Table of Contents
Nearly 4 billion pounds of plastics were consumed by the packaging industry in 1970, about one-fifth of the total plastics production. Urban refuse from the city of Madison, Wis., was found to contain 2.9 weight-percent plastic before drying and 3.3 weight-percent after drying. Plastic waste is a worldwide problem: urban waste contains about 2 percent plastic in the developed countries, generally, and in Japan the amount exceeded 6 percent in 1968. The amount of polyvinyl chloride (PVC) in municipal waste has been estimated at 0.20 to 0.25 percent in the United States, 0.1 to 0.5 percent in Europe, and 2 to 3 percent in Japan. The percentage of plastic waste in urban refuse is predicted to nearly double or triple by 1980. The Bureau of Mines, and others, are developing waste processes that may concentrate the plastic waste into a separate fraction suitable for processing.
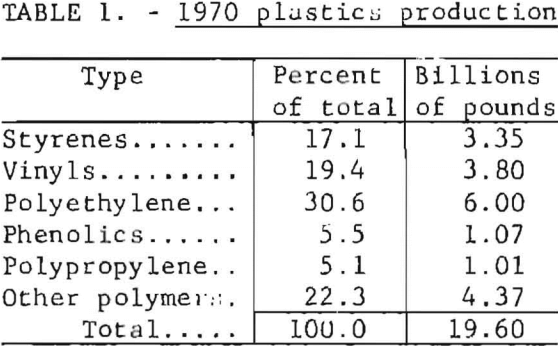
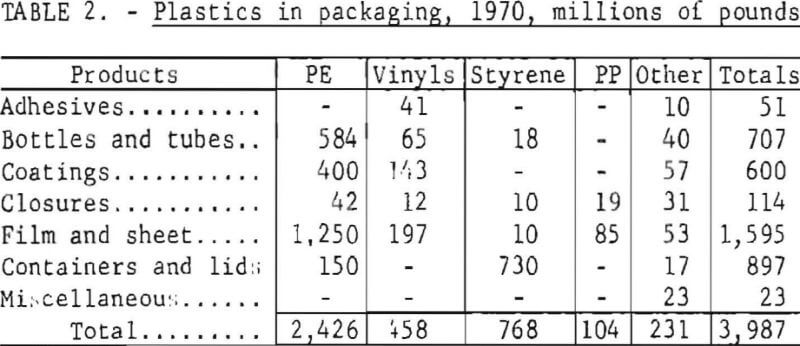
Industrial plastics are of two major types, those that are thermoplastic and can be heat-softened and reformed repeatedly, and the thermosetting varieties that cannot be reshaped after they have been formed. Most of the plastic polymers used in packaging are in the thermoplastic category, of which four major types account for over 70 percent of the plastics production in the United States. They are polyethylene (PE), polypropylene (PP), polystyrene (PS), and PVC. Total plastics production and amounts used in packaging in 1970 are shown in tables 1 and 2. Considering the general physical and chemical characteristics of plastics, their amenability to conventional methods of disposal is assessed as follows.
Incineration
Although some plastics do not burn readily, all can be incinerated and have high heat content. In fact, the addition of plastics to refuse increases its heating value. Modern incinerators encounter little difficulty with the 2 to 4 percent plastics in municipal waste. However, there is evidence that halides liberated by plastics such as PVC can cause corrosion and pollution problems that must be reckoned with, especially in incinerators that are poorly designed and operated.
Pyrolysis
Several reports show that plastics yield useful organic compounds and a combustible char upon pyrolysis, and the Bureau of Mines has contributed in this field. Pyrolysis equipment has been designed that would vent split-out components such as HCl, acetic acid, and plasticizers and pyrolyze the remaining polymers to produce hydrocarbons of various types and uses. A computer-aided paper study by TRW, Inc., in 1969 pointed to PS and PVC as the most likely candidates for profitable thermal decomposition. Polystyrene is one of the few polymers that yields a relatively high percentage of monomer when thermally degraded.
Landfill and Compost
Plastic containers resist compaction, but small quantities of chopped plastics apparently contribute to a stable, dense component for landfill. They do not degrade or decompose and are not suitable for compost, but may have application as decorative ground cover.
Salvage and Reuse
Pure scrap thermoplastics are customarily refabricated and reused in production plants, where trim and rejects from the fabricating processes are chopped and fed back into the forming machines or sold for toy product ion and electrical wire insulation. One manufacturer stated that 50 percent of his company’s plastic supply is recycled in this manner. “Home scrap” mixed with virgin material can easily be reused, but waste scrap of mixed and unknown properties would not be acceptable in most plants using high-speed production methods. The first report of commercial recycling of waste plastic was made in 1970 by a California firm that used high-density polyethylene milk bottles, collected from housewives, in the manufacture of drainage tile.
This operation was later halted by Federal regulations requiring that only clean reworked material, generated from the manufacturer’s own production, may be used. To reprocess the waste plastic in municipal refuse into useful items requires the development of a beneficiation process. While it appear to be a formidable task, this approach is considered to have merit.
Experimental Investigation and Results
Given the mission of devising methods for recovering and utilizing resources in the plastics fraction of urban refuse, the research group at the Rolla Metallurgy Research Center has emphasized the characterization of the waste plastics, methods of cleaning and separating into plastic types, and development of methods of using or recycling the separated products. Figures 1, 2, 3, and 4 illustrate some of the research activities on waste plastics at the Research Center.

Characterization
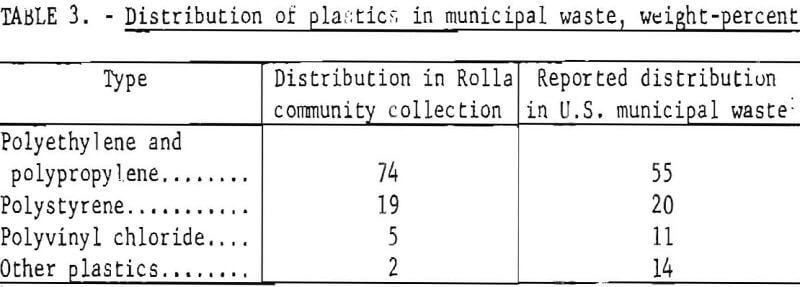
A sample obtained by a waste plastics collection campaign in Rolla, Mo., has been very useful to the research group for purposes of characterizing and experimenting with waste materials. Analysis of the sample revealed that the relative distribution, by weight, of plastic types was as shown in table 3.
Bottles, totaling about 2,000, were the predominant item in the community collection. Detergent bottles accounted for more than half of these, with cosmetic, food, and medicine containers sharing in the other half. Chopping the plastic waste to pass through a ½-inch screen in a commercial granulating machine reduced the space occupied to about one-tenth the original volume.
Most methods of identifying plastics are slow and highly instrumented; for example, infrared spectroscopy, differential thermal analysis (DTA), thermogravimetric analysis (TGA), mass spectrometry, gas chromatography, and

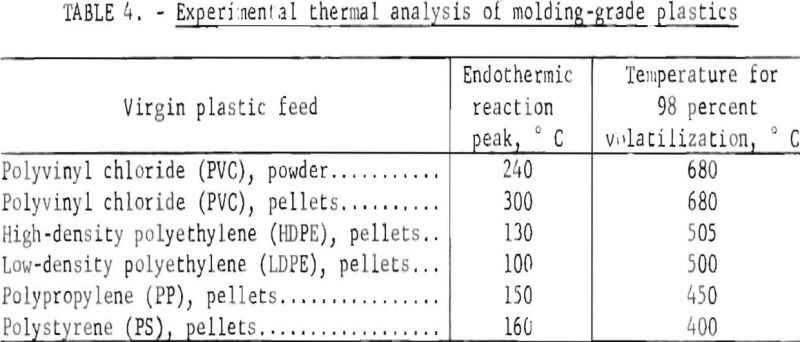
selective dissolution. More rapid and practical but less precise methods were used to sort the plastics in the collection into types, based on feel, sound, appearance, flame behavior, and odor. For example, polyethylene feels greasy and is usually somewhat flexible, whereas polystyrene is brittle and is noisy when dropped or flexed. A scheme of analysis by flame behavior and odor was especially useful for PVC. A hot copper wire touched to PVC and then placed in the blue flame of a gas burner produces a bright green hue. DTA and TGA were used where simpler methods failed; the exothermic and endothermic reaction temperatures give clues to polymer identity.
Table 4 gives the experimental results obtained in the laboratory on several virgin plastic samples, illustrating the values that were used to classify doubtful samples as to plastic type.
Cleaning
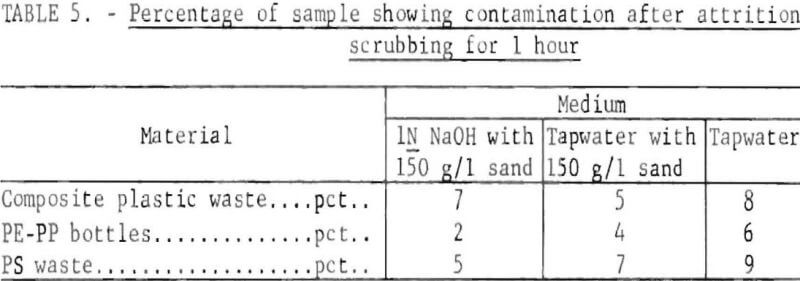
It is necessary to have an initial, washing or cleaning step to remove contaminants such as dirt, labels, adhesives, and residual contents from plastic-waste. Labels, and the adhesives with which they are applied, are difficult to remove, and remnants may be deleterious to subsequent use of the plastics. Numerous small-scale trials of washing solutions and methods of agitation were made on label-bearing polyethylene bottle chips. Hot and cold water, aqueous mixtures of alcohol, acetone, and benzene, and sodium hydroxide solutions were tried with stirring, blending, ultrasonic agitation, ball milling, and attrition scrubbing. Label-covered polyethylene clipped from bottles, chopped to minus ½-inch and air classified to remove loose paper, showed on examination that about one-third of the pieces were free of paper and adhesive. Subsequent attrition scrubbing in the above solutions, and in some cases with sand added to the mixture, generally cleaned upwards of 90 percent of the particles.
In subsequent work on a more practical scale, washing tests were made on 1,200-gram plastic samples in 6,000 ml of solution in a laboratory scrubbing machine. One sample was composed of bottles chopped to minus ½-inch and air classified; the other was a composite from the waste plastics collection containing about the same proportions shown in the community collection in table 3. In table 5, the results of 1-hour scrubbing on samples in three different media are shown in terms of the percentage of pieces still showing contamination. It appears that the use of NaOH would not be justified in view of the cost of adding and disposing of the reagent, but the use of sand in the scrubber may be justified when the scale of operation is expanded.
Separation
Future reuse or recycling of plastics will be influenced considerably by the purity and condition of the plastic types that can be isolated from the collected waste. Methods are being developed to obtain clean fractions of high-density polyethylene (HDPE), low-density polyethylene (LDPE), PS, PP, and PVC from waste. In an actual situation perhaps only PVC would be removed prior to incineration of the bulk.
The optional methods for separating plastics into type are essentially those based on shape, density, selective dissolution, and electrical properties. Chemical dissolution may be feasible for some special situations, such as the recovery of paper from plastic-coated paper milk cartons, but was not considered in this work on raw plastic waste. Electrostatic methods may also be used in separating plastics from other wastes, but the similarity of the plastics to one another hinders this method from use in separating individual types.
A large proportion of the material in collected waste, such as film, fiber, and foam, responds to air classification. A small vertical air classifier, shown in figure 5, has been used with good results in this project.
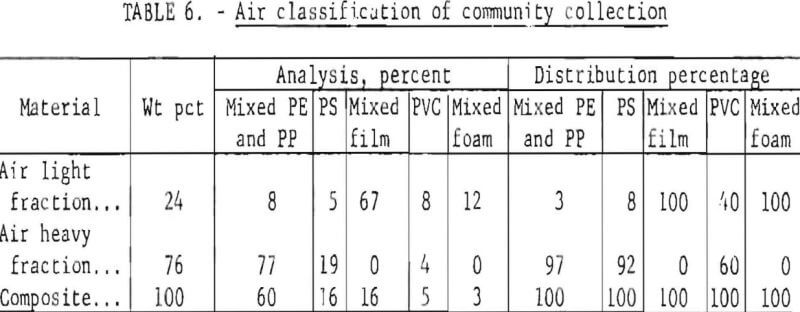
Air classification experiments were conducted on 1,600-gram samples of minus ½-inch material of a composite mixture of the community collection, with the results given in table 6. It was apparent that the shape of the material, tor example, the thickness and area of bottle fragments, strongly influenced the response to the air stream.
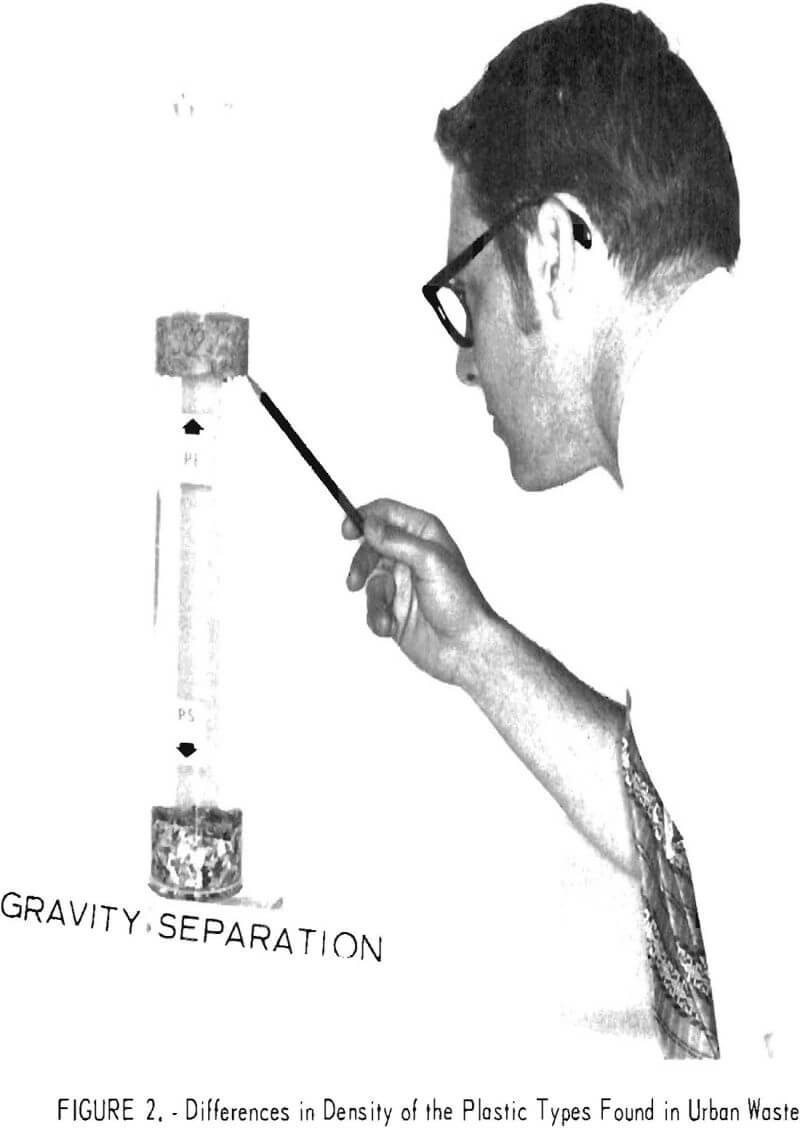
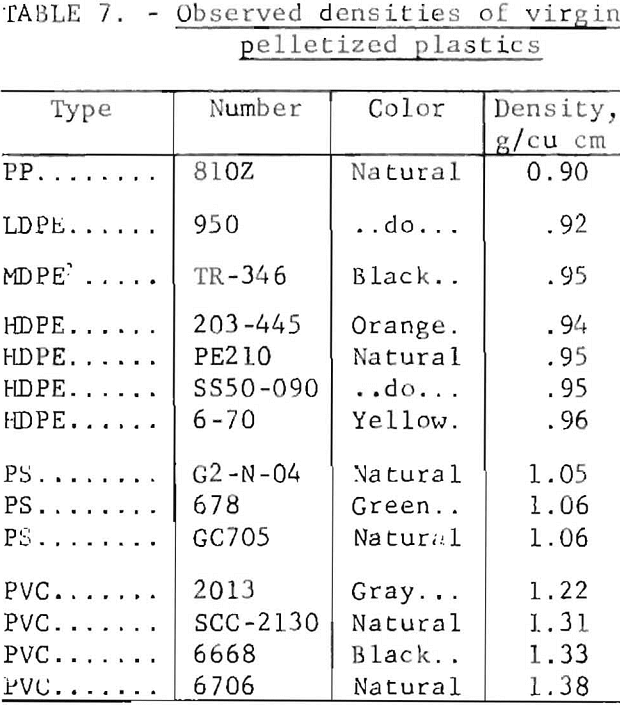
The densities measured in the laboratory on virgin plastic of the types predominant in wastes are shown in table 7. Although additives may alter these densities, results on plastic waste in the laboratory have shown that most products fall in the density ranges given in table 7. The differences in density of the virgin plastics were sufficient to suggest separation schemes based on the buoyancy of plastics in aqueous media. Laboratory investigation of aqueous gravity separation methods was initiated using hand-sorted plastic waste which had been granulated to pass a ¼-inch screen. The experiments were conducted in an unagitated vessel using media of various densities.
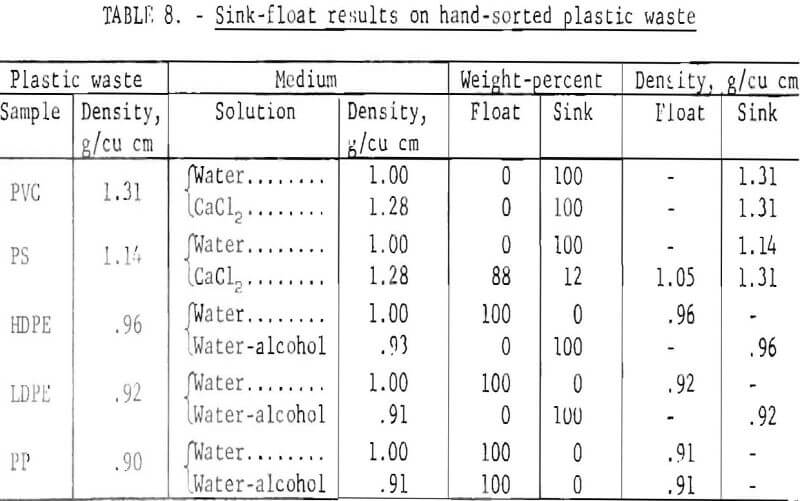
Distilled water, a calcium chloride solution, and two alcohol-water combinations were investigated as media. The results are presented in table 8. It will be noted from the density data in table 8 that, in hand sorting of the plastic waste, the PS fraction was contaminated with heavier material. Both sink and float fractions were obtained in the calcium chloride solution with the float identified as PS and the sink as PVC. A theoretical scheme of separation for the five most plentiful components of waste thermoplastics in packaging wastes is given in figure 6. In actual practice the polyolefin (PP, LDPE, and HDPE) fraction could probably be utilized without further separation.
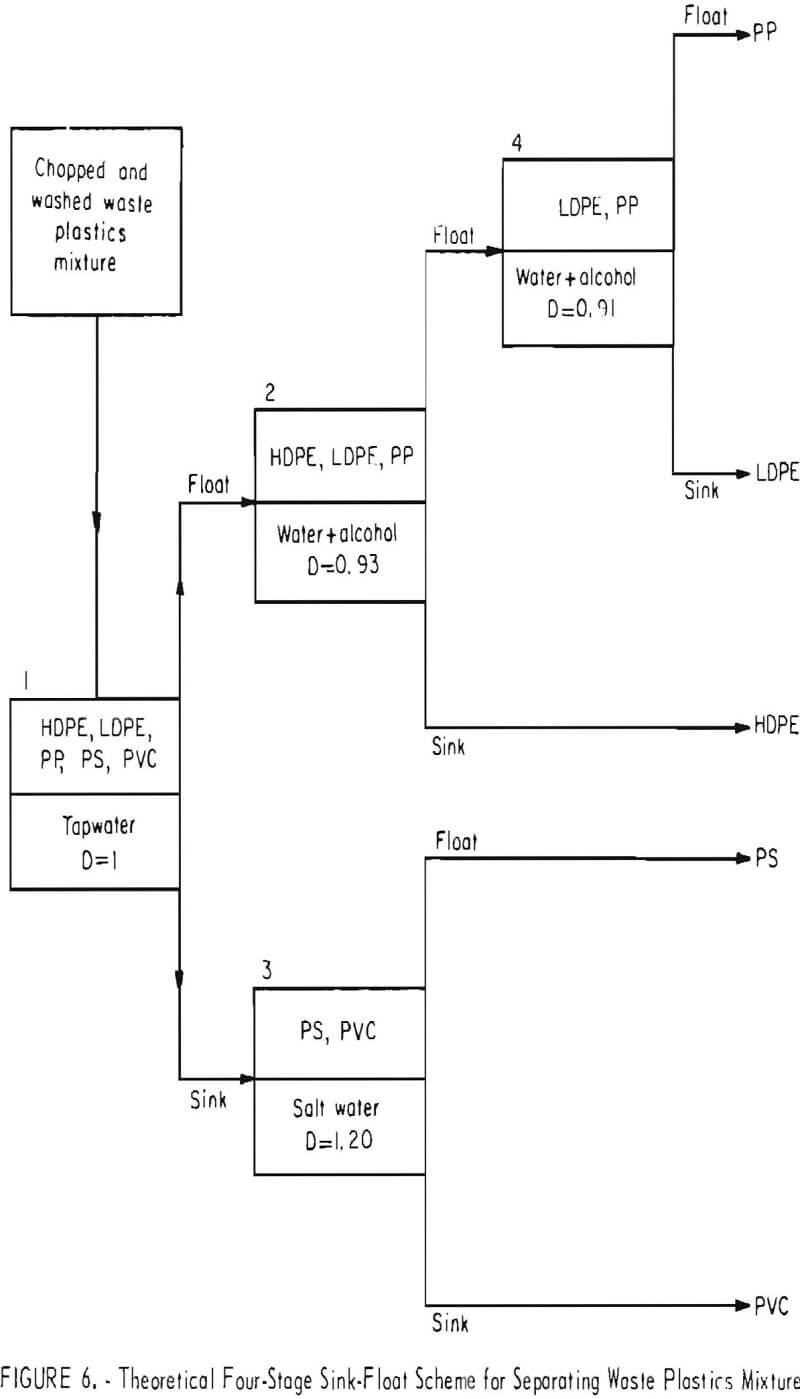
Further investigation of the sink-float process is being done in a 7-gallon capacity chamber with an overflow trough and underflow pipe with an airlift for the sink fraction. Both overflow and underflow are returned to the common reservoir through separate screens where the sink and float fractions are recovered (fig. 7). The plastics sample is presoaked to eliminate

entrapped air and is fed with the recirculated water, which provides adequate agitation for its dispersal, into the sink-float tank. Results obtained on initial runs with granulated virgin pellets (PE, PS, and PVC) were encouraging, with less than 1 percent contamination occurring in the float fraction. It was estimated that the unit can process about 15 pounds of chopped waste per hour. Cursory tests further Indicated that PS and PVC can be separated into clean fractions by water elutriation.
The results obtained to date indicate the sink-float method to be the best approach for separating the plastic waste in urban refuse into useful components.
Disposition of Separated Fractions
When clean, relatively pure plastic types are available from the treatment of raw urban waste, several courses for disposition of the materials can be considered. It may be economically favorable to incinerate all of the plastics except the PVC, if the fuel value exceeds their worth as raw material for refabrication. Thermochemical treatment may offer a profitable route for both PS and PVC. It is also possible that all of the separated types of plastic would be most valuable for refabrication into useful articles.
Refabrication
At this Center, hot pressing has been used successfully to form clean chopped waste plastic into briquets 2 inches in diameter. Polyethylene, polystyrene, and polyvinyl chloride were easily formed in a laboratory press under a pressure of 3,800 psig at 155° C. The briquets retained the bright colors of the chopped particles, and were fused into attractive dense, homogeneous solids. Mixed plastics did not fuse as well as the pure substances. Hot pressing is simple and straightforward, and it is believed that a municipality could dispose of tons of waste PE and PP by this method, producing paving blocks, traffic markers, parking barriers, benches, or other large-volume items.
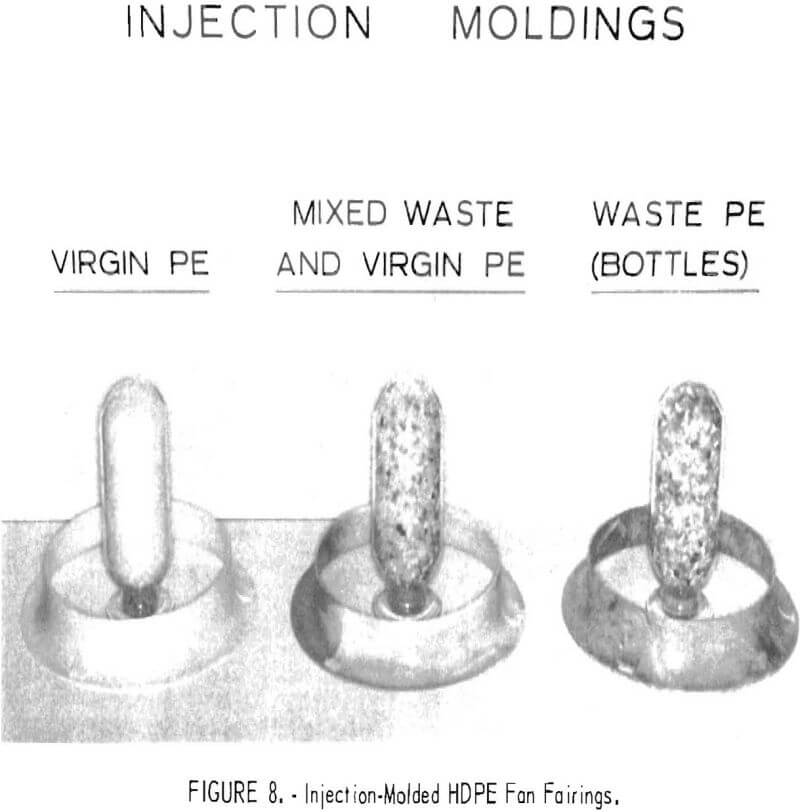
Injection molding experiments with portions of the Rolla waste collection were also encouraging. An 80-pound sample of clean HDPE was prepared from collected bottles from which the labels had been removed. The bottles were chopped to minus ¼-inch and washed in 60° C tapwater. Since a high percentage of the bottles had contained detergent, no additional cleanser was required to aid in the washing. Chemical analyses of the wash water showed Na and Fe contamination was removed by two wash cycles. The cleaned HDPE was taken to the Edward R. Spence Co., in St. Louis, Mo., where arrangements had been made to substitute the cleaned waste HDPE for virgin material in an injection molding production run of fan fairings. A 12-ounce-capacity, plunger-type injection molding machine was used with a two-cavity mold arrangement. As the waste plastic displaced the virgin plastic in the chamber, the mixed products looked normal; however, as higher percentages of waste material were used, incomplete filling of the mold was evidenced. When 100 percent waste material was used, the extremities of the mold did not fill. The operator, at the time, attributed this incomplete filling to the release of gas, which might have been corrected by venting the mold.
Several dozen fairings were made with no problems other than the inadequate flow in the mold. Both barrel temperature and ram pressure were increased on the molding machine without completely relieving the filling problem. The experimental injection moldings are shown in figure 8.
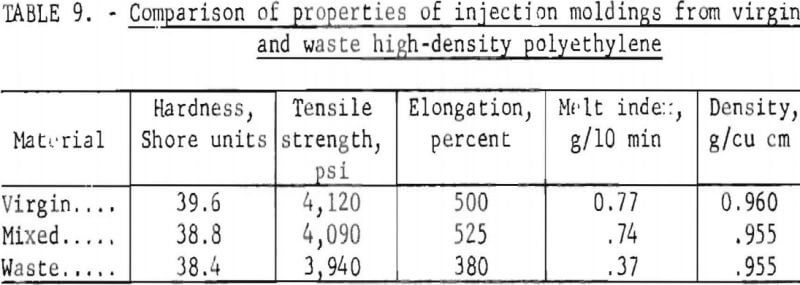
The injection moldings were evaluated by comparing properties determined from sample strips cut from virgin, mixed, and waste HDPE moldings. Tensile strength, hardness, density, and melt index measurements were made on products fabricated from all-virgin, mixed virgin and waste, and all-waste HDPE. The melt index determined on the clean chopped waste HDPE before fabrication was 0.41±0.02 g/10 min, about half that of the virgin material. The data presented in table 9 are based on the results of tests on five samples from each material. They show that tensile strength, hardness, and density were not changed greatly by substituting waste HDPE for virgin HDPE, but that elongation and melt index dropped significantly. The injection process might have been improved by (1) use of a screw-fed injection molding machine, because this produces a more homogeneous mixture of plastic feed than does the plunger type, and (2) the addition of enough virgin PE having a high melt index to bring the average melt index of the material to a more favorable number. The results of the injection molding experiment showed that this is a promising method for the reuse of waste plastics.
Thermochemical Recovery of Values
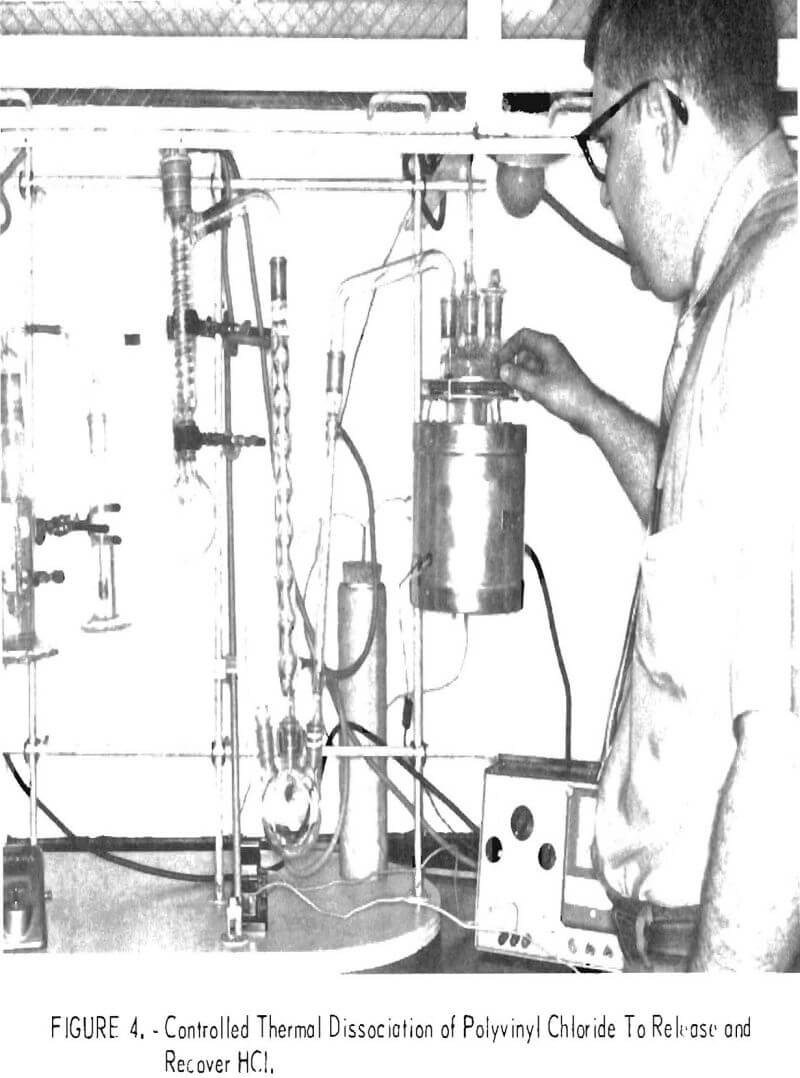
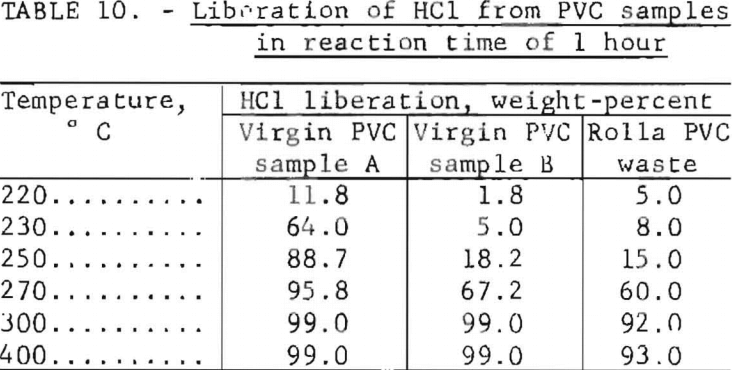
Thermochemical treatments are receiving much attention as a way to obtain useful chemicals and byproducts from municipal and industrial refuse. The prospects of utilizing waste PVC separated from urban refuse as a source of commercial HCl appear most promising. About half the weight of the virgin polymer is composed of HCl, but the addition of fillers and plasticizers in production dilutes this amount. The PVC waste from the Rolla collection contains about 28 percent HCl. Experiments by thermal degradation revealed that HCl evolution begins at about 220° C, and at higher temperatures it proceeds with increasing rapidity. Upon completion of gas evolution a char residue, illustrated in figure 9, remains in the reactor. The results of 1-hour thermal degradation experiments on two virgin samples of PVC molding resins and one sample of Rolla waste PVC at temperatures in the range 220 to 400° C appear in table 10. At 300° C more than 99 percent of the HCl was liberated from the virgin material and 92 percent was freed from the waste.
In addition to the value of the HCl as a resource, the other products from the thermal dissociation-the char, the liquid condensate, and the off gas-have intrinsic worth as fuel. The reactor residue was 40 to 50 percent of the charge weight in experiments at 300° C. At higher temperatures the char became dry and friable with only slight weight loss, making it easy to remove and handle. The residual char from treatment of PVC waste contained 6.3 percent moisture, 15 percent ash, 51 percent volatile matter, and 27 per-cent fixed carbon. Its heat content was 12,300 Btu per pound. Upon evidence that this char contained calcium, titanium, and chlorine, leaching proved to

be effective in removing additional chlorine from the char, indicating that calcium compounds added during manufacturing, combined with some of the HCl during thermal degradation, probably accounted for the 6-percent discrepancy at 400° C in table 10.
The quantity of liquid condensate formed during the thermal decomposition was 4 to 5 percent of the virgin PVC samples and 10 to 15 percent of the Rolla PVC waste samples. The condensate volume increased with reaction temperature. Less than 1 weight-percent of this material remained as ash when the condensate was burned. The off gas was only 2 to 3 percent of the virgin material but was 10 to 19 percent of the waste PVC, and it also increased with temperature. Mass spectral analysis revealed water, methane, ethane, propane, and butane in these gases. The condensate had thermal energy content of 14,000 Btu per pound, and the off gas was estimated to have 20,000 Btu per pound.
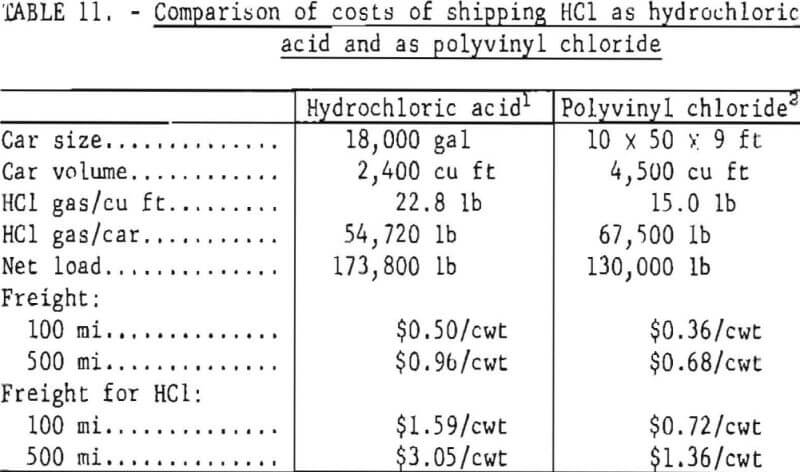
From the above, every ton of PVC recovered from urban waste is a potential source of 600 to 1,000 pounds of HCl, convertible to 1,800 to 3,000 pounds of hydrochloric acid when combined with water, leaving 600 to 1,000 pounds of hydrocarbon residue which could be used for fuel or other pyrolytic processing. For the consumer of HCl in outlying regions this may provide a new way of supplying the gas that has these distinct advantages: flexibility in supply, storage, and use; reduced hazard in shipping and storage; and lower cost of shipping. Waste PVC separated from municipal refuse could be shipped in box-cars or gondolas, stored in bins, and decomposed when HCl was needed at the user’s site. The HCl gas could readily be converted to hydrochloric acid in a falling film or adiabatic absorber. A comparison of the costs of shipping HCl gas in tank cars as concentrated hydrochloric acid (20 Be, 31.45 percent HCl) and in boxcars as polyvinyl chloride appears in table 11, from data on freight rates quoted for the Southwestern United States. The rates shown for PVC apply to virgin material; rates for scrap PVC are virtually the same, however. Per pound of HCl gas, the cost of shipping PVC is less than half the cost of shipping hydrochloric acid.
Summary and Future Plans
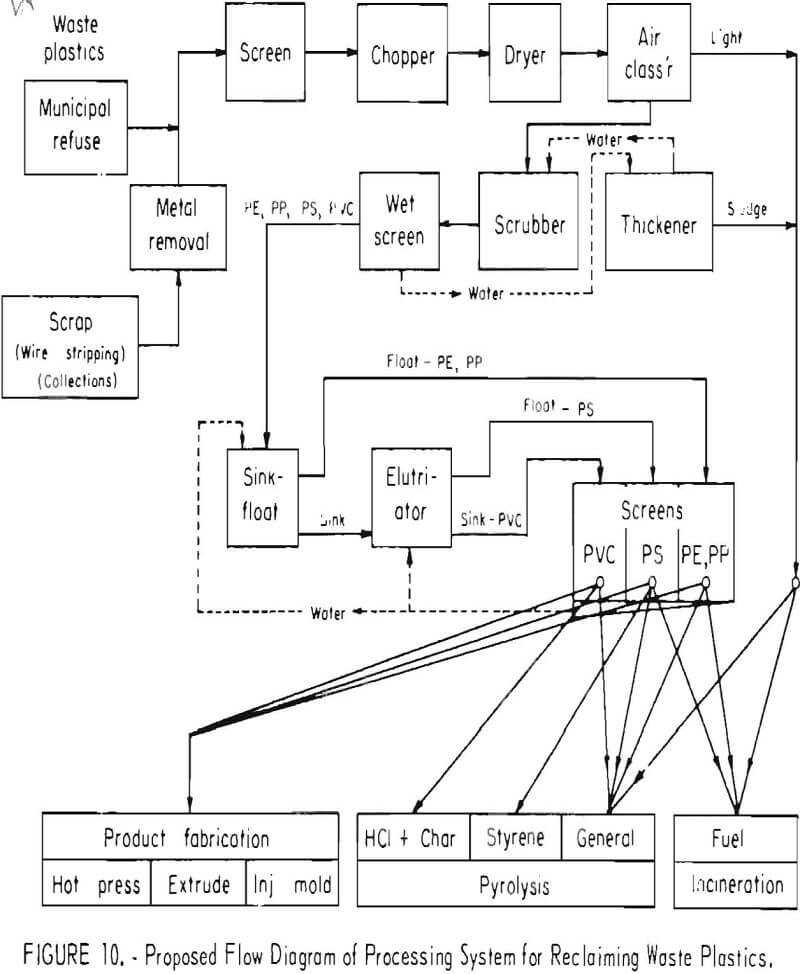
Progress has been made toward solving the problem of separation and reuse of waste plastics found in urban refuse. Possibly some of the methods described can be applied to other types of plastic wastes. The flow diagram (fig. 10) shows a proposed processing recovery system for reclaiming plastic waste from urban refuse by chopping, air classifying, cleaning, and separating into plastic types by the sink-float method. Plastics recovered from processing of raw urban wastes in the Bureau’s pilot plant at College Park, Md., will be treated in a system of this type to obtain data on the quality of the separated materials and to evaluate the economic aspects of the process. Refabrication by compression molding, injection molding, and extrusion will be performed on reclaimed plastic, and quality and market value will be evaluated. Thermochemical decomposition methods will be worked out in more detail for obtaining and utilizing the byproducts from PVC and PS, including economic evaluations of the processes and products.