Table of Contents
Gold spiral electrodes were fabricated as a 3-turn coil of 10 cm long wires of 0.5 mm diameter. Following the hypothesis that a black platinum electrode follows more closely the solution potential than the mineral potential due to its high porosity (area for contact with the solution being much larger than the projected area for contact with particles), black platinum electrodes were made by platinizing 5 cm long straight platinum wires.
During plant investigations it was found that the gold spirals were too fragile to withstand the intensive manipulations involved. To partially solve the problem, one spiral electrode was enclosed in a perforated plastic shield.
Mineral electrodes were fabricated using hand-sorted mineral pieces from the ore under investigation (Al zone ore from Les Mines Selbaie, Quebec). Contact between the mineral and the copper wire was trough a mercury drop.
Epoxy resin was used to seal the contact area between the electrode and the wire, and also to provide a rigid support.
The reference electrode was a porous sleeve Standard Calomel Electrode (SCE) from Fisher Scientific. The pH electrode was a Radiometer model 2401C with a double junction Ag/AgCl 1KCl reference.
Laboratory flotation
These experiments were performed using a Denver D12 flotation machine fitted with the 500 g cell (1 liter of pulp) for roughing flotation and the 250 g cell for cleaning flotation. The impeller speed was 1500 rpm for roughing and 1200 rpm for cleaning. A data acquisition system was used to monitor the potentials exhibited by the electrodes. (See Fig. 1).
Behavior of the black platinum electrode
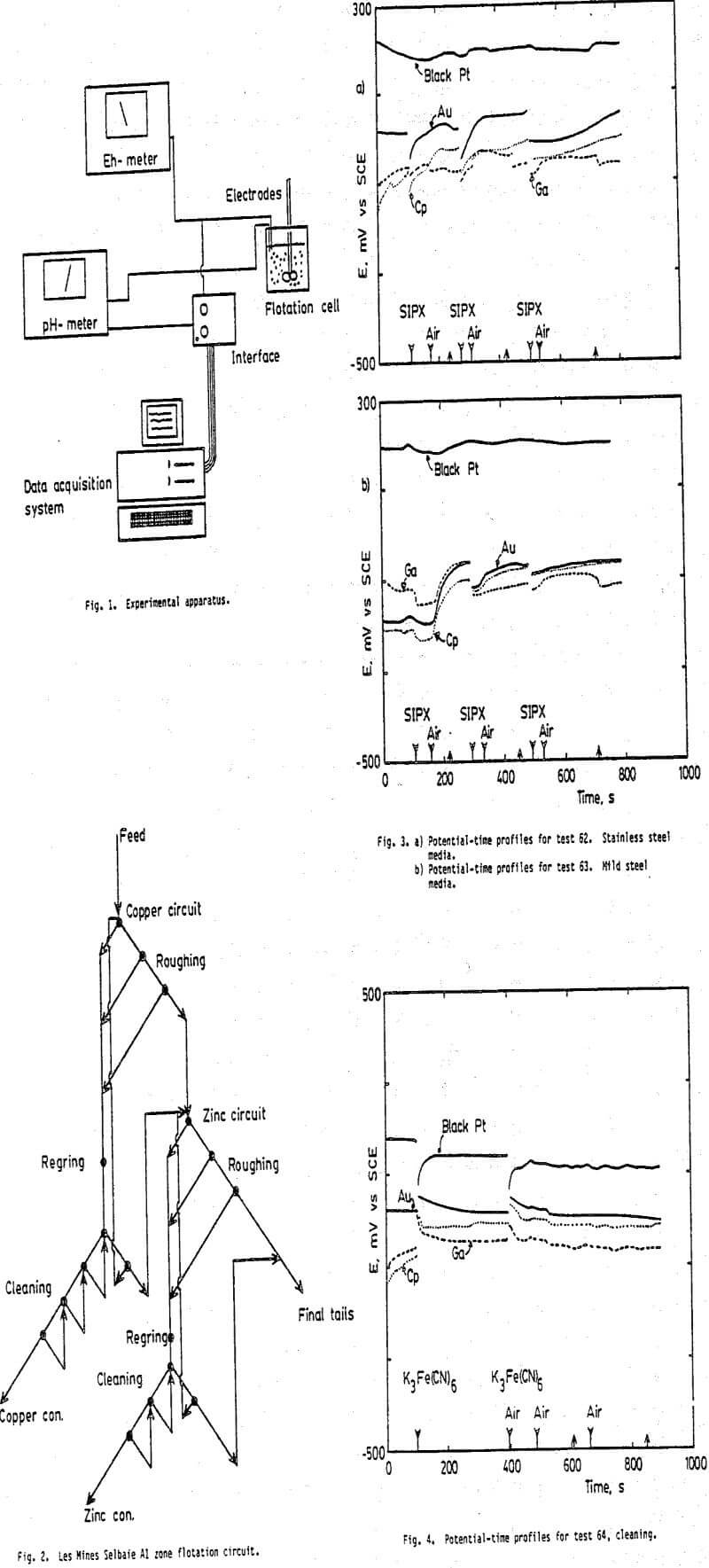
It can be noted (in Table 2) that the potential exhibited by the black platinum electrode is almost constant (184 to 191 mV vs SCE) at the start of rougher flotation (Tests 62, 63, 65, and 68). On the other hand, the potentials exhibited by the gold, chalcopyrite, and galena electrodes vary according to the composition of the grinding media; lower potentials for the mild steel media (Test 63) and higher potentials for the stainless steel media (Test 62). The copper and zinc recoveries after one minute of flotation also vary.
Examination of the potential vs time profiles for these tests (Figs. 3 and 5) show that the potential exhibited by the black platinum electrode is almost constant throughout each test. It does not sense the addition of the collector (SIPX) or the introduction of air for flotation. This behavior is probably due the fact that platinum senses its own Pt-PtO potenial in solutions and hence would not respond to the presence of other ions contrary to mineral electrodes.
The black platinum electrode does sense changes in pH (Figs. 6 and 7) and the introduction of the cyanide ion (Figs. 4 and 7). It appears that the presence of the Cl- ion induces a faster response of the black platinum electrode (Figs. 6b and 7b) in comparison to sulphur based ions (Figs. 6a and 7a). Similarly, the gold and mineral electrodes respond more rapidly in the presence of Cl- ions than to sulphur based ions. This latter observation might be related to the faster leaching kinetics obtained with HCl when compared to H2SO4.
Comparative behavior of the gold and chalcopyrite electrodes
In about half of the tests, the potential exhibited by the gold electrode is nearly the same as that exhibited by the chalcopyrite electrode for the major portion of the flotation time (Figs. 6 and 7). On the other hand, the two electrodes significantly differ when the sulphides are oxidized. This is observed after grinding with a stainless steel media (Fig. 3a) or a weakly reducing media (Fig. 5) or when K3Fe(CN)6 is added to the flotation pulp (Fig. 4). (The ferricyanide was tried to verify if it provided a reverse flotation of sphalerite when secondary copper minerals are present in the ore as indicated by Rey (1980)).
Comparative behavior of the chalcopyrite and galena electrodes
Although copper/zinc separation was attempted, the impossibility of making a sphalerite electrode forced the choice of another minerals the second mineral electrode. Galena was selected. Pyrite could have been used but the behavior of chalcopyrite and pyrite electrodes is extremely similar so that the specific effect of the addition of modifiers onto chalcopyrite could not have been observed.
In general, the potential exhibited by the chalcopyrite electrode differs significantly from that exhibited by the galena electrode. In most tests, the potential of the chalcopyrite electrode is generally higher than that for the galena electrode (See Figs. 3, 4 and 6). This corresponds to the relative position of these minerals on the potential scale for mineral-water systems.