Table of Contents
The use of controlled steel silicon content or the application of borax coatings showed promise in preventing molten copper from forming on the surface of steels containing up to 1 pct Cu when these steels were heated to 2,370° F (1,300° C) , a common temperature for rolling. By preventing copper formation during heating, surface hot shortness of the steel during rolling could be inhibited. Evaluations were based on metallographic studies.
Initially, button-size alloys containing 1 pct Cu were isothermally scaled in air to determine the effect of temperature and steel composition on the location of copper, if formed, at the steel-scale interface. Below the melting temperature of the copper-rich, iron-copper alloy, 2,000° F (1,093° C), the copper-alloy particles did not adhere to the steel surface, but at higher temperatures they wet the surface. For steels containing silicon additions, fayalite, formed on the steel surface by internal oxidation, promoted the formation of iron-rich, iron-copper particles on the steel surface and reduced amounts of copper. Above 2,200° F (1,205° C) , the melting temperature of fayalite, copper was prevented from forming by as little as about 0.2 pct Si, but at lower temperatures complete elimination did not occur with up to 1 pct Si. Aluminum in place of silicon or in conjunction with it was not especially beneficial. Small amounts of boron, 0.04 pct, assisted in preventing copper formation, but steel is hot short with this amount of boron.
Prevention of copper formation on steels containing silicon was found to be dependent upon the heating conditions and the silicon and copper contents of steels when heated per simulated pusher and soaking-pit reheating schedules. As in the isothermal scaling studies, copper could not be completely prevented from forming during continuous heating up to 2,200° F (1,205° C). However, the copper formed below 2,200° F could be eliminated and its further formation prevented when heated to 2,370° F (1,300° C). For steels with 1 pct Cu, 9 vol pct excess oxygen in the furnace atmosphere and about 0.8 pct Si in the steel were necessary. Lesser oxygen and silicon contents were required for steels containing smaller copper contents.
Borax coatings also prevented copper formation after continuous heating to 2,370° F (1,300° C) by either simulated reheating schedule. After pre-scaling the steel specimens to simulate scales formed on ingots during mold stripping, borax glass coatings and coatings containing feldspar or silica plus borax glass were applied at 1,470° F (800° C) and 1,820° F (1,000° C), respectively, using either sprinkling or immersion techniques. When starting at 1,470° F (800° C) to reheat the coated specimens per soaking-pit schedules to 2,370° F (1,300° C), a high silica content, either as feldspar or silica, of the borax coating was necessary to prevent its running off the surface before it reacted with the scale. A borax glass coating without silica was sufficient when using a pusher schedule. To prevent copper from forming on the surface, less stringent steel silicon contents and furnacing conditions were necessary with borax coatings than when only silicon additions to the steel were used.
Studies on the use of silicon additions to prevent copper from causing surface hot shortness are being continued, with emphasis on the effect of cast structures. Mechanical properties of rolled steels containing up to about 0.8 pct Si and 1.0 pct Cu in rolled and heat-treated conditions also are being investigated.
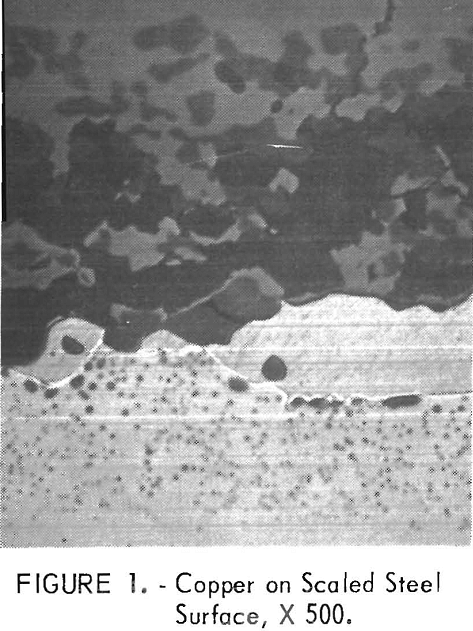
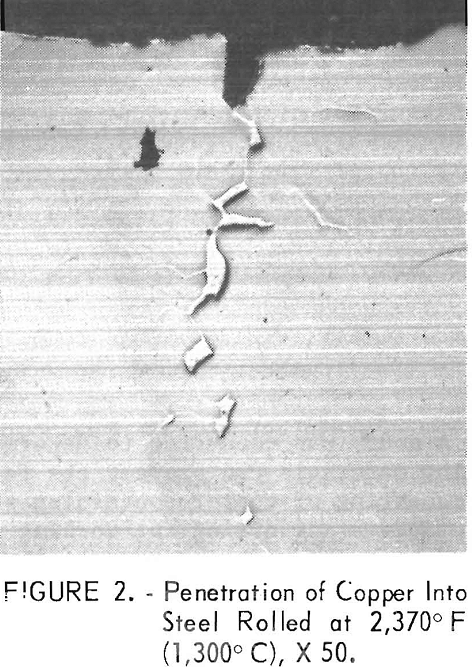
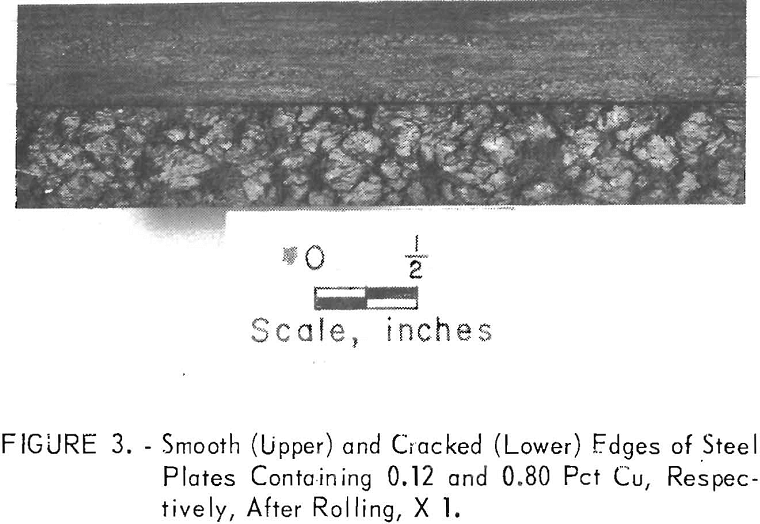
The problem is that copper-containing steels can exhibit surface hot-shortness when such steels are heated and hot-worked. When steel ingots are heated in oxidizing atmospheres to rolling temperatures, unoxidized molten copper is formed on the steel surface as the iron is preferentially oxidized (fig. 1). The molten copper deeply penetrates the grain boundaries (fig. 2), especially on the edges of flanged sections where tensile stresses occur. The copper penetrations weaken the steel surface and deep cracks develop, as illustrated in figure 3, after rolling a steel ingot containing 0.8 pct Cu at 2,370° F (1,300° C). For comparison, the normal smooth edge of a steel with a low copper content, 0.12 pct, is included. The cracked surfaces are detrimental because the cracks promote corrosion or propagate when the steel is bent. Also, the cost of labor and material losses is severe if it becomes necessary to trim off the cracked edge.
Several approaches have been used or studied for removing copper before or during melting, or to make copper innocuous after melting. The predominant method is to remove from the scrap those portions high in copper by hand-sorting or by shredding, screening, and magnetic separation before melting.
Removal by fluxing during melting has met with limited success. Nickel has been added to make the copper innocuous. Although nickel was believed to help by raising the melting point of copper, Salter discovered that nickel was primarily of benefit by increasing the solubility of copper in steel. All these techniques considerably increase the cost of steel.
Steel producers have long observed that surface hot-shortness was less when steel was heated to high temperatures before rolling. Dissipation of surface copper into steel by bulk diffusion or into the scale by occlusion have been considered as explanations. Recently Cox and Winn concluded from their work that the satisfactory hot-working at 1,100° C (2,012° F) of a complex high-copper steel was due to the formation of subscale containing FesSiO4. The steel contained 0.22 pct C, 1.7 pct Si, 1.03 pct Mn, 0.8 pct Mo, and 1.83 pct Cu. The fayalite (Fe3SiO4), formed in the subscale by internal oxidation of silicon and iron, was believed to reduce the outward diffusion rate of iron, which, in turn, resulted in the formation of a viscous iron-copper alloy at the steel surface. The viscous alloy would have less tendency to wet the grain boundaries than copper.
The decision was made by the Bureau of Mines as part of its program to develop methods to promote accelerated use of solid waste, such as scrap automobiles, to study the use of inexpensive alloying additions or coatings to reduce surface hot shortness. Initially, isothermal scaling studies were undertaken over a range of temperatures to further explore the beneficial effect of fayalite (Fe2SiO4), as observed by Cox, in reducing the formation of copper on the steel surface. The study was expanded to include the oxides of aluminum and boron, also formed on the steel surface by internal oxidation of these additions to steel. The silicon contents and simulated ingot heating conditions necessary to prevent the formation of copper on steels containing 0.6, 0.8, and 1.0 pct Cu were investigated. Borax coatings applied to preheated steels with either no silicon or low silicon contents and 1 pct Cu were also studied.
Experimental Procedure
Six series of steels were prepared. Three series of steel buttons with nominal contents of 0.2 pct C, 0.45 pct Mn, and 1.0 pct Cu (table 1) were used for the isothermal scaling studies. The button alloys were used for determining the effects of silicon, aluminum, and combinations of silicon, aluminum, and boron contents as well as of temperature on the formation of copper on the steel surface. Three series of small steel ingots (table 2) were employed for determining the amount of silicon required and the simulated ingot reheating conditions necessary to occlude copper in the oxide scale of steels containing 0.6, 0.8, and 1.0 pct Cu.
The steel buttons and ingots were prepared by different procedures. The elongated steel buttons were made by nonconsumable-electrode arc melting 100-g compacts of the desired compositions. To obtain homogeneous, coarse-grained shapes with a 5/8-in-sq cross section, the buttons were homogenized for 6 hr in a vacuum, forged, and reannealed, all at 2,190° F (1,200° C). The small ingots were prepared by induction melting of SAE type 1020 steel bar stock under a protective argon cover. The 65- and 100-lb heats were aluminum killed and poured into one mold with a 4-in-sq section or two molds with a 3-¼-in-sq section, respectively; both sizes of molds were tapered. The 10-lb hot tops were cropped from the ingots, and, starting at 2,370° F (1,300° C) , the ingots were rolled in eight passes to ½-in-thick plate. Ground, cube-shaped specimens were prepared from the shapes. Sections of worked steels rather than cast steels were used in the scaling tests in order to reduce the number of variables to study. The worked steels used were very uniform in composition, but there was dendritic segregation at the surface of the ingots.
Isothermal scaling was conducted on specimens from the button-size steels in an electrically heated, box-type furnace with good air circulation. The samples were placed on type 310 stainless steel sheet sections, which, in turn, were put on an alumina plate. Because of the stainless steel’s resistance to scaling and good contact of it with the unsealed bottom portion of the specimens, the stainless steel maintained the specimens at the intended temperature by dissipating the exothermic heat of oxidation occurring on the exposed specimen surfaces. The assembly was isothermally heated at temperatures ranging from 1,830° F (1,000° C) to 2,370° F (1,300° C). A Pt-Pt 13-pct Rh thermocouple spot welded to the specimen and/or a calibrated optical pyrometer were used for temperature measurement.
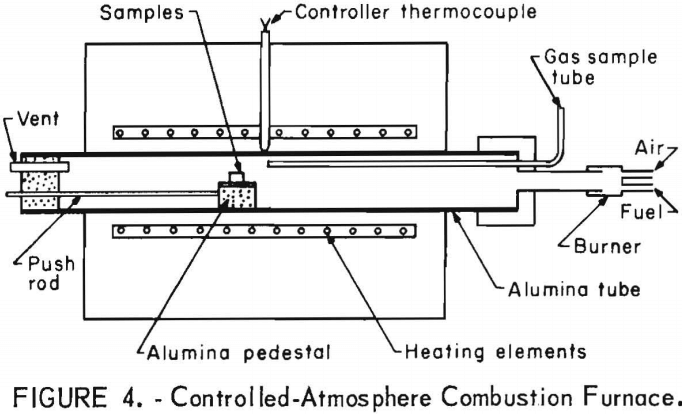
The unit used for simulated ingot-reheating, scaling studies is illustrated schematically in figure 4. The effects of ingot reheating schedules, furnace atmospheres, and steel composition were studied in this unit. Simulated pusher and soaking-pit heating schedules consisted of continuously heating the specimens in burnt natural gas-air mixtures from 390° F (200° C) to 2,370° F (1,300° C) in 2 to 2-½ hr and from 1,470° F (800° C) to 2,370° F (1,300° C) in 8 hr, respectively. Four specimens were placed together, perpendicular to the tubes length, on a stainless steel plate, which, in turn, was put on a rounded-bottom alumina plate. This assembly was placed in the furnace tube at the location of the desired starting temperature, allowed to reach equilibrium, and then mechanically pushed toward the furnace center at a uniform rate. Before pushing the sample, the air and natural gas flows at the burner were adjusted. Natural gas, containing 93 vol pct CH4, and air were burned to produce gases with linear flow rates of 45 to 50 ft/min and 2 or 9 ±1 vol pct excess oxygen contents at the tube center. The burnt gases
contained no carbon monoxide. Temperatures of the samples, atmosphere, and furnace tube are illustrated in figure 5. The distance is the length from
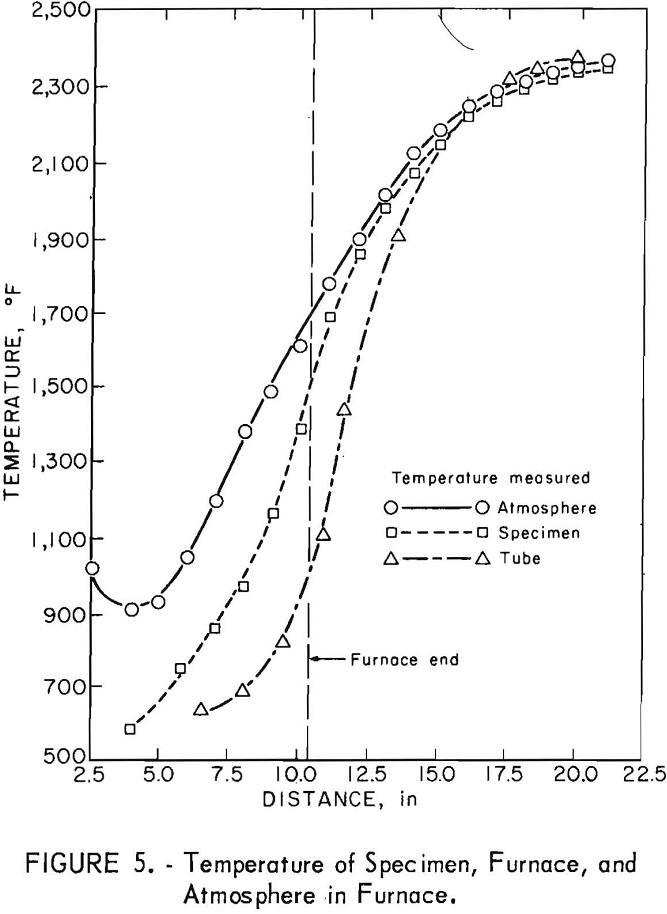
the end of the alumina tube protruding from the furnace. The atmosphere and tube temperatures were determined by placing Pt-Pt 13-pct Rh thermocouples between the specimen and the top of the tube and between the alumina plate bottom and the tube. The same type of thermocouple was spot welded to the specimen to determine its temperature.
When studying borax coatings, the specimens were preheated, then coated. The specimens were preheated in air at 1,470° F (800° C) or 1,830° F (1,000° C) for ½ hr to form a scale similar to that found on ingots after hot stripping, and then they were coated rapidly. For dip coating, the borax salt was heated to 1,830° F (1,000° C) in a fire-clay crucible; then the samples were immersed. Coatings were also applied by sprinkling on the surface at both temperatures. Borax, anhydrous borax, borax glass, and borax glass plus feldspar or silica coatings were studied.
Several techniques were used in preparing and examining the scaled samples. The scaled samples were vacuum-pressure mounted in epoxy for visual and electron-beam microprobe analysis. The mounts were sectioned with a water-cooled diamond saw, remounted in epoxy to seal cracks in the scale, and ground and polished by standard techniques. The polished samples were etched with a 2-vol-pct nital solution when desired, but not for the microprobe studies; plastic-carbon (chromium shadowed) replicas were used for electron microscopy studies. Limited phase studies were made by X-ray diffraction of unground and ground scale surfaces.
Results and Discussion
Isothermal Scaling Studies
General Scale Zones
All the steels produced scale structures similar to those illustrated in figure 6. (The relative thickness of the scale layers and zones to each other are not necessarily those observed.) These are the same structures as reported by Cox and Winn and Moreau and Cogrett. Outward from the steel matrix, the structural zones consisted of the decarburization and internal oxidation zones (which over-lap) , subscale, and scale proper. The decarburization zone is not further discussed in this paper. During preliminary tests of copper
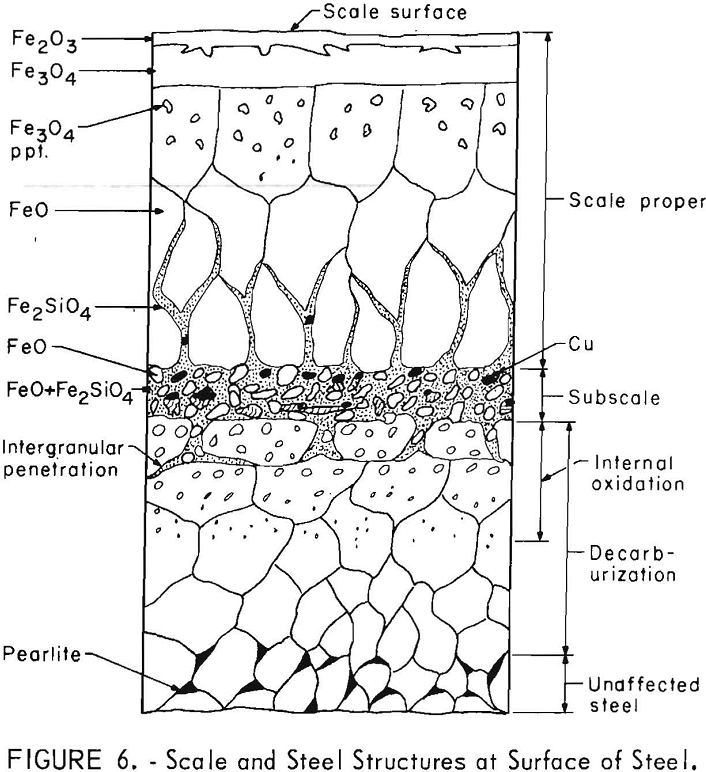
steels with 0.0 and 0.6 percent C, the carbon content was found to have no effect on whether copper was formed on the steel surface or occluded in the scale. Many studies have shown that carbon can cause blistering on ingots by formation of carbon monoxide at the steel surface, but blistering was not observed in the present study.
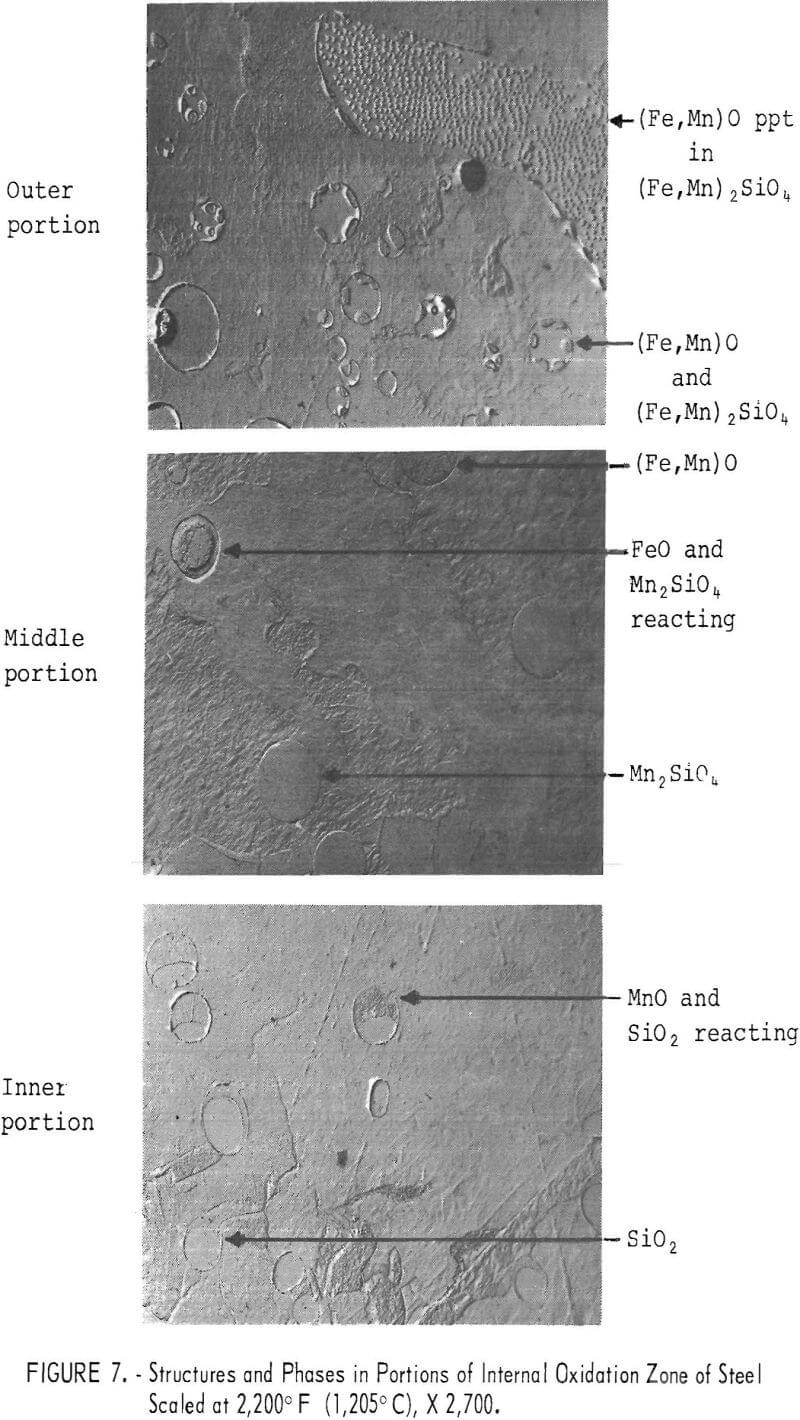
The phases occurring in the internal oxidation zone were very complex. The solutes with high, negative free energies of oxide formation (Si, Al, and B) oxidized in the innermost portion. These then reacted with manganese oxides and then with iron oxides as the oxides of Si, Al, and B progressed outwardly during scaling. Typical examples of structures and oxide phases, as identified by microprobe analysis, in portions of the zone are illustrated in figure 7 for a steel containing silicon. Because of oxidation of iron and the solutes other than copper, the concentration of copper increased to the steel surface. As the oxides grew in size near the surface, they tended to coalesce, especially with higher silicon and aluminum contents. The molten oxides, when formed, tended to penetrate steel grain boundaries to shallow depths but no deeper than the internal oxidation zone. The thickness of the internal oxidation zone at any temperature was dependent upon the silicon and aluminum content. The higher these contents, the thinner the zone.
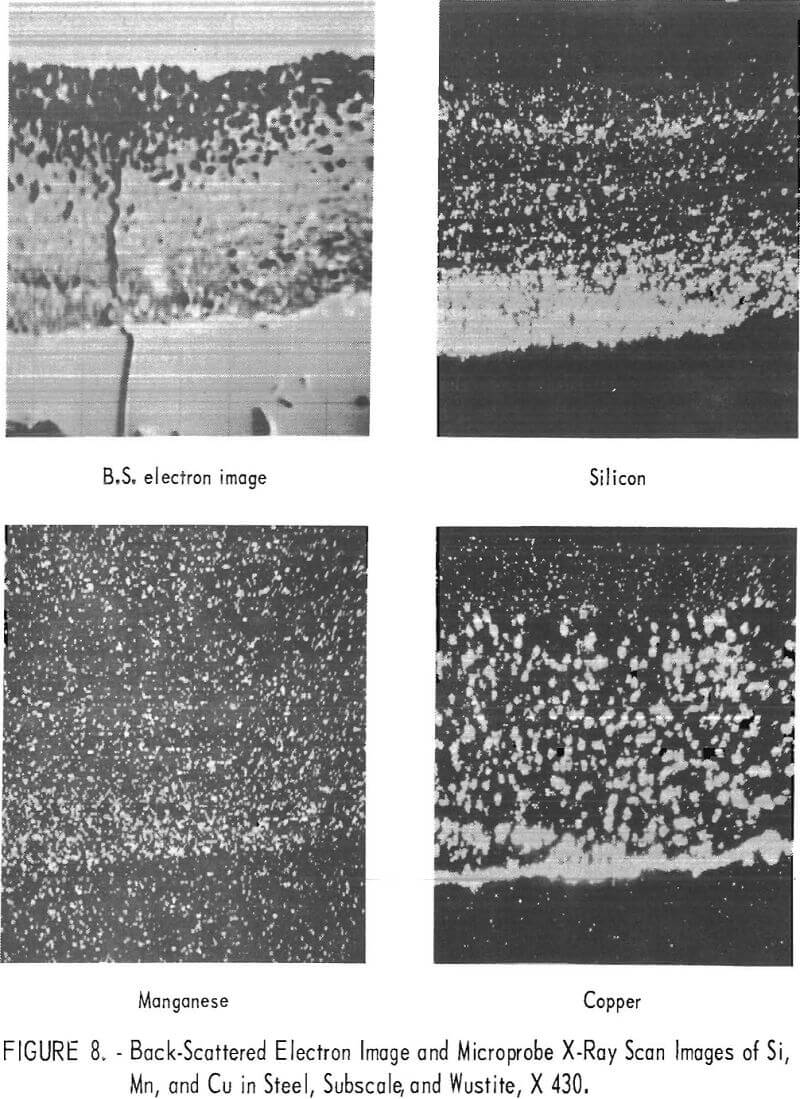
The oxide phases present in the subscale were the same as those finally formed on the exterior portion of the internal oxidation zone. Almost all of the oxides of Si, Al, and B, as well as a portion of the FeO, concentrated in the subscale. These respective oxides were combined with FeO (wustite) to form Fe2SiO4 (fayalite), FeAl2O4 (hercynite), and complex borates. Manganese oxides were partitioned between wustite and fayalite or hercynite. A back-scattered electron image and X-ray-scan images of the distribution of Mn, Si, and Cu in a steel scaled at 1,830° F (1,000° C) are illustrated in figure 8. Concentration of these elements is indicated by the density of white dots. As can be observed in the subscale, the silicon content is higher and the manganese content is similar to that in the other scaling zones. A similar distribution of these elements was observed at all scaling temperatures. The distribution of copper and iron-copper metallic particles as well as the melting temperatures of the oxide phases will be discussed further in the text.
The scale proper consisted of Fe2O3 (hematite), Fe3O4 (magnetite), and FeO (wustite) layers, which occur inwardly from the scale surface in the order named. Once oxidation started, further scaling depended upon diffusion of oxygen through the hematite layer and diffusion of iron through the magnetite and wustite layers. The hematite layer was remarkably thin and uni¬form, but the magnetite was thicker. The wustite layer was usually many times thicker. Molten oxide phases penetrated into the wustite layer grain boundaries when those phases formed in the subscale.
Occlusion of Copper by Scale
Solid metallic copper formed on the steel surface below the melting temperature 2,000° F (1,094° C) of the copper-rich, iron-copper alloy, which contains about 4.0 pct Fe at this temperature. When the steel contained no silicon, the copper remained on the steel surface. The copper dispersed into
the subscale formed when Si, Al, and B were alloyed with the steel. This is illustrated in figure 9 for steel B4, which contains 0.48 pct Si, when scaled at 1,830° F (1,000° C).
Above 1,981° F (1,083° C), when copper melts and wets the steel surface, interactions at the steel surface become more complex. The observations made will be discussed in the succeeding sections according to the preferably oxidizable solutes alloyed with the steel.
Silicon (steel series I): The quantity of molten copper formed on the steel surface between 1,981° F (1,083° C) and 2,150° F (1,177° C) was

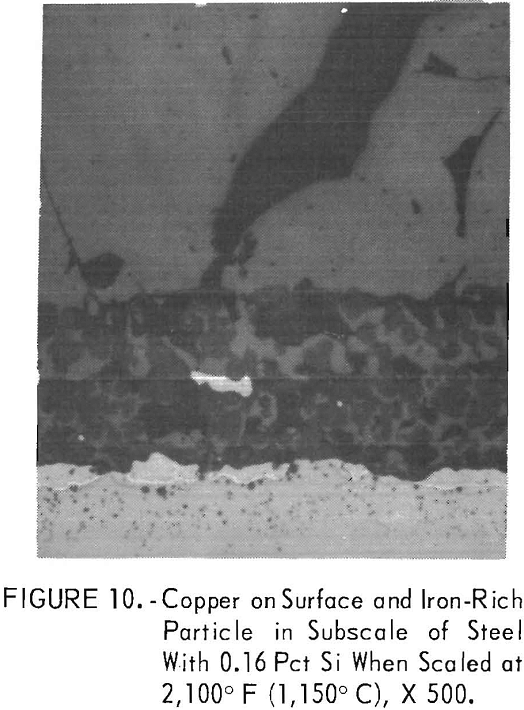
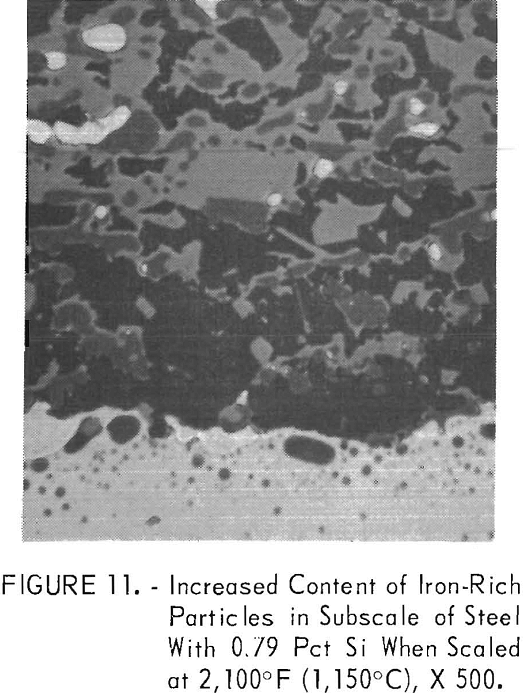
dependent upon the silicon content of the steel. Almost all of the molten copper stayed on the steel surface when 0.16 pct silicon was present (fig. 10, steel B2) . With increasing silicon contents, the amount of copper decreased. The decrease was due to the fact that in some outer portions of the metal in the internal oxidation zone, the solubility of copper in iron, about 8.5 pet, was not exceeded. As hypothesized by Cox and Winn, the reduced copper content of the steel matrix may be caused by solid fayalite reducing the outward diffusion rate of iron, upon which oxidation is dependent at the scale-steel surface. Simultaneously, the iron-rich, iron-copper particles tend to become entrapped in the subscale (fig. 11, steel B6) with increasing silicon contents by linking up of coalescing oxides at the outer portion of the internal oxidation zone.
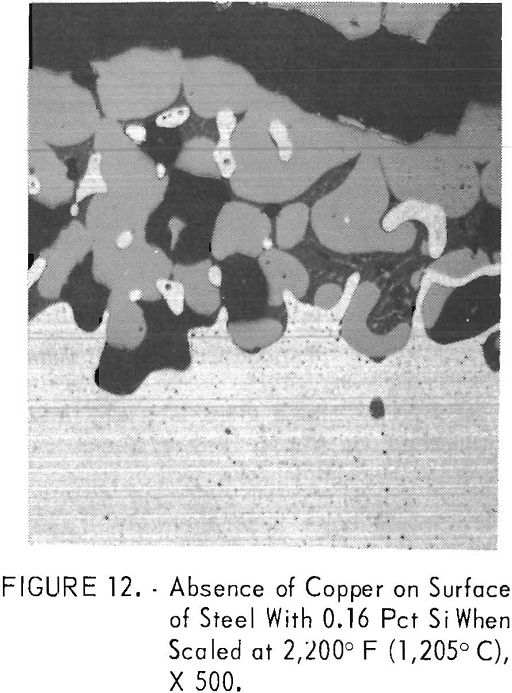
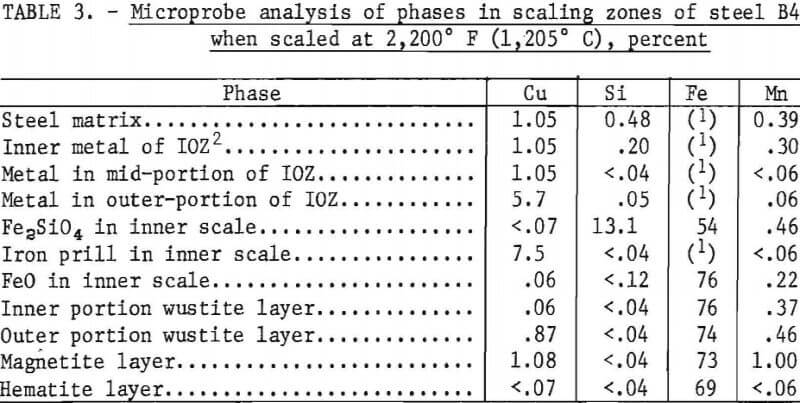
Much less copper was formed above 2,150° F (1,177° C) when molten oxides started to form on the steel surface by a eutectic reaction between fayalite and wustite. Complete prevention of copper formation did not occur for 0.79 pct Si steel B6 at temperatures between 2,150° F (1,177° C) and 2,200° F (1,205° C), the melting temperature of fayalite. In contrast to the observations of Cox at 2,012° F (1,100° C), the scaling rate increased at 2,200° F (1,205° C) and above, and only iron-rich, iron-copper alloys formed on the steel surface when molten fayalite was present. Iron-rich, iron-copper particles formed on the surface of steel B2, which contains 0.16 pct Si (fig. 12). The copper content of the iron- rich phase is about 7.5 pct. With increasing silicon content, the copper content of the iron-rich phase became even less, as shown for steel B4, which contains 0.48 pct Si (table 3). Also, distribution of the elements in all scale zones is shown. In contrast, only copper formed on the surface of steel Bl, which contains 0.05 pct Si.
The reasons for copper not forming on the steel surface in the presence of molten fayalite were not clear. Certainly molten fayalite would not increase the diffusion rate of copper and dissipate it into the steel matrix. Most probably the activity of molten fayalite is sufficient that copper as well as iron are dissolved in it and transported to the outer portions of the wustite layer into which the molten fayalite penetrates. And, the greater the fayalite content, the greater the depth of penetration.
High copper contents in the outer wustite layer are shown in table 3. The molten oxides also tended to penetrate deeply along steel grain boundaries within the internal oxidation zone. These penetrators tended to completely encircle metal grains as scaling proceeded, especially in the steels with low silicon contents; for example, 0.2 pct.
Aluminum (steel series II): Hercynite (FeAl2O4) formed in the sub-scale of steels containing aluminum. As with solid fayalite, it also reduced the scaling rate of steel and promoted the formation of iron-rich alloys on the steel surface. However, complete prevention of copper formation did not occur up to the highest temperature investigated, 2,370° F (1,300° C). Below this temperature, melting of hercynite or a eutectic reaction between it and wustite does not occur.
Silicon plus Aluminum, Boron, or Boron plus Silicon and/or Aluminum (steel series III): This series of steels was made to determine if the melting temperature of the oxide phases formed in the subscale layer could be lowered substantially below the fayalite and wustite eutectic, 2,150° F (1,177° C). The amount of copper formed on the steel surface at temperatures between the melting point of copper and the melting point of the oxide phase and the difficulty of removing the copper at higher temperatures may then be less during continuous heating of ingots.
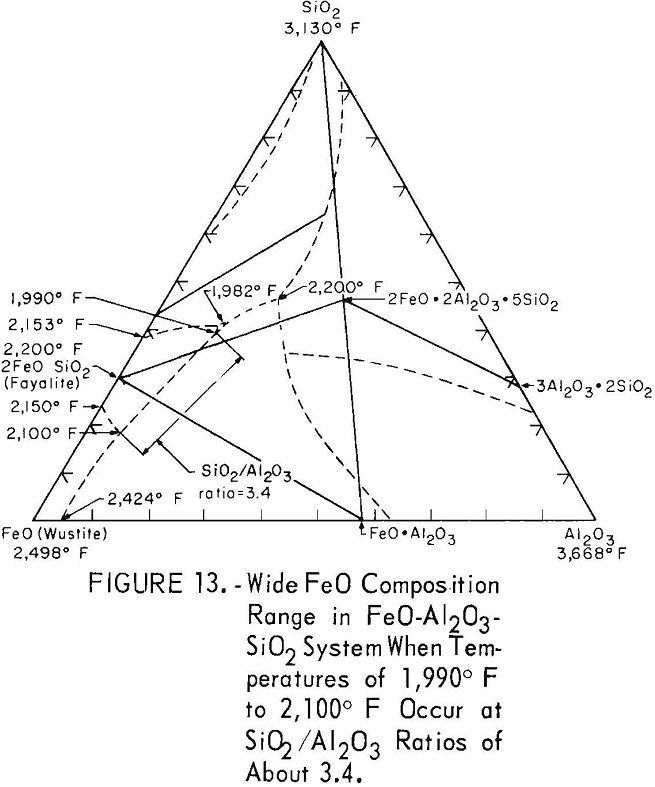
Melting may occur at temperatures of 1,990° F (1,088° C) to 2,000° F (1,093° C) over wide FeO contents in the FeO-Al2O3-SiO2 system when SiO2 and Al2O3 are present at ratios of about 3.4 to 1 (fig. 13). These oxide ratios may be attained in the subscale when steel containing silicon and aluminum contents with ratios of about 3 to 1 are scaled. However, molten oxides were not observed below 2,100° F (1,150° C) when steel B17 of series III, containing silicon and aluminum in these ratios, was scaled. Lower temperatures were found to not occur because the SiO2 + Al2O3 content of the subscale was too large.
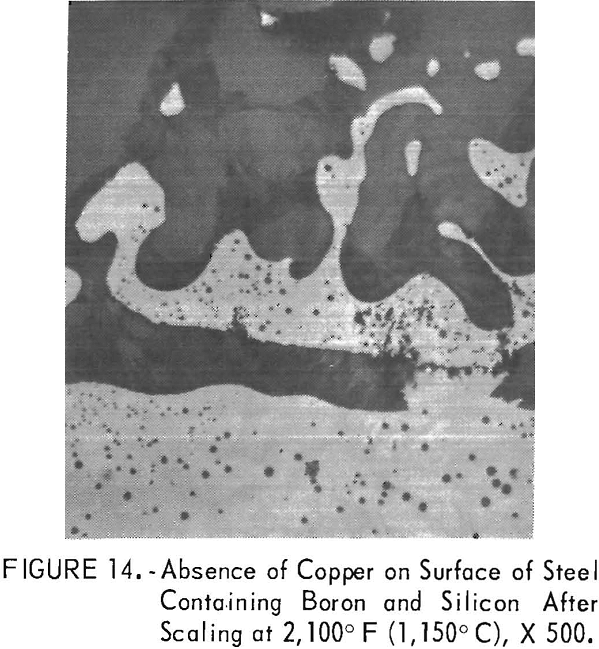
Excellent copper removal occurred in the steels B13 and B15, which contain boron and boron plus silicon, respectively. The excellent removal of copper as iron-rich, iron-copper particles and the complex structure of the solidified boron-oxide phases in steel B15 after scaling at 2,100° F (1,150° C) is illustrated in figure 14. Further study of boron additions to steel was not pursued as carbon steels with over 0.007 pct B are hot short above 1,922° F (1,050° C) because of a eutectic reaction between Fe and Fe2B. Because of the excellent occlusion of copper by molten borates, the investigators conceived the idea that the same benefits may be achieved by coating steels with borax coating. This will be discussed later.
Simulated Ingot Heating Studies of Copper Steels Containing Silicon
The steel surfaces were subjected to all the temperature zones discussed under “Isothermal Scaling Studies.” When solid fayalite forms, copper is occluded in the subscale below its melting temperature. Above this temperature, 1,980° F (1,083° C), but below the melting temperature of fayalite, copper tends to wet and adhere to the steel surface. This tendency is reduced with increasing silicon contents. Above the melting temperature of fayalite, 2,150° F (1,177° C) , an iron-rich, iron-copper phase, not copper, forms on the steel surface, even with quite low silicon contents.
During preliminary simulated-heating studies, it was obvious to the investigators that reduction of the amount of copper formed on the steel surface between the melting temperatures of copper and fayalite could be accomplished by reducing the amount of scaling. Less scaling could result from reducing the heating time and oxidizing potential of the atmosphere between these temperatures. However, neither of these variables can be readily controlled in pusher-type furnaces as presently designed. In soaking-type furnaces, both of these methods could be used; however, reducing the heating period is not very feasible if large ingots are heated. Reduction of time means higher atmospheric and ingot-surface temperatures, which result in large thermal gradients in the ingots and may in turn cause internal ruptures. Reducing atmospheres would be more easily applied. But this approach is also undesirable commercially because the burning efficiency is reduced. No attempt was made during subsequent studies to use atmospheres without excess oxygen at any temperature or to reduce the heating time employed between the melting points of copper and fayalite.
The silicon contents of steel series IV, V, and VI (table 2) and the heating conditions necessary to prevent copper from forming on the steel surfaces on heating to 2,370° F (1,300° C) per pusher or soaking-pit furnace operations are shown in table 4. As can be observed, the conditions necessary for a steel with a given copper content were not significantly different for either type of operation. Only 0.18 pct Si, a normal content in commercial steels, and a 2-vol-pct O2 content of the atmosphere were necessary for a 0.56-pct-Cu steel for either type of furnace operation. Considerably more stringent requirements were necessary for 1.0-pct-Cu steels.
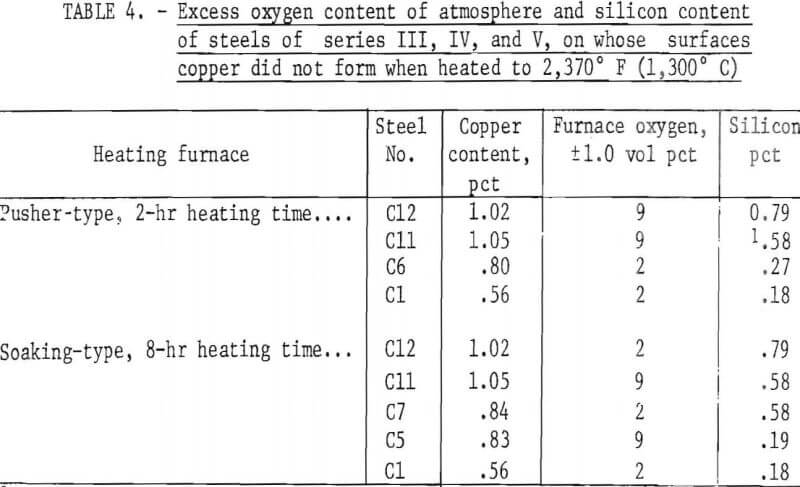
The copper formed on steels containing <0.28 pct Si and 1 pct Cu was especially difficult to remove when continuously heated above 2,200° F (1,205° C). The copper tended to collect at the root of molten oxide stringers that formed along the steel grain boundaries, as illustrated in figure 15 for steel C9. The deep copper penetrations were removable but only by increasing the scaling time above 2,200° F (1,205° C).
Whether or not copper formed on the surfaces of the steel specimens placed adjacent to each other depended upon their fit. When together with their surfaces parallel, little scaling occurred and no copper formed. When separated enough to limit accessibility of these surfaces to the atmosphere, less copper formed on these surfaces when heated to 2,200° F (1,205° C) than
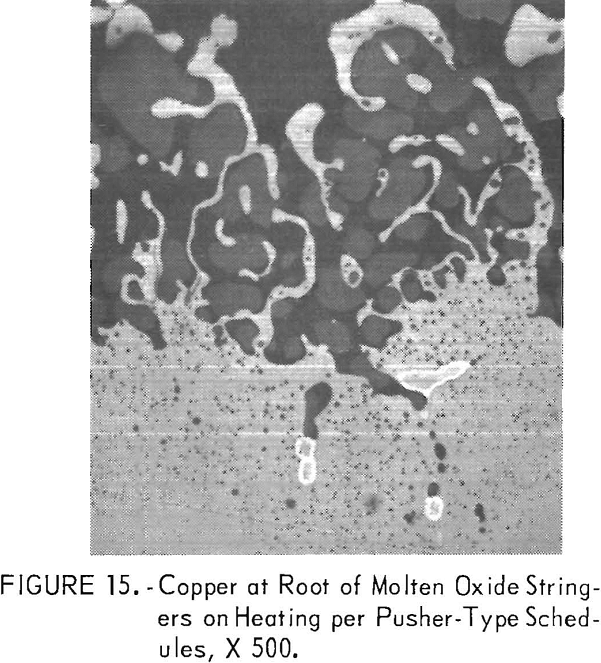
fully exposed surfaces, as expected. However, the copper tended to remain on the adjacent surfaces when heated to 2,370° F (1,300° C). These observations indicated that steel surfaces require full exposure to or complete exclusion from the oxidizing atmosphere when heating to rolling temperature; this prevented copper from forming on the steel. For these reasons, copper would tend to form on the contacting surfaces of ingots (whose surfaces are usually irregular) when heated in pusher furnaces unless they are separated.
From the studies, it was concluded that surface hot shortness by copper could be minimized by (1) increasing the silicon content of the steel, (2) increasing the heating time and oxygen content of the atmosphere above 2,200° F (1,205° C) , and (3) allowing full exposure of the steel surfaces to the furnace atmospheres.
Borax Coating Studies
Because boron was found in the isothermal scaling studies to be an excellent ingredient for preventing copper formation on the steel surface, it was decided to determine if the same benefit could be accomplished with coatings containing borax and possibly other ingredients. The authors expected that borax coatings would adhere to the thin scale present on steel ingots after they have been hot stripped from molds. On heating, the coating containing borax could react with the iron oxide scale to form an iron borate and other iron oxide compounds which would stay on the steel surface as an inner scale during subsequent scaling.
Originally, coating tests were performed on an Fe 1-pct Cu alloy. This alloy was selected so that performance of the coating could be evaluated by eliminating interferences from carbon, manganese, and silicon oxides formed in the inner scale during scaling. The alloy was prepared in the same manner as the button-size alloys. Sections of the alloy were coated with powders of hydrous and anhydrous borax and borax glass. The powders were applied by a “salting” action after the sections were heated to 1,830° F (1,000° C) for ½ hr. Afterwards, they were heated at 2,280° F (1,250° C) for 2 hr and examined metallographically. Copper was found on the surface when the alloy was coated with hydrous and anhydrous borax but not with borax glass. The structures with borax glass were similar to those shown in figure 14, but much coarser.
Next, coated sections of the Fe 1-pct Cu alloy and steel C9 (containing 0.28 pct Si and 1.09 pct Cu) were heated to 2,370° F (1,300° C) per simulated pusher operations as previously described. The coatings consisted of borax glass and borax glass plus 25 pct feldspar. The coatings were applied by salting at 1,470° F (800° C) or immersion of samples in melts heated in a crucible to 1,830° F (1,000° C) after scaling the samples for ½ hr at the same temperatures. Prevention of copper formation varied according to the type of coating, coating method, and application temperature. Fair results were obtained on the Fe 1-pct Cu alloy after coating with borax glass by dipping, but not by salting. The subscale produced was very thin. Dip coatings containing 25 pct feldspar gave better results. The greater viscosity imparted by the silica content of the feldspar prevented the coating from running off the steel scale until the coating could react with scale to form less fluid iron borates and silicates during initial heating stages. The best results were obtained when the same coating was applied to steel C9. The structures near the scale-steel interface were very similar to those illustrated in figure 14. Retention of borax as complex phases in the subscale was demonstrated by microprobe analysis, listed as follows:
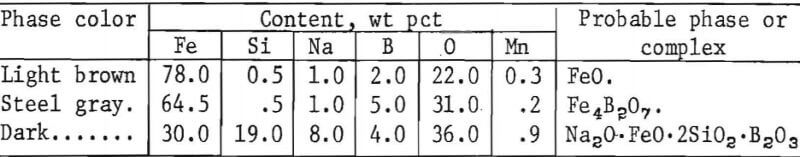
Afterwards, coated sections of steel C9 were heated by a soaking-pit operation. The coating consisted of borax glass and borax glass plus 25 pct feldspar. The samples were applied by dipping or salting at 1,470° F (800° C) or 1,830° F (1,000° C), respectively, after scaling for ½ hr at these temperatures. The subscales produced when heated to 2,370° F (1,300° C) were very thin, and complete copper removal was not observed at any of the conditions used. Because of the initial temperature, 1,470° F (800° C), at the start of the simulated heating schedule, the investigators believed that the coatings were too thin and ran off the sample before the coating could react with the scale to form less viscous iron compounds.
The effect of varying feldspar and silicon contents of borax coatings on simulated soaking-pit operations was then studied. Sections of steel C9 were scaled at 1,830° F (1,000° C) for ½ hr, and the coatings were applied at the same temperature by salting. Of the compositions studied, complete copper removal was obtained with those borax coatings containing either 50 pct feldspar or 30 pct silica.
Thus, copper could be prevented from forming on the steel surface during either simulated pusher or soaking-pit furnace operations by using borax coatings. High silicon contents in the coatings, either as feldspar or silica, were necessary to prevent copper formation when using soaking-pit furnacing. In contrast, borax glass alone would suffice for the pusher operation. Thus, the potential of using borax coatings to prevent the formation of copper was demonstrated. Borax coatings were effective for steels containing about 1.0 pct Cu and 0.3 pct Si and as low as 2 pct excess O2 in the furnace atmosphere, whereas much higher silicon and oxygen contents were necessary when using silicon additions to prevent copper formation on the steel surface.
