Table of Contents
- Extraction of Mineral, Metal, and Energy Value from Urban Refuse
- Municipal Incinerator Residues
- Unburned Municipal Refuse
- Reclamation and Reuse of Waste Glass
- Refining of Metals from Refuse
- Utilization of Discarded Plastics
- Pyrolysis of Solid Wastes
- Conversion of Organic Refuse to Oil
- Upgrading and Utilization of Automotive and Related Ferrous Scrap
- Junk Car Incinerator
- Increased Use of Ferrous Scrap in Electric Furnace Steelmaking
- Production of Foundry Pig Iron From Junk Car and Other Ferrous Scrap
- Destructive Oxidation of Ferrous Scrap
- Utilization of Ferrous Scrap as a Reductant
- Ferrous-Scrap-Operation Surveys
- Chemical Stabilization
- Recovery and Reuse of Values From Industrial Wastes
- Precious Metal Recovery From Scrap
- Recovery of Sulfur Dioxide From Smelter Gases
- Current and Recently Completed Solid Waste Research Grants and Contracts
The Interior Department’s Bureau of Mines has always considered the waste products and scrap generated by the mineral and metals industries and the consuming public as potential resources, The Bureau has been active in reclaiming values from byproducts of mineral, metal, and energy processes for over 50 years. This statement is supported by the continuity of publications by Bureau authors on environmental subjects dating back to 1912. The Bureau has pioneered the fields of secondary metals recovery and solid waste reclamation and has laid valuable groundwork in several major solid waste areas, including urban refuse; junk cars; mine, mill and smelter wastes; and industrial solid and liquid wastes. The authority and responsibility for conducting research on separation recovery, and recycling of such products, however generated, is inherent in the Organic Act of May 16, 1910, as amended in 1913 and 1915, which established the Bureau of Mines.
Almost from the date of its founding, the Bureau has been involved in preservation of the environment and conservation of natural resources by recycling. Its activities have encompassed not only solid wastes but also means of alleviating air and water pollution, especially those which originate from industries based on the utilization of minerals, metals, and fossil fuels. Pollution caused by gaseous, liquid, and solid wastes continues to be of major concern to the Bureau. The fact that air and water pollution are excluded from the scope of this report necessitates the elimination from the appended bibliography of the many Bureau publications on these subjects.
Although this summary is devoted to solid wastes, brief reference is made to related Bureau accomplishments that cannot realistically be separated from the solid waste aspect. For example, the acid-mine-drainage problem has occupied a prominent place in the Bureau’s work since 1920. Inasmuch as the product of neutralizing acid mine waters is a solid waste sludge, its direct relationship to the Bureau’s authority for conserving and developing mineral resources is evident.
The case is equally definite for air pollution caused by emissions of sulfur oxides from metallurgical operations and burning of fossil fuels. Means of alleviating such problems were sought by Frederick G. Cottrell, associated with the Bureau of Mines from 1911 to 1921, and Director. 1920 to 1921. More than any other individual, Dr. Cottrell succeeded in finding means for abating air pollution. He invented and developed the electrostatic precipitator which bears his name, now used for almost five decades throughout the world to remove acid fume and particulate matter from stack gases. The Bureau has continued such work uninterruptedly and is presently conducting research studies on removal of sulfur oxides from stack gases with subsequent recovery of elemental sulfur. Other means for abatement of noxious emissions from many types of industrial operations are also in an advanced developmental stage.
One of the more important functions of the Bureau is to conduct programs of inquiry and research that will stimulate the production of an adequate supply of metals and minerals to meet our national needs. The Bureau’s authority and responsibility in this area was recently renewed, strengthened, and explicitly restated in the recently passed Mining and Minerals Policy Act of 1970 (P.L. 91-631). The Congress recognized the Bureau’s special competence and responsibility in minerals research and supply by assigning a major role to the Department of the interior in the Solid Waste Disposal Act of 1965 (P.L. 89-272), and more recently in the Resource Recovery Act of 1970 (P.L. 91-512).
The Bureau’s Metallurgy Research organization is well equipped to deal with problems relating to metal- and mineral-waste products. Included are the Divisions of Metallurgy and Solid Wastes, which is made up of about 780 full-time employees at eight research facilities strategically located across the country. About half of the personnel are metallurgists, mineral engineers, geologists, chemical engineers, chemists, and physicists. Other members of the Bureau’s staff are engaged in coal, petroleum, and mining research and have capabilities to deal with solid waste problems relating to energy recovery, utilization, and disposal of mining wastes.
With passage of the Solid Waste Disposal Act, the Bureau accelerated and expanded its research efforts in separating, recovering, and recycling the values contained in a variety of metal-, mineral-, and energy-laden wastes. Carrying out this program required extensive retraining of a group of its engineers and scientists to cope with the wide range of problems, methods, and materials that characterize the secondary materials industry, but which have traditionally escaped consideration in the curricula of colleges and universities. The approach taken by the Bureau to solve these problems has always been that of improving and expanding the technology of extractive metallurgy and in modifying known techniques to the solution of difficult secondary materials problems. The success of this approach is evident in achievements during 50 years of secondary metals research as detailed in hundreds of publications, patents, and presentations resulting from these studies. Documentation is provided in the bibliography.
Rather than consider metal and mineral wastes as environmental problems, as many do, the Bureau of Mines views them as opportunities to bolster our supply of natural resources. These opportunities exist not only in the processing and recycling of proposed new products but also in processing the present generation of scrap more effectively. Since the scrap materials of tomorrow are being manufactured as durable goods today, we are afforded a real opportunity to examine them for potential future benefits. These materials are accumulating as goods-in-use at a rapid rate and collectively are reaching enormous proportions. We must be prepared to recover them efficiently and completely.
The importance of secondary metals which represent our only growing metal resource can best be assessed by comparing the gross production of the major metals with the quantities reclaimed from secondary sources. According to production estimates, on an annual basis, over 50 percent of lead, 40 percent of copper, +5 percent of iron and steel, and 25 percent of zinc and aluminum fabricated into new products last year were derived from secondary sources. Equally important are the estimated quantities of these metals accumulating in the “in-use” channels of the economy. About 40 million tons of copper and 4 million tons of lead and zinc are presently in this category, which represents a constantly growing “man-made mine” of future raw materials.
Since the mineral resources, once mined, are irreplaceable, it is apparent that a primary solution to the problem of scarcity is the development of methods for recovering, recycling, and reusing the values contained in wastes. To some extent, the secondary metal industries have traditionally served this purpose and have done a remarkable job, but the concept must be more widely applied. It is probable that present mine tailings dumps, junk-car graveyards, and possibly even municipal landfills may be looked upon in the future as man- made mines for minerals and metals whose natural ores have been depleted or remain in deposits that can be mined only at greater cost than required for recycling of wastes.
The Bureau of Mines considers solid wastes as resources out of place we are simply trying to put them back.
In-House Research Program
Extraction of Mineral, Metal, and Energy Value from Urban Refuse
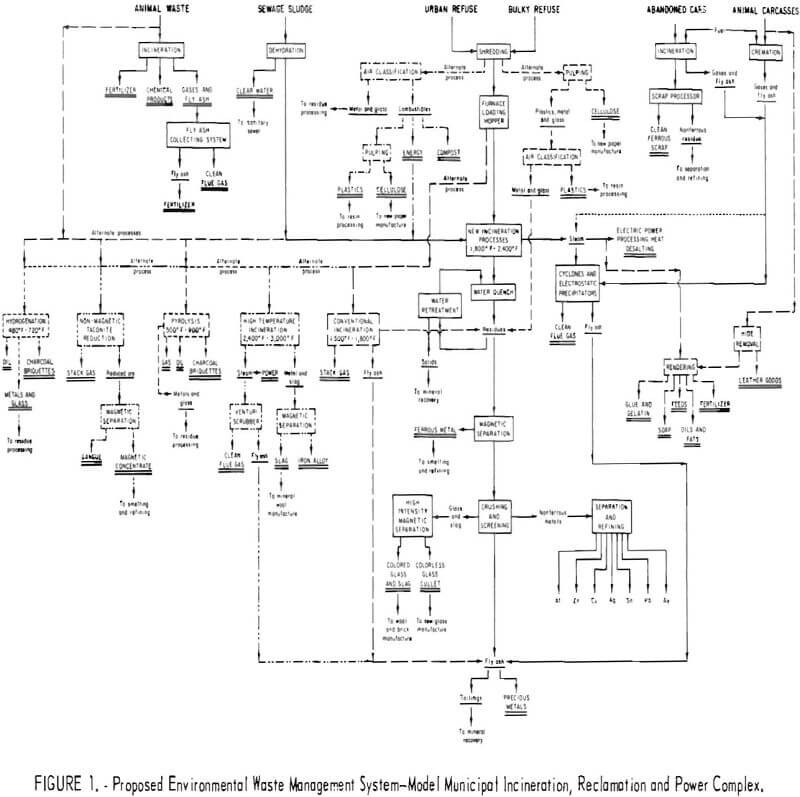
The Bureau is directing a major research effort toward developing methods to recover metal, mineral, and energy values from municipal incinerator residues and raw, unburned refuse. Specifically, the program seeks to (1) develop and demonstrate new or improved techniques to State and local governments and private industry for the recovery and recycling of values contained in urban refuse; (2) reduce environmental pollution resulting from inadequate treatment and disposal of a major solid waste; (3) develop technology and provide training in solutions to problems involving the use and reuse of urban solid wastes; and (4) alleviate the acute problem of urban solid waste disposal. A conceptual systems approach for managing all urban wastes Is presented in figure 1. The principal components of the urban-refuse research program are the following.
Municipal Incinerator Residues
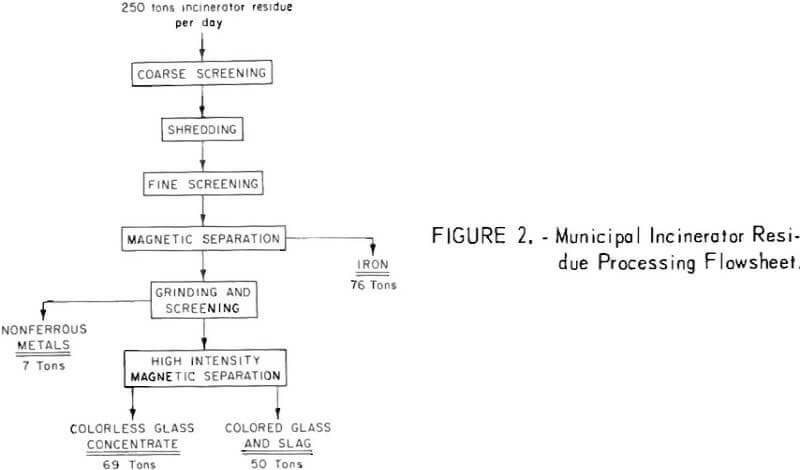
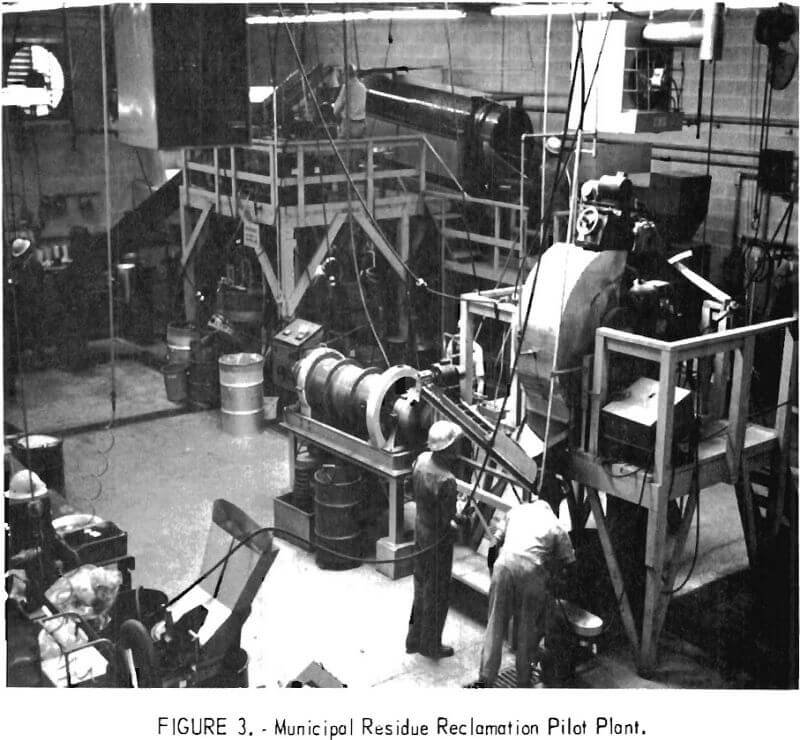
A pilot plant with a capacity of one-half ton per hour, which treats municipal incinerator residues in much the same manner that minerals are separated from their ores, has been operated successfully by the Bureau for over a year (figs. 2-3). The Inputs to the plant, consisting of a heterogenous mixture of tin cans, iron and steel products, aluminum, copper, lead, zinc, glass, and ash, are automatically separated and recovered on a continuous basis. A sophisticated component of the plant equipment separates the glass into clear and colored fractions. The process, in addition to being technically sound, shows favorable economics.
Unburned Municipal Refuse
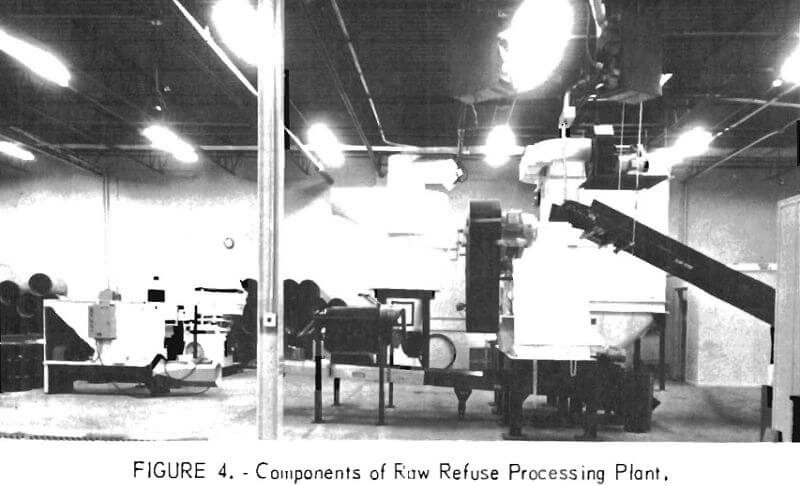
Also, under development is a process for mechanically separating and recovering the metals, glass, paper, and plastics from raw, unburned refuse on a continuous basis. The initial step in this process is the liberation of all of the materials in the refuse by coarse shredding in a mill with double opposed chains. Various combinations of air classification, magnetic separation, screening, optical sorting, and gravity concentration separate and reclaim the individual values. The major equipment required for pilot plant operation has been purchased, and installation is nearing completion (fig. 4).
Reclamation and Reuse of Waste Glass
Glass, which is the major constituent of incinerator residues and one of the major components of refuse, is being given special attention. Methods to obtain clean separations of clear and colored glass are nearly perfected. The fine-size colorless glass concentrates obtained from the pilot plant are already of a quality acceptable as cullet for making new glass containers by the industry. Research, has also been conducted on making such products as bricks, glass wool, reflecting beads, and tile from the waste glass fractions. Attractive colored bricks have been produced that meet ASTM (American Society for Testing and Materials) specifications for “severe weather” facing brick, and high-quality glass wool has also been produced from the waste glass product (figs. 5-6). An optical electronic sorter, which is not yet part of the


flowsheet, has been successfully employed in batch tests to preconcentrate the colorless glass.
Refining of Metals from Refuse
Emphasis is being placed on refining and utilizing the ferrous and non- ferrous values recovered from incinerator residues and raw refuse (fig. 7). Conversion of the metallic fractions of refuse into marketable products is another of the prime objectives of the urban refuse research.
Several methods developed by the Bureau for refining the iron fractions that contain small amounts of copper and tin have shown considerable promise. Preliminary tests indicate that an excellent separation of high-quality aluminum product from the mixed nonferrous metals can be made by using heavy media techniques. The sink fraction of the separation is a copper-rich product containing zinc, lead, tin, and small amounts of stainless steel. The lighter aluminum is collected in the float fraction. Other techniques including vacuum refining, intermetallic compound formation, and flux refining are also being applied to the separation of the complex nonferrous metal fraction of the residues.

Other utilization research is focused on developing uses for the fines and ash products. Grants have been awarded to the Universities of Missouri, Stanford, Alabama, and Wisconsin to seek ways of utilizing these and other values contained in refuse.
Utilization of Discarded Plastics
The Bureau is investigating methods to utilize the vast tonnages of plastics discarded annually. Preliminary tests have shown that polyethylene, polystyrene, polyvinyl chloride, and polypropylene, which represent over 95 percent of waste plastics, can be separated and reshaped by application of heat and pressure (fig. 8). Characteristics of the reshaped plastics are comparable to those made of virgin materials. It has also been discovered that polyvinyl chloride can be broken down into anhydrous hydrogen chloride by heating at 300 C (fig. 8). Half the weight of this polymer is made up of hydrogen chloride gas which is released on destructive distillation, yielding a residual char suitable for use as a fuel.
Pyrolysis of Solid Wastes
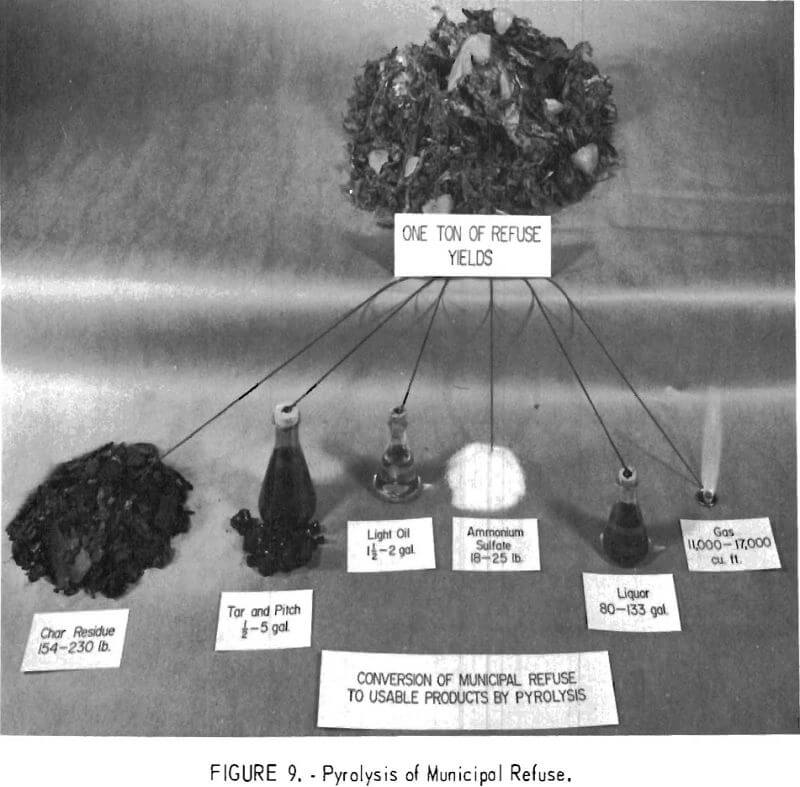
The Bureau is applying the process of pyrolysis, the destructive distillation of materials in the absence of air, to the disposal of a variety of waste products including refuse. This process involves the thermal conversion of materials into usable forms of char, oil, and gas. It can be applied to a variety of waste materials such as municipal refuse, wood bark, sawdust, scrap tires, mulch, and plastics (fig. 9). The process generates enough byproduct fuel gas to supply the required heat for the reaction. The Bureau’s work on pyrolysis originated more than 50 years ago when it was applied to the destructive distillation of coal.
Conversion of Organic Refuse to Oil
A novel process for converting the organic material contained in refuse (garbage and cellulose) into crude oil is also being investigated (fig. 10). The process involves the reaction of carbon monoxide and steam at 500° F and at 1,500 psi. The technique can also be applied to sewage sludge, manures, and wood byproducts. One ton of dry refuse, minus the glass and metal content, will produce 2 barrels of oil with a sulfur content of only 0.1 percent. Even higher yields are recoverable from manures. The Bureau is presently operating a continuous laboratory process for treating waste organic materials by this method.
Upgrading and Utilization of Automotive and Related Ferrous Scrap
The Bureau of Mines has been conducting research on problems relating to ferrous scrap for many years. The primary objective is to devise economic methods to recover and upgrade the values contained in junk cars and related ferrous scrap in order to increase the utilization of this commodity and maintain traditional markets.
The total inventory of junk cars in the United States has been estimated at about 15 million cars, and is still growing, so that a substantial loss of

valuable metals results. Even when a car enters the normal scrap cycle, much of its aluminum, copper, and zinc content is not recovered, but is discarded in dumps along with nonmetallic components of the vehicle.
The Bureau is conducting an in-house research program directed toward expanding the market for junk auto and large-appliance scrap, as well as the 7 million tons of tin cans generated annually, by improving the preparation of these scrap materials for conventional markets, and increasing the recovery of nonferrous metals associated with such scrap. Research efforts are underway on (1) an improved junk car and railroad car incinerator; (2) increased use of ferrous scrap in electric furnace steelmaking; (3) production of synthetic pig iron from ferrous scrap; (4) removal of contaminants from molten ferrous scrap; (5) development of an air separator for junk car shredder residue; (6) recovery of copper from copper-containing ferrous scrap by cryogenic techniques; (7) recovery of copper from starters, generators, and alternators by a molten salt method; and (8) high-temperature, oxidation-reduction studies aimed at producing iron oxide and clean scrap products that can be used as sources of iron in the steel industry. Current Bureau research in this area is as follows.

Junk Car Incinerator
The Bureau has developed and has been successfully operating for over a year a prototype low-cost (estimated at $22,000), smokeless, junk car incinerator (fig. 11). The facility was constructed in cooperation with a scrap processor in Utah and operated as part of the scrap yard equipment. Over 20,000 scrap cars have been processed in the facility without adverse air pollution effects. Work is continuing on the unit to (1) determine amounts of non-ferrous metals melted during incineration, (2) increase the capacity of the incinerator, and (3) lower operating costs. Burning of old railroad cars, trucks, and buses prior to final processing is another area in which the Bureau-designed incinerator has potential and on which research is underway. The prototype incinerator is capable of burning four cars every 20 minutes with a particulate emission discharge of less than 0.01 grain per cubic foot. The cost of burning, including fuel for the afterburner, has been reduced to less than $2 per car.

Increased Use of Ferrous Scrap in Electric Furnace Steelmaking
The electric-furnace steelmaking industry is one of the major consumer of purchased scrap. However, growing competition from pelletized iron ore, which can also be fed to these furnaces, may drastically reduce the auto-scrap market for such purposes because prereduced iron pellets can be charged continuously to furnaces whereas much of the present scrap cannot. This fact gives the pellets an advantage over scrap even when prices are equal. Bureau engineers believe that properly prepared auto and large appliance scrap, as well as tin cans, can also be charged continuously to electric furnaces, and that a mixture of scrap and pellets would be a suitable charge in most cases. Current research on this project has emphasized (1) the effects of continuous charging of ferrous materials at low and elevated temperatures to a conventional, tilt-able, open-arc furnace; (2) the technical feasibility of continuous steelmaking in a stationary open-arc furnace; and (3) process evaluation for production of secondary pig iron from fragmented auto hulks in a submerged-arc furnace. Preliminary tests in which prereduced iron ore in various forms and shredded autos were added continuously at a controlled rate to a bath of molten auto scrap showed that, compared with conventional batch practice, the duration of heats and energy consumption can be substantially lowered and tramp metal content can be reduced to levels acceptable for most steel products. Further improvement in the efficiency of electric furnace steelmaking appears practical by utilizing the sensible heat in furnace offgases during continuous charging to preheat feed material.
Production of Foundry Pig Iron From Junk Car and Other Ferrous Scrap
The objective of these studies is to determine the optimum charges and processing variables required to make specification-grade foundry iron from auto scrap and subcommercial scrap iron at commercially attractive costs. This research is being conducted in a modified size “0” hot-blast cupola having an 18-inch inside diameter (fig. 12). The cupola can accommodate 330 pounds of scrap, together with coke and fluxes, to produce approximately 300 pounds of metal. Preliminary tests have shown that the copper content of metal produced in the cupola, using prepared scrap, meets the specifications for gray iron but not for malleable iron. The tests also indicated that specifications for sulfur content for gray iron can be achieved.
Destructive Oxidation of Ferrous Scrap
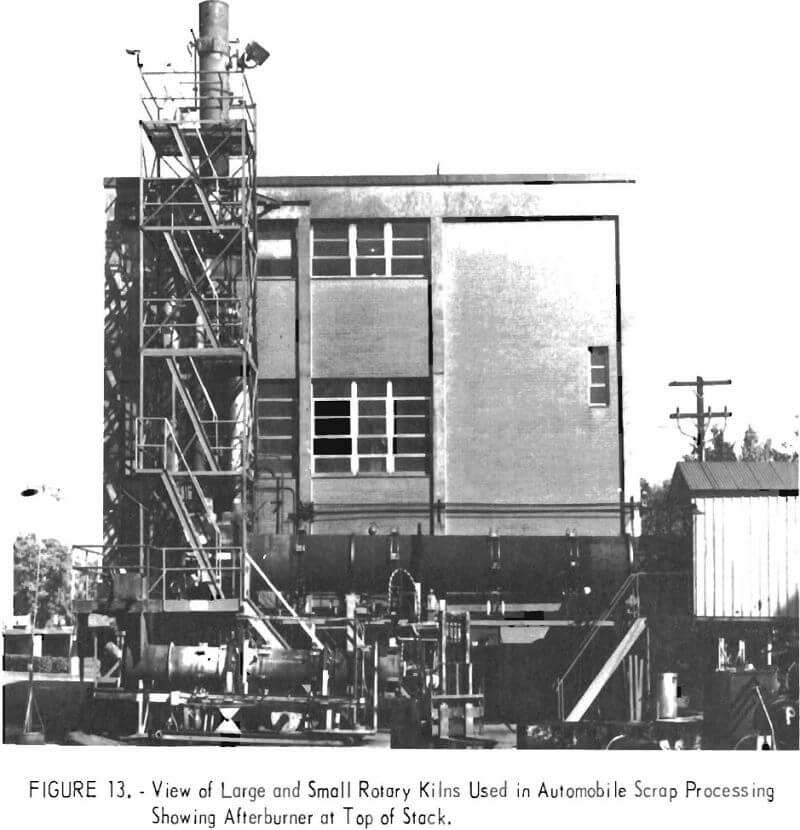
Work is progressing on a process for converting junk-car and other low-grade scrap in a rotary kiln at 1,000° C (fig. 13) to yield coproducts of high-grade iron oxide and dense residual steel scrap of high purity. Destructive oxidation need not be limited to treatment of automobile bulks but can accommodate other low-value ferrous scrap such as refrigerators, stoves, tin cans, turnings, and borings. The light-gage material yields only one kiln product consisting of high-grade iron oxide. Heavier ferrous scrap charges are 70 percent oxidized in one pass through the kiln and can be either removed as a usable product or recycled through the kiln. Recovery of the heavier members of automotive scrap from the kiln roast product not only provides feed suitable as heavy melting stock for electric furnace or foundry use, but also gives a material that is substantially more valuable than the original auto scrap.

Utilization of Ferrous Scrap as a Reductant
The Bureau has done considerable work on the utilization of ferrous scrap as a reductant for converting nonmagnetic taconite into a product that can be separated from associated gangue minerals. In the process, the mixture of ore and scrap iron is heated to 1,000° C in a rotary kiln (fig. 13). The scrap is oxidized to iron oxide and the nonmagnetic ore feed is reduced to the magnetic form. Past experience has shown that the best scrap charge is thin-gage auto scrap, tin cans, borings, and turnings. The thinner gage scrap is oxidized more rapidly in the process. Although the heavier ferrous scrap may not be completely oxidized in the kiln, it can be collected and used as high-quality, heavy-melting stock for electric furnace feed. Future work, particularly on the use of auto scrap as a reductant, will emphasize the recovery of valuable nonferrous metals present in the iron oxide concentrate.
Recovery of Copper and Iron from High-Grade Ferrous Scrap
The aim of this work is to recover high-grade copper and iron from copper-bearing ferrous scrap, such as generators, starters, alternators, etc., by a preferential melting technique in molten salts, slags, or other suitable media. Although the basic method was developed some time ago, the Bureau is continuing this work with emphasis on improving the economics of the process. Calcium chloride has been found to be a suitable substitute for the more costly barium chloride salt used in early work. It has also been determined that pretreatment of the scrap with sodium sulfate prior to immersion in the molten bath for sweating off the copper increases the copper recovery.
Removal of Contaminants from Molten Ferrous Scrap
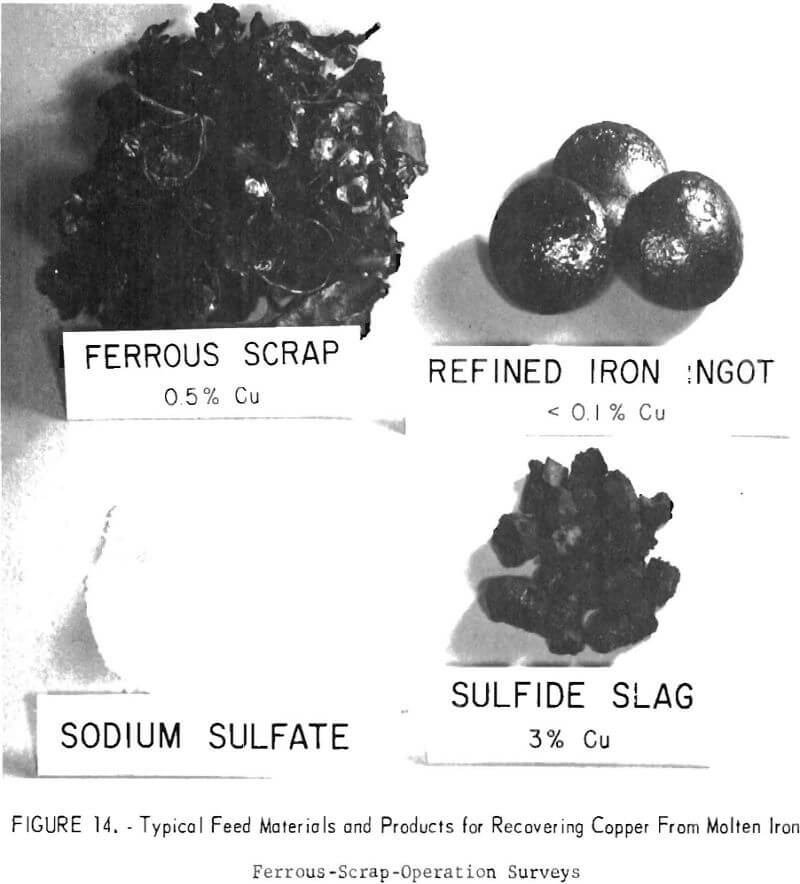
The Bureau is also working on a metallurgical refining method for removing copper and other contaminants from molten junk car scrap and other ferrous materials. One promising approach, currently under investigation, is the use of an alkali slag to remove copper from molten iron (fig. 14). Technical feasibility of the technique has been demonstrated and its possibilities for commercial use are being explored. The process involves the addition of sodium sulfate to a molten iron bath to form copper sulfide, which is removed along with the copper. Work is continuing on means of lowering the sodium sulfate addition to the bath and on increasing the recycling of the sodium salt from the slag. The ultimate aim will be to develop a process that will permit electric furnace melting of low-grade ferrous scrap.
Air Separator for Automobile Shredder Residue
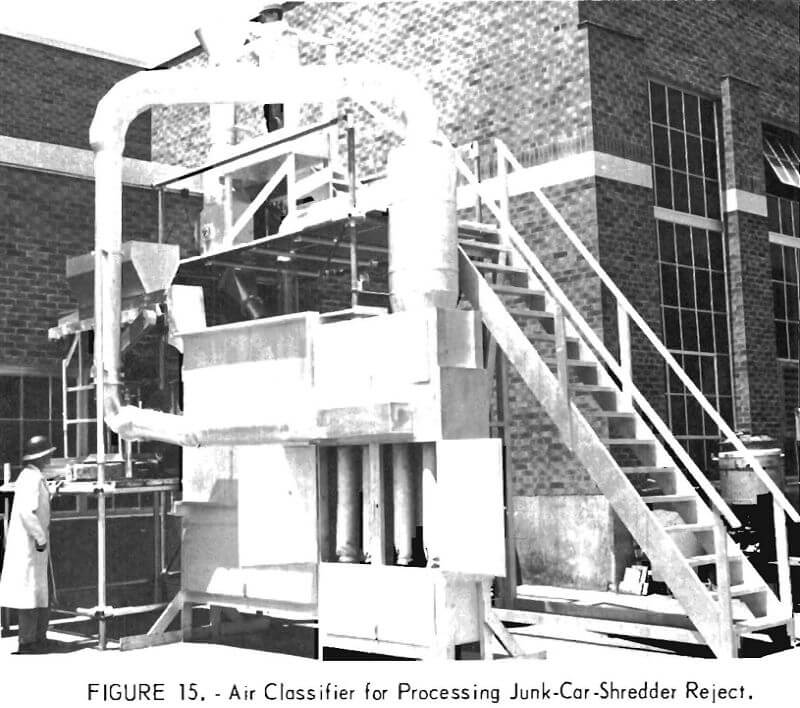
In most junk-car shredding operations, large quantities of nonferrous metals, combined with the other nonmagnetic residues from the shredded vehicle such as glass, plastics, and rubber, are disposed of in dumps or landfills. It is estimated that 300,000 tons of nonferrous metals are lost annually in this manner. The Bureau has designed an inexpensive pilot air classifier with a capacity of 16 tons per hour, in which the nonmagnetic residue from shredding operations can be automatically separated (figs. 15-16). Tests have shown that more than 95 percent of the nonferrous metal of the residue can be collected in a single pass through the device. Methods for physical separation of the individual metals from the nonferrous metal concentrate are being examined.
Recovery of Copper, Aluminum, and Other Nonferrous Metals by Cryogenic Techniques
Preliminary tests have shown that quick freezing of copper-ferrous scrap in liquid nitrogen, or in mixtures of acetone, methanol, and dry ice, greatly enhances recovery of the copper. Copper and aluminum wire can also be cleanly separated from plastic and rubber insulation by freezing and shredding. The method is particularly suited to recovery of copper from small motors.
Ferrous-Scrap-Operation Surveys
The Bureau, in conjunction with its surveys of mineral industries, has recently conducted an examination of nine representative junk-car shredding operations across the country. The rated capacity of the sample from nine shredders was 21 percent of the total capacity of 89 existing shredders. Collection and dissemination of information will be continued on various types of ferrous scrap operations that include junk cars.
Utilization and Stabilization of Mine, Mill, and Smelter Wastes
Another phase of the Bureau’s solid waste program is directed toward the stabilization and utilization of the nearly 25 billion tons of mining and mineral solid waste accumulated across the country. Surveys have identified many of the existing wastes, and characterization studies have provided much information and data on their nature. Many such wastes can be used to make

attractive structural materials, and others can be stabilized by several promising methods. Excellent progress has been made in the development of low-cost methods to stabilize fine waste such as tailings. Large acreages of uranium, copper, and lead-zinc tailings piles have been successfully stabilized by vegetative and chemical means. The Bureau also has a substantial research and demonstration program on coal mine subsidence and on controlling fires in coal waste banks. Current research on mineral wastes includes the following.
Vegetative Stabilization
Successful vegetation stabilization procedures have been developed for tailings piles from mining of copper, uranium, and lead-zinc ores. Many varieties of grasses, wheats, and ryes have been grown in laboratory tests and on large-acreage tailings plots (fig. 17) . Vegetation that shows promise includes sweet clover, winter wheat, rye, and barley; grasses such as sorghum, Kentucky blue, and orchard; and shrubs such as sagebrush, rubber rabbit brush,

and Siberian pea tree. A special barley, Charlottetown 80, has been effectively grown in acid wastes at a pH as low as 3.0. Laboratory work is also underway to determine which salt:; or organic reagents in the tailings most affect plant growth. Considerable research has been conducted to devise means of overcoming the deleterious effects of salts and acids within mill tailings. Both sewage sludge and compost made from municipal refuse were tested as additives to the tailings and proved beneficial to both vegetative germination and growth.
Research is in progress to determine the feasibility of reclaiming and revegetating coal refuse banks and adjacent affected lands. Coal refuse was examined for composition, texture, acidity, moisture content, and plant nutrient levels. Experiments have been conducted by applying various amounts of fly ash and soil conditioners and mixing these components with coal refuse to varying depths to promote plant growth. Grasses and legumes were successfully established on a formerly barren coal refuse bank.
Chemical Stabilization
Chemical stabilization of fine-grained mill tailings is a promising method to reduce pollution from such materials. In many instances the tailings, because of contained chemicals or climatic conditions, are not amenable to vegetative treatment. At costs of cents per square yard, some of the additives found to be effective surface stabilizers are Coherex, a resinous adhesive; DCA-70, an elastomeric polymer; calcium, ammonium, and sodium lignosulfonates; and cement and lime. A 34-acre tract of uranium mill tailings in Arizona was effectively stabilized with DCA-70 and calcium lignosulfonate (fig. 18).
Combined Stabilization Methods
One of the most innovative studies of the Bureau in this area has been the development of a combination chemical-vegetative stabilization method. The use of chemicals in conjunction with vegetative growth can have several advantages in (1) preventing sandblasting of plants, (2) helping to retain moisture by decreasing evaporation, (3) aiding in germination by adsorbing heat, (4) adding organics to the wastes, and (5) preventing reflection of the sun’s rays back to the plant. A combination system involving vegetation and an application of Coherex was successfully used on a 10-acre copper tailings plot in Nevada. Smaller plots of lead-zinc tailings have also been stabilized with the combination methods.
Work has also been conducted on physical stabilization of fine tailings to prevent air pollution. The most useful materials are rock and soil obtained from adjacent areas. Granulated smelter slag has been employed as an effective stabilizer as well at bark covering, straw, placement of windbreaks such as old tires, and spreading of a mixture of limestone chips and sodium silicate as a coating.

Utilization of Mineral Wastes
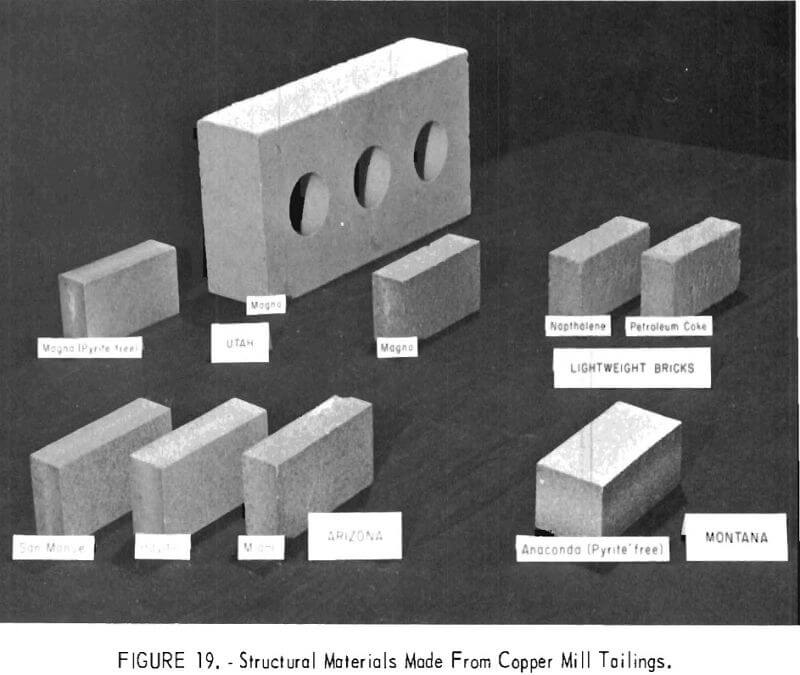
Research is underway on the use of mineral wastes for producing ceramic and construction materials. As most mineral wastes are usually low in clay and plasticity, the Bureau’s work has been directed toward developing dry- pressing and steam-curing methods to produce .structural products. The feasibility of using copper mill tailings for making bricks from six different sources by a dry-press method has been demonstrated (fig. 19). Tailings containing 1.5 percent or less sulfides were used as received, mixed with calcium lignosulfonate and water, and subjected to pressure and curing.
Steam-cured, silicate-bonded building bricks have a]so been made in the laboratory from porphyry copper mill tailings from Utah and Arizona. A method was devised for producing quality wall tile from phosphorus furnace slag.
Studies on mineral wastes include development of low-cost methods for recovering copper, as well as molybdenum, gold, and silver from mining and milling wastes. Procedures comprising crushing, screening, and flotation are being employed to obtain rich concentrates. Percolation leaching of coarse, residual, dump material is also being investigated. Improved, low-cost techniques for recovering uranium from wastes such as mill tailings and mine water are under development.
Mine Subsidence Control
Mine refuse from past anthracite coal mining operations is being used to backfill voids in abandoned mines to prevent subsidence of the surface in populated areas (fig. 20). To date, 12 projects have been completed on flushing crushed mine refuse into abandoned underground mine voids to provide surface support under populated areas. Surface water leaches sulfides in the waste banks to form acid drainage; the waste banks contribute silt to adjacent land areas and streams; and, when ignited, the banks contribute to pollution of the air. Thus, three benefits evolve from backfilling operations-disposal of waste, control of subsidence, and elimination of hazardous conditions.

Control of Burning Coal Waste Banks
Waste accumulations from coal mines contaminate air and water, pose hazards to public health, create scenic blights, and interfere with community development. Because of the carbonaceous content of these piles, they are susceptible to ignition by spontaneous combustion or by irresponsible trash burning. Once ignited, a coal waste bank may burn for decades, polluting the atmosphere with smoke and noxious gases (fig. 21). According to the most recent survey conducted by the Bureau, 292 burning coal waste banks are located throughout the United States.
Two demonstration projects have been completed in Pennsylvania’s anthracite region to develop effective methods for extinguishing fires in coal waste banks. One consists of water-quenching and compaction and the other is based on constructing ponds on the leveled surface of the bank, A third project involves testing the effectiveness of using yet another technique-water sprays and a water injection system. In addition, a survey of the ambient air at the project site is being conducted to gather data on emissions of gases, such as sulfur dioxide and carbon monoxide, from the coal refuse fire.
Recovery and Reuse of Values From Industrial Wastes
Industrial wastes present serious disposal problems because each type of waste requires a different solution. In this category are included piles and acres of sludges, slags, scrap plastics, scrap metal, rags, and waste paper, as well as chemicals, oils, solvents, and other liquids resulting from manufacturing processes.
The Bureau has developed methods to recover values from industrial waste products over a period of more than 30 years. However, these have been mostly metal- and mineral-bearing wastes and were directed to reclamation of high- value materials. In fact, many of the procedures used by secondary processors to recover metal values from industrial wastes are Bureau developments. In recent years, the Bureau has also directed its attention to a variety of troublesome low-value industrial waste materials. Current investigations include the following.
Scrap Metal Identification
An important project involves a study on developing rapid, simple identification methods for scrap metals. Although this is a vital step in the reuse cycle, and errors can be serious and cumulative, the methods in common usage are neither scientific nor reliable. Tests that permit rapid identification of the major alloying constituents of aluminum alloys have already been developed. An inexpensive device, (fig. 22) requiring only 15 seconds per test, was designed for electrographic sampling onto filter paper. Current work is directed toward methods for Identifying the large number of copper alloys that are collected in scrap processing yards.


Precious Metal Recovery From Scrap
Methods are under investigation to recover and recycle the vast quantities of precious metals that are presently lost when electronic equipment is discarded. Military electronic equipment is presently being scrapped at a rate of 15,000 tons per year. This scrap averages per ton, 100 troy ounces or silver, 5 troy ounces of gold, 1 troy ounce of palladium, and lesser amounts of other precious metals (fig. 23). The most promising approach for recovery of these valuable metals is a smelting process, followed by electrolytic treatment to separate the precious metals from copper (fig. 24).
Reuse of Waste Lubricating Oils
The Bureau has recently initiated research to develop the best methods and techniques for recovery and reuse of the 8 million barrels of automotive and industrial lubricating oils presently dumped onto land surfaces or into streams each year. Further attention is being directed toward the

sludge-disposal problem resulting from the re-refining of used oil. The products are being examined for a variety of uses.
Recovery of Sulfur Dioxide From Smelter Gases
Another serious problem is that of collection and recovery of sulfur dioxide emitted from smelters. The Bureau has developed a process for recovering elemental sulfur from smelter stack gases by absorbing the SO2, in a solution of sodium citrate and sodium carbonate. By reacting the absorbed SO2 with H2S elemental sulfur is precipitated. Stripped citrate solution is recovered for recycle, and part of the sulfur is converted to H2S for use in the precipitation step. A pilot plant based on the Bureau’s process was built and operated for several months in cooperation with Magma Copper Co. to obtain technical and economic data on the process.

Reuse of Ferrous and Nonferrous Dusts
Studies identifying the nature and magnitude of solid wastes issuing as flue dusts in steelmaking operations are underway. Aside from tho loss of iron contained in these wastes, significant amounts of zinc, copper, and lead, originating from the scrap additions to the steelmaking processes, are also present. Techniques such as sulfuric acid extraction of copper and zinc are being used to treat and recover these dusts so they can be returned to the steel furnaces as high-grade pelletized iron ore. Processes to treat flue dusts from brass smelters to form metallic zinc, lead, and copper products are also being investigated. Emphasis is placed on methods that allow the dusts to be treated in the plants where they are generated.
Recovery of Values From Waste Plating Solutions and Sludges
Work continues on recovery of metallic values from waste electroplating solutions by application of the Bureau’s waste-plus-waste technique. This method involves the combination of two waste liquids to produce recoverable products. Research has been initiated on recovery of the valuable constituents in the sludges that result from electrochemical maching (ECM) and electrical discharge machining (EDM) of stainless steels and nickel- and cobalt-base alloys. Approaches to be used in solving this problem include selective dissolution, solvent extraction, and controlled-potential electrolysis.
Recycling Titanium Scrap
Attention is also directed toward improved methods of recycling titanium and titanium-based alloy scrap. One procedure employs the use of the Industo-slag process for continuous direct feed and melting of scrap titanium.
Another project involves developing a fused-salt, electrorefining process to recover titanium from scrap alloys containing aluminum and tin.
Recycling High-Temperature Alloy Scrap
Methods to recover and recycle the individual components of high-temperature-alloy scrap are receiving attention. Studies include sequential oxidation and reduction of elements from molten salts based on their position in the emf (electromotive force) series.
Treating Aluminum Scrap
Research is continuing on electrolytic processing of aluminum scrap and alloys in a large, single-compartment cell. Emphasis is placed on extraction of aluminum and precious metals from aluminum-base electronic scrap and the nonferrous fraction of municipal incinerator residues. Tests are also being conducted to determine process conditions necessary for effective reparation of aluminum and aluminum oxide from high-chloride salt slag by molten slag filtration techniques. Refining slags and fluxes for treating aluminum drosses are under examination.
Lead-Battery Scrap
Work has been initiated on developing a new method for processing lead-battery scrap to allow recovery of lead and sulfur and eliminate the current problem of sulfur dioxide emissions. Optimum conditions for leaching, smelting, sulfur recovery, and regeneration of leaching agents are being examined.
Reuse of Fly Ash, Coal Bottom Ash, and Other Wastes
Other research and development projects include the use of waste glass, coal bottom ash, and fly ash to product, brick by appropriate mixtures of clay and the waste materials.
Research on the production of portland and regulated set-cement from fly ash is proceeding. The use of waste silicofluorides and other mineralizers in the production of cements and refractories appears to be highly promising. Reclamation of iron foundry core sands is under investigation.
Current and Recently Completed Solid Waste Research Grants and Contracts
The Bureau’s work on utilization and disposal of solid wastes is supplemented by grants and contracts to educational and other organizations. The program has two objectives. One is to involve educational institutions in the recovery and recycling of solid wastes. The second is to provide incentives to graduate students and their associates to prepare for industrial and public service careers concerned with environmental and mineral supply problems.
