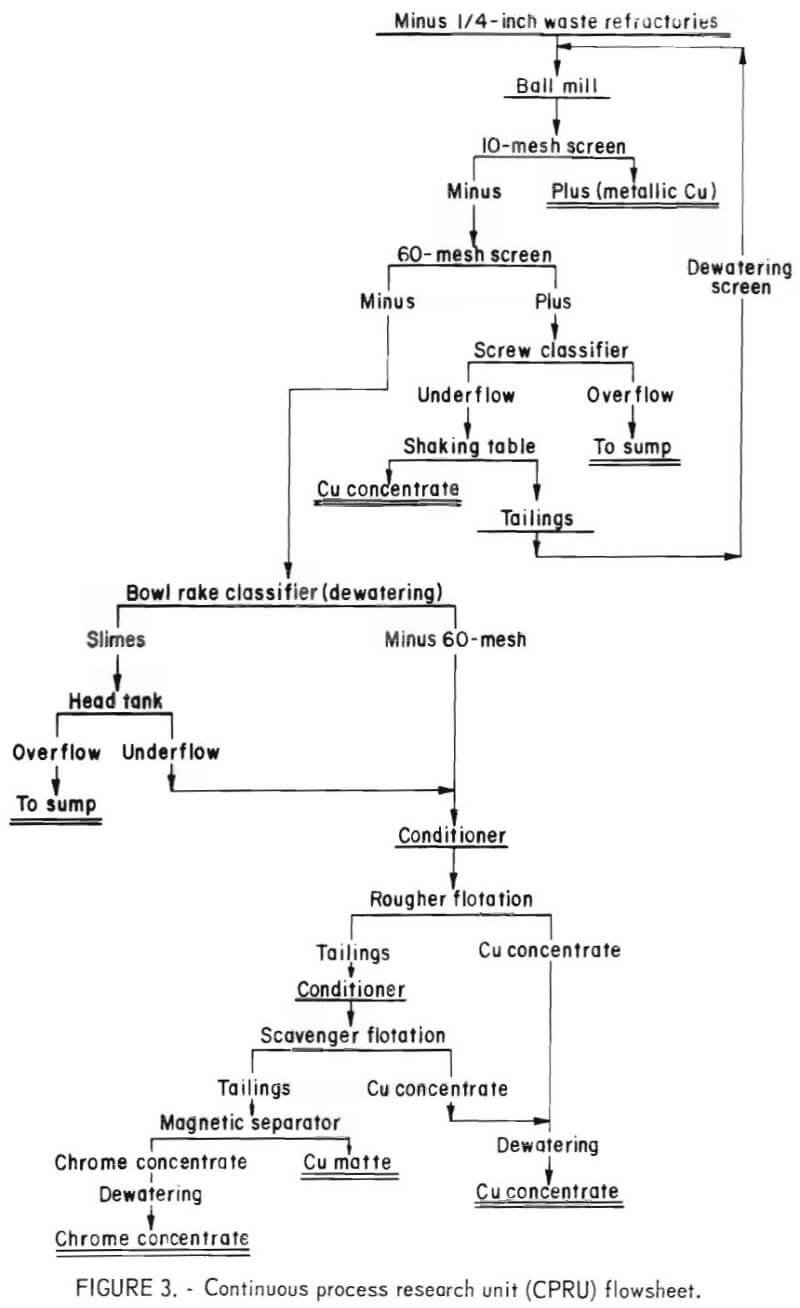
The data contained in tables 4 and 5 show that contaminant removals and chrome recoveries are similar to those obtained in laboratory batch testing. The copper concentrates were of sufficient grade to be recycled to the copper smelting furnaces. The chrome concentrates from tests 1 through 3 of table 6 were obtained using the circuit presented in figure 3, while those of tests 4 and 5 were obtained without the use of a scavenger float or the 10-mesh screen.
Leaching
The fine-grained nature of the copper in the waste refractories required grinding to pass 65 mesh to achieve liberation for physical beneficiation. Since refractory producers prefer to have raw materials that contain particles up to 6 mesh in size, a series of ammonia-ammonium carbonate leach tests were conducted on the minus 6-mesh waste refractories as an alternative to the flowsheet in figures 2 and 3. Prior to leaching, the magnetic fraction was separated by low-intensity wet magnetic separator and retained as a final product. The nonmagnetic fraction was agitation leached and was retained as the chrome concentrate.
Ammonia-ammonium carbonate leaching of copper bearing materials has been thoroughly investigated. The kinetics and mechanism of copper dissolution were studied in each investigation. Technology for recovering copper from pregnant ammonia-ammonium carbonate solutions is common practice throughout the copper industry.
The effects of leaching variables were determined in a series of leach tests at constant aeration rates. The ratio of ammonia to copper and ammonia to carbon dioxide was varied at several levels, and the leaching times were 6, 12, 18, and 24 hours. An analysis of variance of the data indicated that the leaching time was the most significant variable, followed by the ammonia to copper ratio, and the ammonia to carbon dioxide ratio. The analysis also indicated that there was no significant interaction between the variables. Figure 4 presents the copper recovery versus the ammonia to copper ratio at three ammonia to carbon dioxide ratios for 24-hour leaching tests.
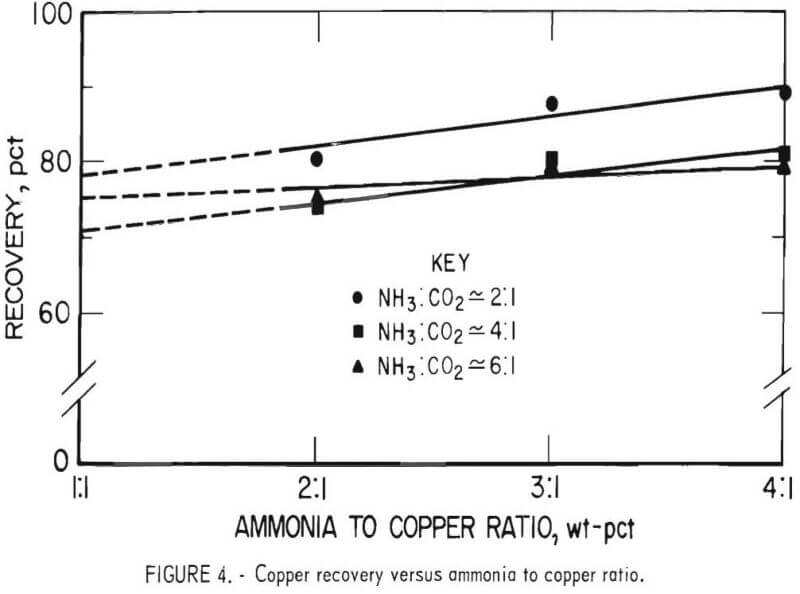
The leached solids or chrome concentrates graded as low as 0.5 pct Cu at an ammonia to copper ratio of 4 to 1 and ammonia to carbon dioxide ratio of 2 to 1 after leaching for 24 hours. Data showed that increases in leaching time and the ammonia to copper ratio resulted in increased copper removals, while increases in the ammonia to carbon dioxide ratio, beyond 2 to 1, showed a decrease in copper removal. Table 7 presents a material balance for a 24-hour test made at an ammonia to copper ratio of 7 to 1 and an ammonia to carbon dioxide ratio of 2 to 1. The test period was 24 hours.
Refractory Studies
Preparation of Samples
The beneficiated chrome concentrates from the copper furnace linings were mixed with 3 pct polyethylene glycol and pressed into 1- by 2- by ¼-inch thick briquets at 20,000 psi and sintered at 1,500° and 1,700° C. The briquets were then sectioned into ¼- by ¼- by 2-inch-long bars for MOR, density and porosity determinations. Chemical, X-ray diffraction, scanning electron microscope, and energy dispersive X-ray analyses were also used to study changes in mineralogy and intergrain bonding.
Commercial refractory bricks of identical or similar chemical composition were obtained from several manufacturers and crushed to the same particle size distribution as the beneficiated material; then, following the procedure described previously, samples were made and used as standards for comparison.
The effect of particle size distribution of the chrome concentrates on the refractory properties of sintered samples was demonstrated early in the research. In order to eliminate this parameter from the results, all samples, both from the beneficiated and “standard” materials, were carefully stage-ground by hand to yield the distribution shown in figure 5. It should be noted that this minus 65-mesh distribution is not typical of the distribution used in the production of full-size refractory brick, but it was required because of the small sample size used in this preliminary evaluation.
Chrome Concentrates From Copper Smelter Linings
Since no information could be found in the literature on the effect of copper impurities on the hot strength of mag-chrome refractories, CuCO2:Cu(OH)2 was added to crushed and sized “commercial standard” brick to produce a series of samples with copper contents ranging from 0.05 to 1.0 wt-pct. While copper additions had no effect on MOR values at 25° C, a dramatic effect was noted for samples fired at 1,500 C and tested at 1,350° C.
Figure 6 shows the MOR values. Adding as little at 0.05 wt-pct Cu substantially reduced the MOR for test bars produced from minus 65-mesh material, and the addition of only 0.4 wt-pct Cu reduced the MOR to 20 pct of the value for uncontaminated samples. Modulus of rupture values at 1,350° C on similar samples fired 200° C higher to 1,700° C were unaffected by copper additions up to 1.0 wt-pct. The fact that high-temperature (1,350° C) MOR values for samples fired to 1,700° C were not affected by copper additions, while high-temperature MOR values for samples fired to 1,500° C were significantly affected by even small copper additions, indicated that the copper affected the glassy silicate bond present between refractory periclase-chrome spinel grains. The copper acts as a flux in the glassy phase, lowering the softening point or refractoriness of the bond phase. When samples are fired to a higher temperature, the glassy bond is replaced by a direct grain-to-grain periclase-chrome spinel bond with some forsterite present at the grain boundaries, as shown in figure 7.
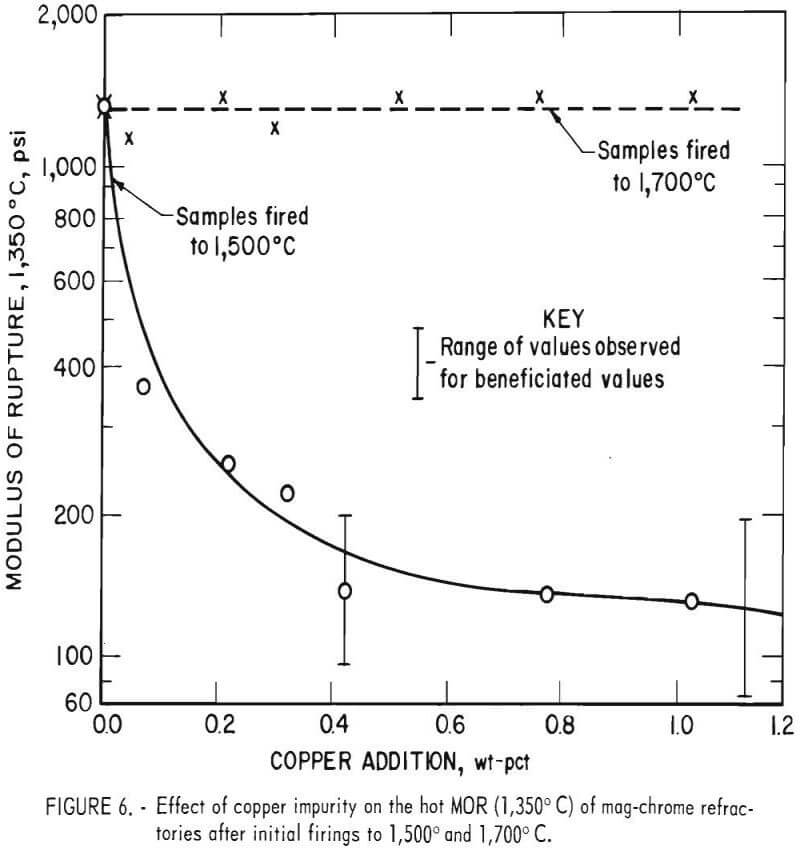
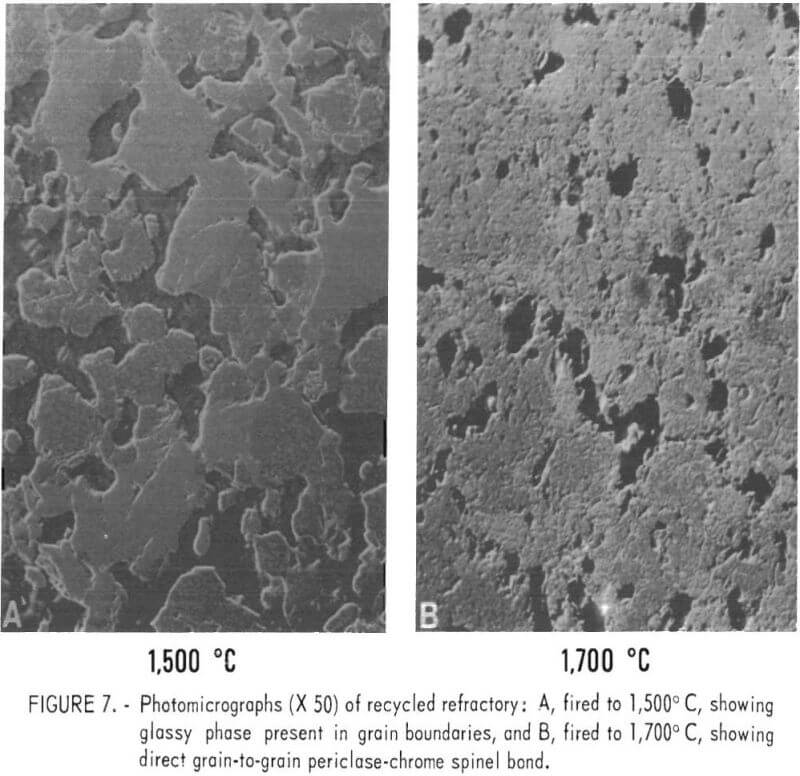
Table 8 shows the 1,350° C MOR, bulk density, and apparent porosity values for two chrome concentrates from magnetic separation and froth flotation and one from leaching, along with samples prepared from two commercial bricks. The copper content in the leached sample was reduced to a very low level (<0.2 pct), but no corresponding improvement resulted in the refractory properties. As shown, MOR values for samples fired to 1,500° C are low; however, when the samples were fired to 1,700° C, their MOR values were higher than those of the standard samples produced from commercial bricks.
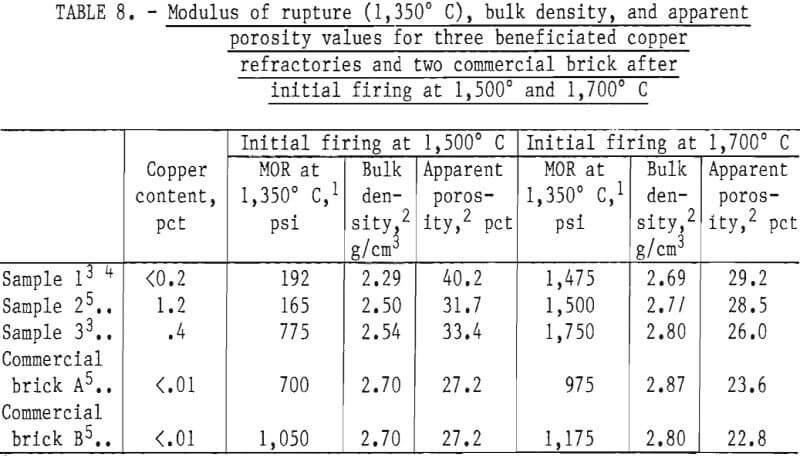
As previously stated, in order to liberate the majority of copper and allow removal by magnetic separation and froth flotation, it was necessary to grind the waste copper refractory linings to minus 65-mesh, which creates an additional problem in the recycling of the material. Preliminary sintering of the concentrate would be required to obtain the particle-size distribution needed for refractory brick fabrication. Beneficiation by leaching offers the only feasible alternative for lowering the copper content to acceptable levels in a coarser material. This may increase the cost of beneficiation, and any advantages would have to be weighed against the cost of prefiring the minus 65-mesh material to form a refractory grain.
Environmental Considerations
As part of this study, investigations were made to determine minerals and/or elemental associations in the refractory wastes that might present environmental problems in recycling efforts. Identification of such associations were made by semiquantitative spectrographic, X-ray, and energy dispersive X-ray analyses; none of these methods detected minerals or elements of the nature that might prove environmentally toxic. Quantitative analyses were made on the samples of waste refractories by a commercial research laboratory and the analytical sections of several Bureau of Mines Research Centers. Each of these facilities reported the presence of minute quantities of arsenic and water-soluble chromium (Cr+6). The data are summarized in table 9.
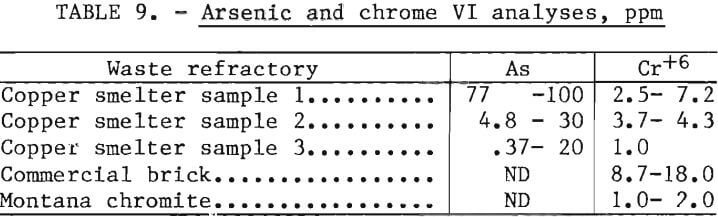
During the pilot plant studies the discharge waters were analyzed for arsenic and chromium to ascertain the extent these elements were being leached from the used refractory. Analyses of these discharge waters were made on seven runs over a 5-week period, as shown in table 10. These values are surprisingly low and are well below the drinking water standards of 0.05 mg/l (ppm) for these two elements. These elements are apparently precipitated as insoluble compounds by reaction with flotation reagents or with metallic elements in the machinery.
During the crushing of a 10-ton sample of waste refractories, officials from the U.S. Department of Labor, Mining Safety and Health Administration (MSHA), monitored dust level and employee exposure to arsenic and chromium by sampling the crushing area. The time-weighted average exposures ranged from not detected to 0.00015 mg/m³ for arsenic and from 0.0004 to 0.02 mg/m³ for chromium, which was well below the Threshold Limit Value (TLV) of 0.5 mg/m³ for each of these elements.
Recycling Waste Refractories in Copper Smelting Furnaces
Copper recovery from waste smelter refractories was readily achieved by standard physical beneficiation techniques. Since grinding to 65 mesh is required to liberate the metallic copper, the beneficiated refractory material will require briquetting, calcining, and crushing to prepare a coarse refractory grain for reuse in mag-chrome refractories. Thus, the recyling of the chromite concentrate from waste copper smelter refractories is at an economic disadvantage with respect to chromite concentrate obtained directly from ore.
The alternative processing method, consisting of magnetic separation and ammonia leaching, removed over 90 pct of the copper from the used refractory after it had been crushed to minus 6-mesh. The chrome concentrate contained 0.4 pct Cu. However, the economics of this process is not very promising, either.
The presence of copper impurities as low as 0.05 wt-pct can dramatically reduce the hot-strength of mag-chrome refractories containing a glassy bond phase. It was determined that firing these refractories to 1,700° C, which eliminates the glassy phase and produces a direct periclase-chrome spinel bond, allows copper impurities of up to 1.0 wt-pct not to affect the high- temperature properties.