Table of Contents
This investigation has demonstrated that finely divided titanium metal, titanium lower chlorides, or a mixture thereof can be produced at a temperature between 105° and 205° C. in a continuous operation. The relative quantities of metallic titanium and lower chlorides produced by the reaction are dependent upon the ratio of the feed materials used. For example, with a sodium-to-titanium tetrachloride molal ratio of about 4 to 1, the titanium in the product is nearly all in the metallic state; with a sodium-to-titanium tetrachloride molal ratio of 2 to 1, the titanium in the product is mainly in the form of lower chlorides. Saint-Claire Devilie attempted the reduction of titanium tetrachloride with sodium in 1855, but it was not until 1910 that Hunter succeeded in preparing relatively pure titanium metal by that method. In recent years researchers have employed many variations of technique in the sodium reduction process. The U. S. Industrial Chemical Company and Quin employed a technique involving the use of a sodium coating on finely divided inert solids.
The Bureau started work on sodium reduction of titanium tetrachloride at low temperatures in ball-mill reactors in an attempt to develop a continuous process for the production of titanium metal as well as a titanium lower chlorides-sodium chloride mixture for use in a titanium electrorefining cell as an electrolyte. Because of atmospheric contamination of the product through mechanical difficulties that appeared to be inherent in the ball-mill reactors, this approach was abandoned in favor of the reduction technique using a sodium coating on inert solids.
The early work on reduction of titanium tetrachloride with a sodium coating on inert solids was conducted in a horizontal reactor on a batch basis. Results showed its advantages over the ball-mill reactors, and further improvements were made by placing the reactor in a vertical position and making the process continuous with automatic feeding of the reactants and discharging of the product.
This report includes information on the construction and operation of the vertical reactor and analytical data on the reduction products.
Theoretical Aspects
Reduction of Titanium Tetrachloride With Sodium
Production of metallic titanium and titanium dichloride may be represented by the following overall reactions:
4Na(l) + TiCl4(g) = Ti(s) + 4NaCl(s)…………………………………………(1)
and
2Na(l) + TiCl4(g) = TiCl2(s) + 2NaCl(s)…………………………………….(2)
In producing titanium dichloride, some titanium trichloride is also formed by the reaction,
Na(l) + TiCl4(g) = TiCl3(s) + NaCl(s)…………………………………….(3)
The free energies and heats of these reactions, calculated from thermodynamic data reported by Kelley and Mah are presented in table 1.
The values indicate that the reactions are exothermic and should proceed spontaneously, at low temperatures.
High-Surface Sodium
The term high-surface sodium (H.s.s.), is applied to films of sodium approaching colloidal dimensions (0.5 to 1,000 millimicrons), spread over inert solids of high surface area.
Sodium in this form possesses a very large reacting surface. When the thickness of the sodium films and the exothermic nature of the subsequent reaction with titanium tetrachloride are considered, it becomes apparent that, once the reaction starts, the heat of reaction will supply more activation energy for reaction and the reaction will proceed very rapidly to completion.
Practical Considerations
Reduction of titanium tetrachloride with H.s.s. is accomplished by adding molten sodium and vaporized titanium tetrachloride simultaneously to an agitated bed of finely divided inert solids. Sodium coats the inert solids in exceedingly thin films and reacts with the titanium tetrachloride.
Sodium chloride is used as the H.s.s. carrier for starting the operation afterwards, the reaction products are used. Since in either case the carrier is one of the reaction products, no additional separation problem is incurred by the use of a carrier.
By keeping the reaction temperature below the melting points of the reaction products, titanium lower chlorides and sodium chloride, and by keeping the reaction mixture sufficiently agitated to prevent agglomeration of particles, the product mixture is maintained in a free-flowing state, which makes possible the continuous discharge of the products.
Heat may be added or taken away from the reaction system by an external heat exchanger; however, adjusting the feed rates of the reactants allows a more effective control over the temperature.
Apparatus
A schematic drawing of the apparatus is shown in figure 1.
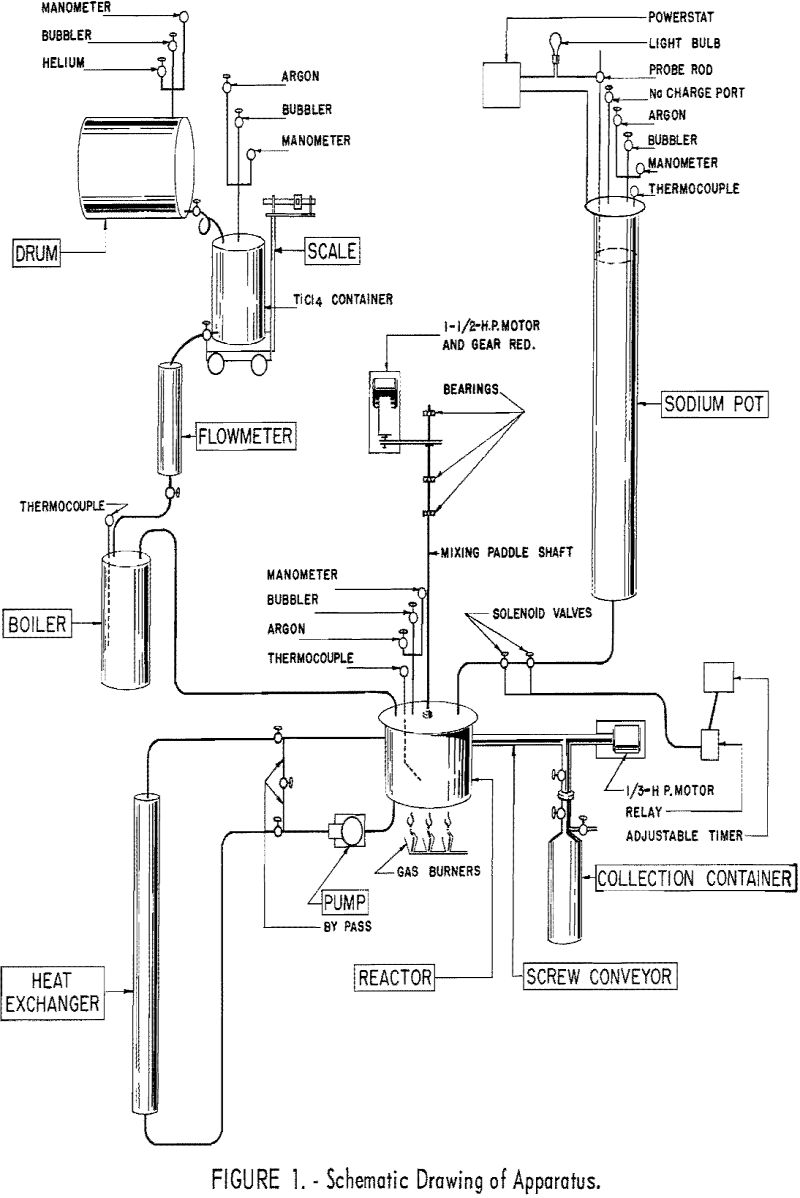
Titanium Tetrachloride System
Purified titanium tetrachloride was stored under helium in a steel drum which was connected with a flexible steel hose to a 2-gallon steel feed container mounted on a scale. The outlet of the weighing container was connected with a flexible steel hose to a flowmeter that discharged into a mild steel boiler. The vaporized titanium tetrachloride was fed to the reactor. The boiler and the pipe carrying the vaporized titanium tetrachloride were electrically heated with nichrome elements.
Sodium System
Molten sodium was charged under argon to a mild steel pot, 6 inches in diameter by 36 inches long. In the cover of the sodium pot, fittings were provided for a thermocouple well, a manometer, an argon inlet, an oil bubbler, a sodium-charging port, and a sodium-level measuring rod. The upper end of this probe rod was wired, in series, to a 25- to 50-volt power source, an electric light bulb, and the sodium pot; the lower end of the rod was inserted into the sodium pot through a rubber seal and an insulating glass tube. When the sodium level was being measured, the probe rod was pushed down to make contact with the surface of the molten sodium, closing the electric circuit, as indicated by lighting of the electric bulb. The weight of sodium was then calculated from the measurement of the sodium level.
Liquid sodium was transferred to the reactor through a ½-inch mild steel pipe connected to the bottom of the sodium pot. In this line, there were two 3/8-inch solenoid valves connected to an adjustable automatic timer and relay switch for controlling the flow rate. Heat was applied to the sodium pot and the line carrying sodium by nichrome electric heating elements.
Reactor
The reactor was fabricated from a mild steel pipe section, 14 inches in diameter and 9 inches high, with a ½-inch thick mild steel plate welded to the top and the bottom. A baffled jacket was provided at the walls and the bottom of the reactor.
A U-shaped, mild steel mixing paddle was installed in the reactor with only 1/8 inch of clearance from its walls. The mixing paddle was chain-driven by an electric motor and was held in alinement at the upper end of the drive shaft by three bearings, which were supported on the superstructure of the reactor stand. Sealing the mixing-paddle shaft to the reactor cover was accomplished by a water-cooled packing gland.
Other fittings in the reactor cover were: A titanium tetrachloride vapor inlet, a sodium port for feeding the molten reducing agent near the center of the reactor, a thermocouple well, and lines for argon, bubbler, and manometer. The product-discharge port was located in the reactor wall, 1 inch from the top.
Gas burners below the reactor were used to supply heat to the reaction system when needed, as in the starting-up period.
Heat-Exchange System
The medium used in the heat-exchange system was a commercially available, chlorinated biphenyl. A pump circulated this heat-transfer medium through the reactor jacket and through a water-cooled heat exchanger when cooling of the reactor was desired. The water-cooled heat exchanger was bypassed, and gas burners beneath the reactor were used to supply heat when heating of the reactor, was desired.
Product-Discharge Mechanism
For discharging the product, a 2-inch-diameter pipe was connected at a 90-degree angle to the reactor wall 1 inch from the top. This pipe housed a horizontal, motor-powered screw conveyor, whose shaft end was sealed by a packing gland. The screw conveyor discharged the product into a vertical tube. This vertical tube had a rubber-sealed, ball-type valve and a flange for bolting on a product-collection container. The neck of the product-collection container also had a flange and a ball-type valve as well as a connection for evacuation of air and backfilling with inert gas. Thus, the product-collection container could be attached or detached with a minimum atmospheric contamination to the product and to the reactor system.
Materials for Reduction
Sodium was purchased in drums with the following information furnished by the supplier on its impurities; Nitrogen, none; calcium, less than 0.04 percent; and oxygen, approximately 0.2 percent. It was melted in the drum under argon and then passed through a sintered stainless steel filter into the sodium pot.
The titanium tetrachloride used was purified from technical-grade titanium tetrachloride by treatment with hydrogen sulfide and subsequent fractional distillation. Chemical analysis of this purified titanium tetrachloride showed it to contain the following percentages of impurities: Iron, less than 0.02; silicon, less than 0.10; sulfur, less than 0.02; vanadium, less than 0.10; and free chlorine, approximately 0.01.
Experimental Procedure
The reactor body and its cover (along with the stirring mechanism) were alined and welded together. After the reactor was proven to be leak-free, about 9 pounds of previously dried, minus-100-mesh, c.p.-grade sodium chloride was charged into the reactor. The titanium tetrachloride system, the sodium system, the product-discharge and collection mechanism, and the lines for argon, bubbler, manometer, and vacuum pump were then connected. A check for leaks in these connections was made before heat was applied to the reactor. When the temperature reached 110° C., the reactor was evacuated to approximately 27 inches of mercury for 2 hours and then backfilled with argon.
The mixing paddle then was set in rotation at 175 r.p.m. to stir the sodium chloride.
The sodium pot was heated to about 135° C. and the pipe between the sodium pot and the reactor to slightly above 100° C. An argon pressure of 3 p.s.i.g. was maintained in the sodium pot, and the sodium level was measured. Feeding of the sodium to the reactor was regulated by adjusting the timer to have the solenoid valves open for 1 second and closed for 60 seconds. About 3-½ ounces of sodium was added to coat the sodium chloride before starting titanium tetrachloride feed.
After the weighing container was filled with titanium tetrachloride, it was closed off from the storage drum and then opened to an argon source to establish and maintain a pressure of 3 p.s.i.g. The flow of titanium tetrachloride to the boiler, which was preheated to about 275° C., was set at about 20 ml. per minute. The line carrying the vaporized titanium tetrachloride to the reactor was heated to about 150° C.
These control settings for feeding sodium and titanium tetrachloride were used for reducing titanium tetrachloride to metal as expressed in equation (1).
Shortly after the initial period, heat of reaction would raise the temperature and pressure in the reactor. The pressure was relieved to ¼ p.s.i.g. through an oil bubbler in order to maintain an approximately constant pressure difference between the feed systems and the reactor to avoid excessive fluctuations in the feed rates. When the temperature in the reactor reached 160° C., the gas burners were turned off, and the heat-transfer medium was circulated through the water-cooled heat exchanger to maintain the reactor temperature between 105° C. and 205° C.
Finally, the motor powering the product-discharge conveyor was turned on to carry the product to the collection container. Thus, the H.s.s. reactor was set in a continuous operation.
To shut down the operation, the feeds and the product-discharge mechanism were turned off, but the mixing paddle and the water-cooled heat exchanger were kept in operation to cool the reactor and to prevent the residual material in the reactor from forming a solid mass. When the reactor had cooled to about 75° C., or well below the solidification temperature of sodium, the mixing paddle and the heat exchanger were turned off.
To remove product, the valves on the product-discharge tube and the neck of the collector were closed, and the collector was detached. The product was unloaded in an airtight box under argon.
For subsequent operation, the powder retained in the reactor was used as the H.s.s. carrier. When a different reaction product was desired, for example, titanium lower chlorides or a mixture of titanium lower chlorides and metallic titanium, the sodium-to-titanium tetrachloride feed ratio was adjusted to the corresponding values.
When the feed ratio was changed to produce a different product, about four times the weight of the material retained in the reactor had to be put through the reactor before the final product reached a consistent composition.
Analytical Methods
The products were powdery and pyrophoric. Samples were taken and weighed under an argon atmosphere. The analytical methods used were as follows:
Sodium – the zinc-uranyl acetate method.
Chloride – the silver chloride gravimetric method.
Total titanium – the zinc amalgam reduction-ferric ammonium sulfate titration method.
Soluble titanium – sample was dissolved in an acidic, ferric sulfate solution and filtered. (Ferric sulfate was used to inhibit the dissolution of the finely divided metallic titanium and to improve filtration.) The filtrate was reduced with zinc amalgam and then titrated with ferric ammonium sulfate.
Indicated titanium – sample was dissolved in potassium dichromate solution; diphenylamine sulfonate indicator and dilute sulfuric acid were added, and the excess dichromate was then titrated with ferrous ammonium sulfate solution.
AEV – the average effective valence of the soluble titanium (or the apparent average valence of the titanium existing as dichloride and tri-chloride) was determined by the following formula:
AEV = 4 – percent indicated titanium/percent soluble titanium
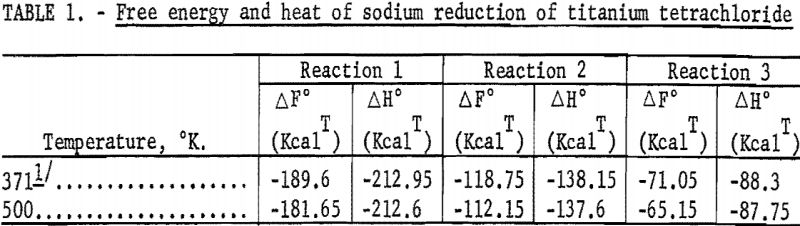
The product as discharged from the reactor was very difficult to analyze for indicated titanium; hence the value for the average effective valence of the soluble titanium (titanium in the chloride form) was determined from the analysis of a fused sample. This sample was prepared by melting a portion of the as-discharged product at a temperature of 820° C. under argon. Some reaction perhaps took place while the sample was being heated, but use of the fused sample was justified for the following reasons: (1) A melted sample could be more easily handled and more accurately analyzed for indicated titanium than the highly reactive as-produced powder. (2) Analytical data on the fused sample were useful, since any practical use of the H.s.s.-reduction product would entail the melting of the salt.
Results and Discussion
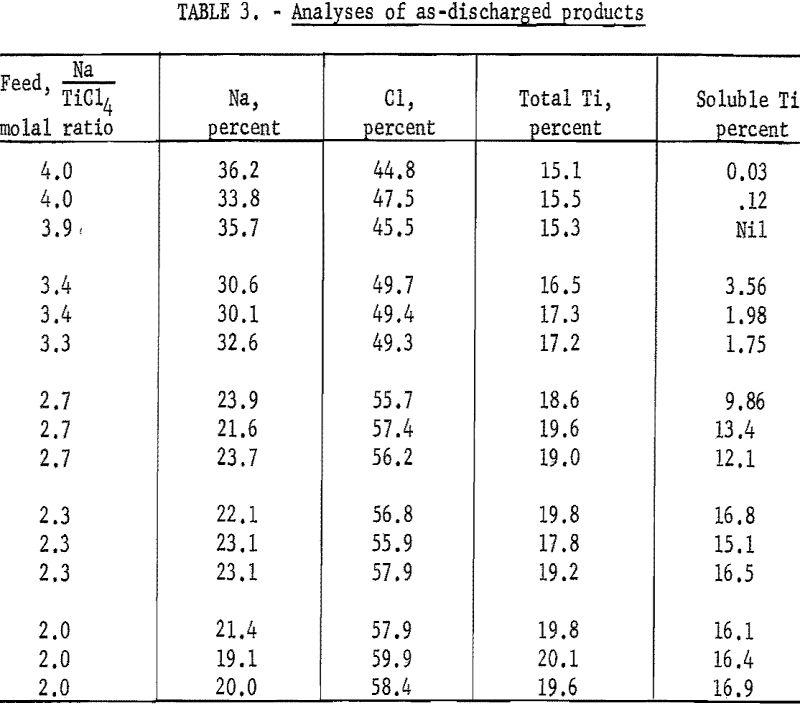
Reductions using sodium-to-titanium tetrachloride feed molal ratios in the range of 1.9 to 3.9 were made in the H.s.s. reactor at temperatures between 105° and 205° C. and at pressures between 0 and 2 p.s.i.g. When using feed ratios in the range of 2.4 to 3.3, the sodium and titanium tetrachloride feed rates and the product-discharge rate remained fairly constant, once the regulating instruments were adjusted. In operations using feed ratios of 1.9 and 3.9, frequent adjustments of the regulating instruments were required because of the pressure fluctuations that occurred. The operating data are presented in table 2.
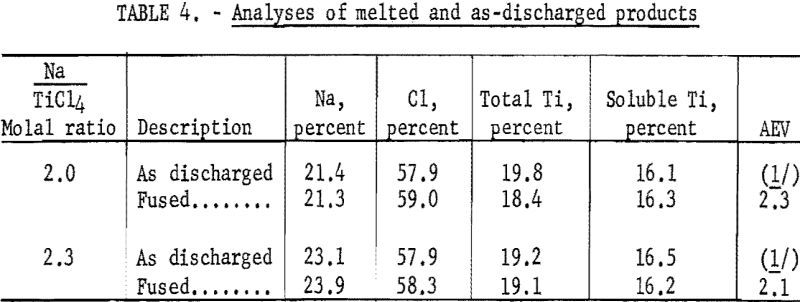
The reaction products as discharged from the reactor were in the form of fine particles in the micron size range. They were very reactive in air but could be handled safely under an inert atmosphere. Analytical data on the products, representative of operations using various feed ratios, are shown in table 3. These samples were taken after steady-state conditions in the reactor had been obtained.
The average effective valence of the titanium as lower chlorides in the products was calculated from the values of the indicated titanium and soluble titanium of fused samples. The analytical results of two samples are shown in table 4.
Attempts to extract titanium metal in the pure form from the powdery product mixture containing salt and metal by leaching or vacuum distillation proved unsuccessful. This metal, however, should be amenable to a recovery method reported by Hansley and Schott wherein the salt-metal mixture was heated at a temperature of about 850° to 1,050° C. to sinter the metal into a sponge mass before the molten salt was drained from the sponge metal under an inert atmosphere.
Product mixtures containing titanium lower chlorides and sodium chloride could be melted and then reduced with additional sodium to produce crystalline titanium metal or could be used as a source of titanium lower chlorides in preparing electrolytes for fused-salt electrorefining of titanium.