Table of Contents
A process was developed in which iron-contaminated zinc was refined to readily acceptable purity. Aluminum added to the molten impure metal reacted with the iron, forming a solid intermetallic compound, Fe2Al5, which floated to the surface of the melt. Small amounts of residual aluminum were eliminated from the product easily by fluxing with zinc chloride.
Filtration was evaluated as one method for separating the solid compound from the pure zinc. In an 80-pound test, zinc recovery was 95 percent. The iron content of the metal was lowered from 0.37 to 0.02 percent.
A centrifuge was designed and tested as an alternate method for making the solid-liquid separation. The centrifuge was a “dipping” type which, when lowered to the surface of the melt, pumped the floating solids to the interior where they were retained by a glass cloth filter medium. Product purity by centrifugation was comparable to that gained by filtration, but zinc recovery tended to be slightly lower.
This particular type of centrifuge would probably be most practical where it is desired to remove a relatively small amount of floating impurity from a large amount of molten metal. Filtration would be more commercially attractive than centrifugation, where the amount of solids to be removed is relatively large.
Filtration residues will still contain significant amounts of zinc. Recovery of this metal should be accomplished readily by normal distillation practice.
Previous Bureau of Mines research resulted in the development of a process for simultaneously refining zinc-base, die-cast scrap and galvanizers’ dross. The unique feature of the process is that the impurities contained in each material act as refining agents. When melted together, the aluminum in the die-cast scrap reacts with iron in the dross and forms one or more solid intermetallic compounds which, being lighter than zinc, will float to the surface of the melt. The solid compounds can then be separated readily from the residual zinc by some physical means, such as skimming, filtration or centrifugation.
The first investigators of this technique were Bornemann, Rutherford, and Schmidt who demonstrated that adding aluminum to galvanizers’ dross would cause the formation of solid compounds which they identified as FeAl3. The solid phase found in work done by the Bureau of Mines was identified as primarily Fe3Al5. In the Bureau’s work, the solid and liquid phases were separated by filtration. Optimum operating conditions were established for the process, and it was demonstrated that under these conditions 88 to 92 percent of the zinc can be recovered, containing only 0.01 percent iron and less than 0.2 percent aluminum.
The present report describes results of work in which the technique was studied further as a means for purifying zinc contaminated with smaller amounts of iron. Filtration was again used as a means for making the solids-liquid separation. Centrifugation, employing a novel centrifuge was developed as an additional separation method.
Present Practice for Removing Small Amounts of Iron from Zinc
Zinc produced by most smelting processes contains varying amounts of iron, depending on the process. Even the product from horizontal retort processes is frequently contaminated with iron as a result of the molten zinc contacting the charge, from corrosion of iron tools used in drawing and casting the condensate, and from entrainment of iron in the zinc vapor issuing from the mouth of the retort. The intense heat generated in some electric smelting processes will cause considerable contamination by iron as well as other impurities.
To make a marketable zinc product, most of these impurities, especially iron, must be removed. The two most widely used methods for refining a low-grade zinc product are liquation and redistillation. Liquation is the simplest means of lowering iron and lead contents to specification limits. Sometimes it is also used as an intermediate step prior to redistillation for crude zinc products containing considerable amounts of iron, thus minimizing the volume of residues to be removed from the retort. This operation normally consists of melting the metal in a reverberatory furnace and holding it undisturbed at a temperature just above the melting point long enough for the heavier lead and iron-zinc compounds to settle to the bottom. The refined zinc is drawn off and furnace temperature is increased to allow further concentration of lead and iron impurities. A small fraction of impure liquid zinc in the remaining top layer is withdrawn to be recycled. The furnace is cooled, freezing the iron-zinc dross and allowing the lead to be tapped. Finally the iron-zinc dross is remelted and withdrawn.
While this current practice is simple, it has two serious drawbacks. A fraction of the zinc must be recirculated to the smelting operation; also, the final iron-zinc dross ties up 35 to 50 pounds of zinc for each pound of iron removed, even though the amount of zinc in the solid iron-zinc crystals is only 12 pounds per pound of iron. A process that would eliminate or minimize these disadvantages would significantly reduce the amounts of dross requiring reprocessing.
Use of aluminum to react with iron would free the 12 pounds of zinc tied up in the iron-zinc compound of the final dross, and efficient filtration or centrifugation techniques would further free a large part of the 23 to 38 pounds of pure zinc associated with the dross. In addition, there would be no need to make the second zinc draw from the liquation furnace, thus eliminating this product that required recirculation.
Materials Tested
Iron contaminated zinc obtained for testing was made by an electric furnace process. Two separate lots of 55-pound slabs were received, and several slabs from each lot were cut into 1-inch cubes. Saw cuttings were saved for sampling. Average analyses of each lot are given in table 1. One lot contained considerably more iron than the other.
Commercially pure aluminum was used as the refining agent. This was alloyed with commercially pure zinc in a ratio of 1 part aluminum to 2 parts zinc for filtration tests. Enough alloy was prepared to satisfy the needs of the filtration tests. Alloy for centrifugation tests was prepared by melting aluminum with part of the zinc to be refined.
Segregation and Filtration Studies
Segregation Tests
The first experimental work was limited to segregation tests to verify that adding aluminum in the form of a 1- to -2 aluminum zinc alloy would be

a satisfactory method for causing the aluminum to react with iron in molten zinc. It was also of interest to learn if this method would result in the growth of sufficiently large crystals of the aluminum-iron intermetallic compound to permit effective filtration.
Iron-contaminated zinc was melted and heated to about 550° C in a tapered crucible. The stoichiometric amount of zinc-aluminum alloy reagent was added to provide enough aluminum to react completely with iron to form Fe2Al5. The melt was stirred for half an hour to allow uniform blending of the constituents. It was then allowed to cool for an hour undisturbed and to solidify. The solid ingot was sliced vertically in half, and the sectioned surfaces were polished and etched. The degree of segregation is shown in figure 1. The dark, narrow band at the top represents the solid aluminum-iron compound that floated to the top of the ingot. The lower part is shown to be practically free of the aluminum-iron crystals, demonstrating that the crystals were of sufficient size and had an adequate specific gravity difference to allow them to float readily to the top and to concentrate in a pasty, semisolid layer.
Description of Small-Scale Filter
The filter used for small-scale tests is shown in figure 2. This is identical to the one described in the report on refining of dross and
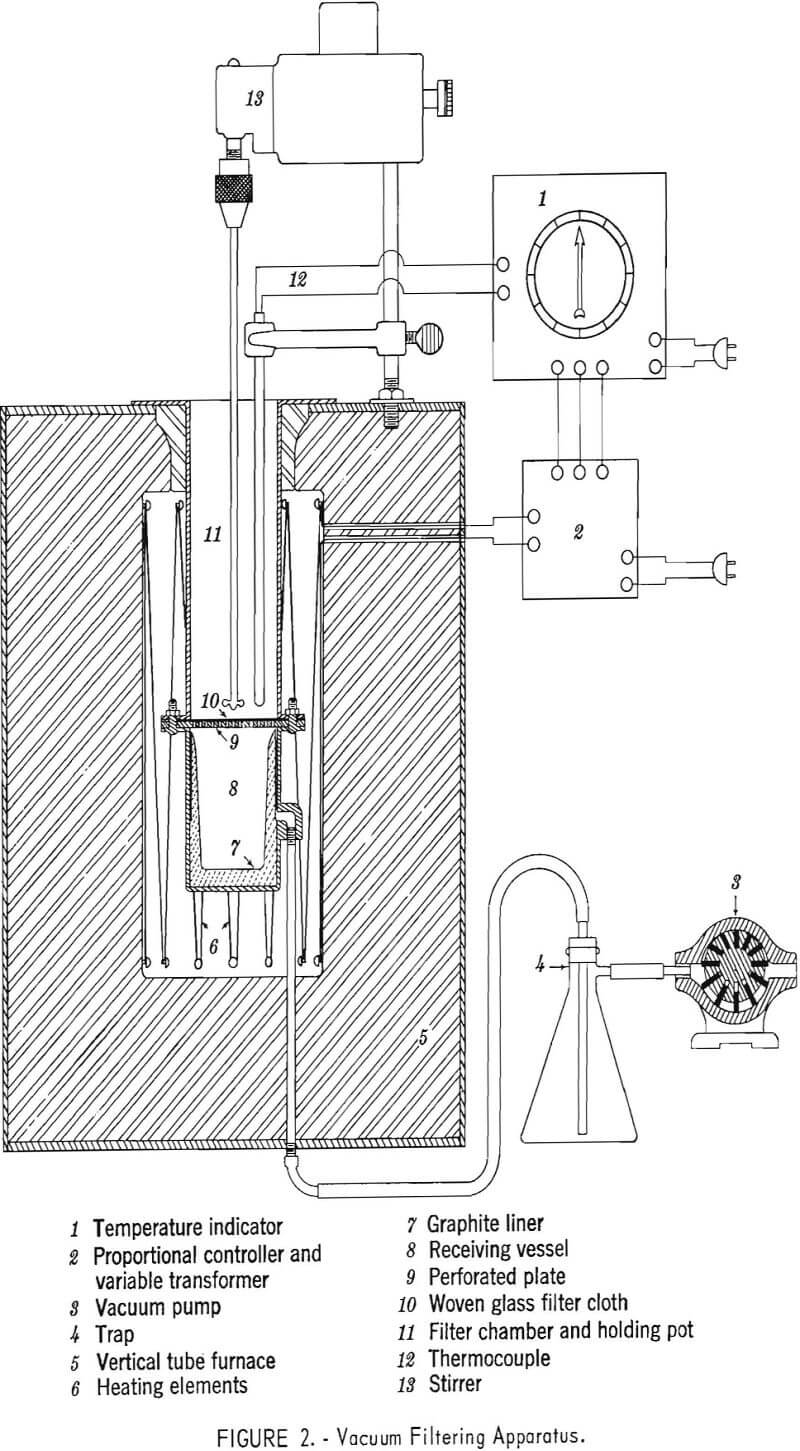
die-cast scrap. It was made of stainless steel and comprised an upper cylindrical member 3 inches in diameter which functioned both as a holding pot and as a filtering chamber and a lower graphite-lined receiving vessel. Both members were flanged, and a perforated stainless steel disk was bolted between the two flanges in order to support a layer of woven-glass filter cloth. Circular asbestos paper gaskets were used on the face of each flange. The inside of the upper chamber was coated with a bone ash compound used in the diecasting industry to prevent molten zinc from adhering to steel dies. The entire apparatus was contained inside a specially built, electrically heated crucible furnace. A motor-driven glass shaft with attached impeller was provided to agitate the contents of the holding pot. Temperature was regulated by means of an automatic indicating temperature controller with its thermocouple immersed in the melt. The lower chamber of the filter vessel was connected, through a trap, to a rotary vacuum pump.
Experimental Filtration Procedure
The effect on zinc recovery and purity of the following variables was studied: (1) Amount of aluminum-zinc alloy added, (2) holding or alloying temperature, (3) residence time at alloying temperature, (4) cooling rate to filtering temperature, and (5) temperature of filtration. Initial tests were made with no addition of aluminum to determine the amount of zinc retained in the iron-zinc filter cake. In each of the other experiments, a charge of 750 grams of iron-contaminated zinc was placed in the upper member of the filtering apparatus and heated to the holding temperature. After the zinc became molten, the stirrer was started. When the holding temperature was reached and became stabilized, the aluminum-zinc alloy was added. The melt was held and agitated at this temperature for a predetermined time and then allowed to cool. When filtration temperature was reached, the vacuum pump was turned on and suction was applied for 2 minutes. Filtration took place under a vacuum of 24 inches of mercury. Because of the fineness of the filtering cloth, no metal leaked into the lower chamber prior to filtration.
During filtration, the liquid zinc was drawn through the filter cloth and perforated plate into the lower chamber. The solid Fe2Al5 intermetallic compound remained behind in a disk-shaped filter cake about one-eighth inch thick. After completing the experiment, the filter apparatus was removed from the furnace and disassembled. The filter cake and filtrate were removed, weighed, and sampled for chemical analyses.
All tests made in this series were made using the low-iron (0.37 percent Fe) zinc sample referred to in table 1.
Results of Small-Scale Filtration Tests
Results of small-scale filtration tests are given in table 2. The first three runs were simple filtration tests with no aluminum added. Zinc recoveries were lower than in any other refining tests. The filter cakes from these tests contained a lower percentage of iron, verifying that the solid iron-zinc alloy ties up much more zinc in the cake than the solid Fe2Al5 intermetallic.
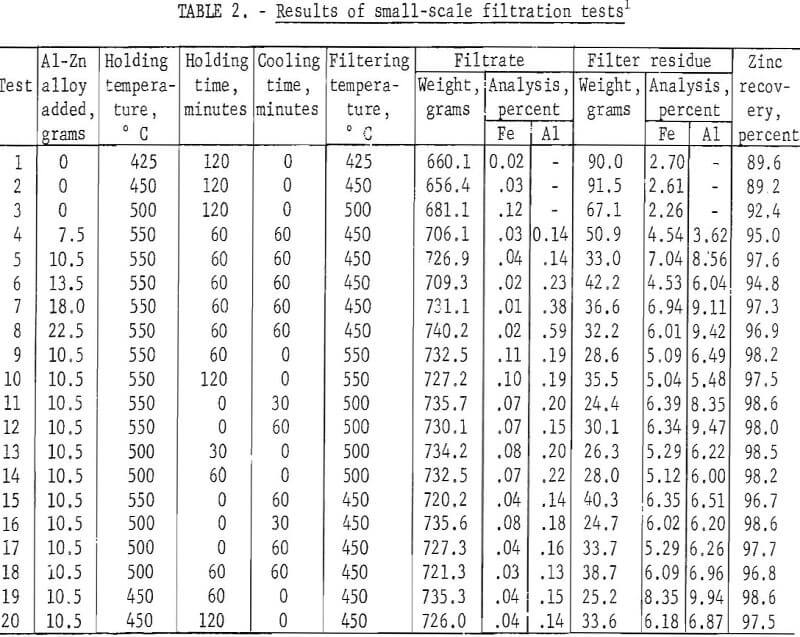
The first variable studied in other refining tests was the amount of aluminum added to establish the optimum or required amount. Test 5 shows that using 10.5 grams of the aluminum-zinc alloy resulted in the highest recovery of zinc with good purity. This amount of alloy contained 3.5 grams of aluminum, the calculated theoretical requirement to form Fe2Al5 is 3.35 grams. Except for filtration temperature, the effects of all of the other variables in the ranges studied appear to be insignificant. At filtration temperatures above 500° C, the iron content in the filtrate tends to increase.
Large-Scale Filtration Test
A final filtration test was made on a larger scale, using the apparatus described in the report of previous work. All of the features of the small-scale filter had their counterparts in the larger apparatus. The principles of operation were the same.
The object of this test was to establish if zinc recovery and purity on this scale would be comparable to that observed on the smaller scale.
Eighty pounds of iron-contaminated zinc was treated with 508.5 grams of the 1- to -2 aluminum-zinc alloy. Filtration was performed at 450° C under a vacuum of 22-½ inches of mercury. Zinc recovery was 95 percent. Iron content of the refined zinc was 0.02 percent, and the aluminum content was 0.13 percent. The filter cake contained 8.83 percent iron and 9.47 percent aluminum. These results demonstrate that recoveries and purities of metal on a larger scale can be expected to compare favorably with those obtained on a small scale.
Centrifugation Studies
In this refining method the amount of solids to be separated from the liquid zinc was relatively small, suggesting that centrifugation might be a
practical separation method. An inverted type of centrifuge, capable of skimming the solids from the top of the melt, appeared to provide the most effective design. Experiments in which floating cork was separated from water with crudely fashioned conical centrifuges provided data that were used as a guide in design of the molten metal centrifuge.
Description and Operation of the Centrifuge
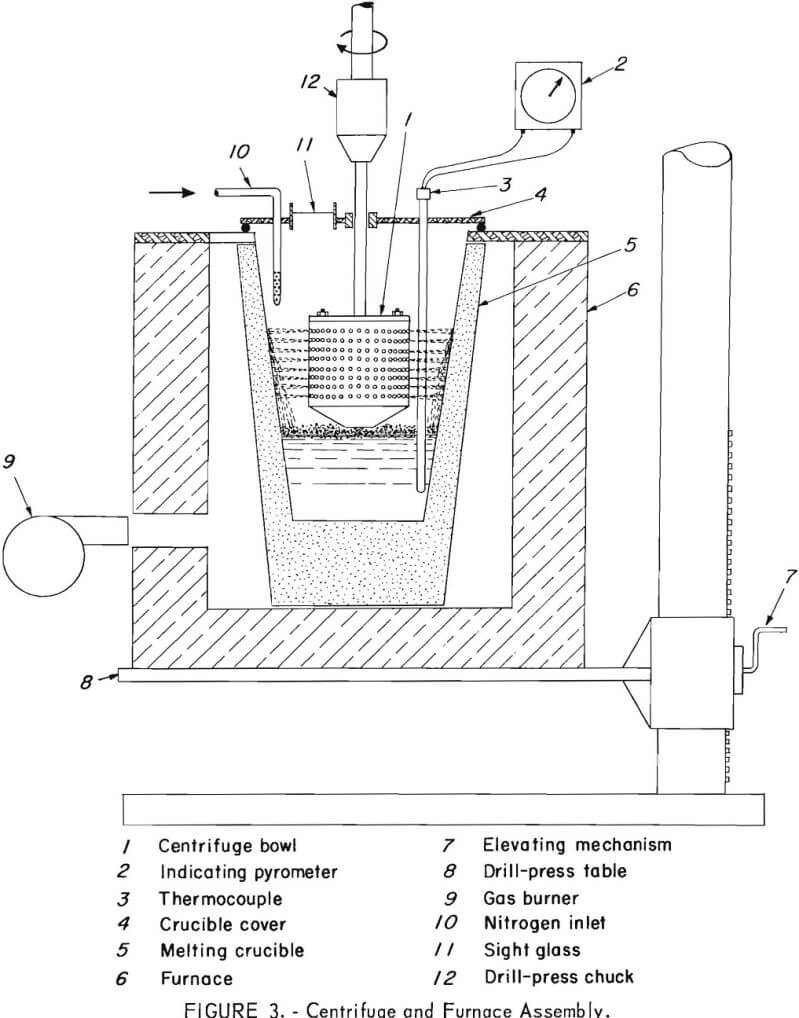
The melting furnace, melting crucible, and centrifuge were constructed as an integral unit as shown in figure 3. A medium-size drill press performed the dual function of spinning the centrifuge bowl and supporting the gas-fired melting furnace. The furnace was constructed of insulated fire brick and rested directly on the drill-press table which could be raised and lowered to permit proper contact between the bottom of the centrifuge bowl and the top of a molten charge in the crucible.
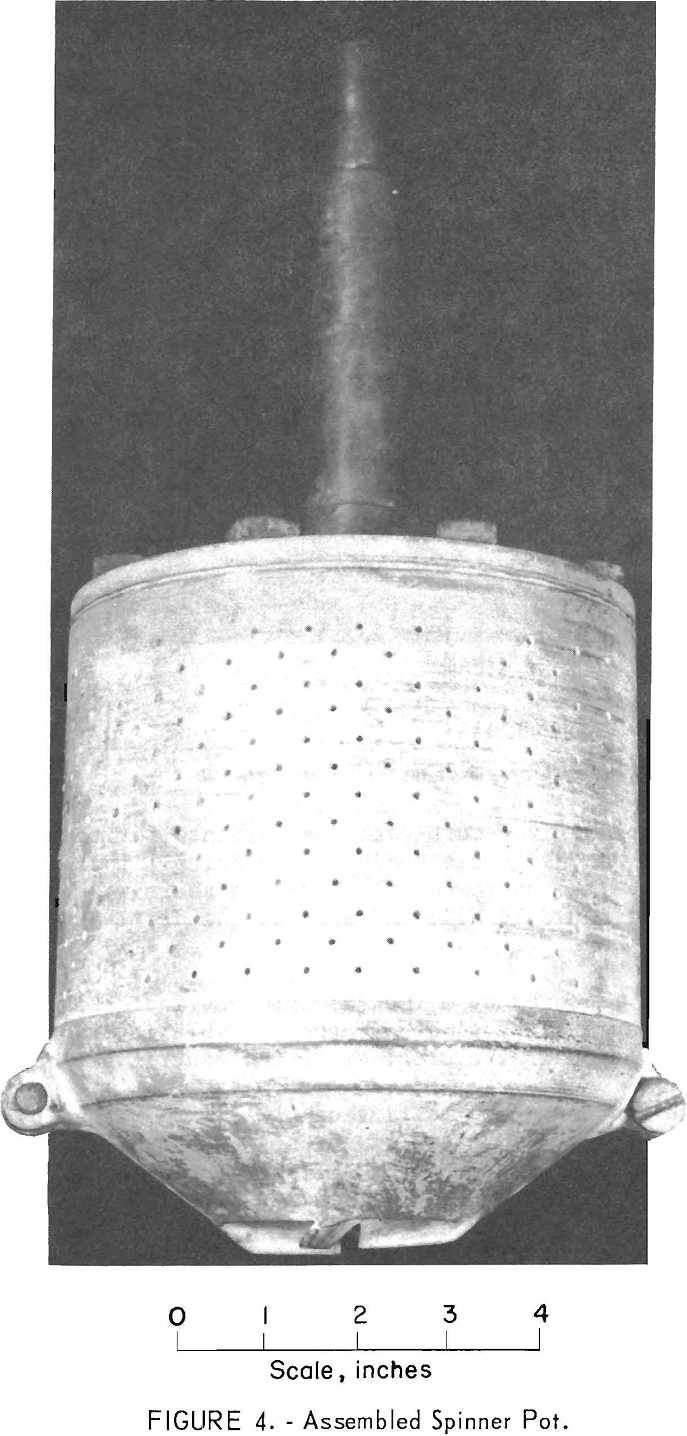
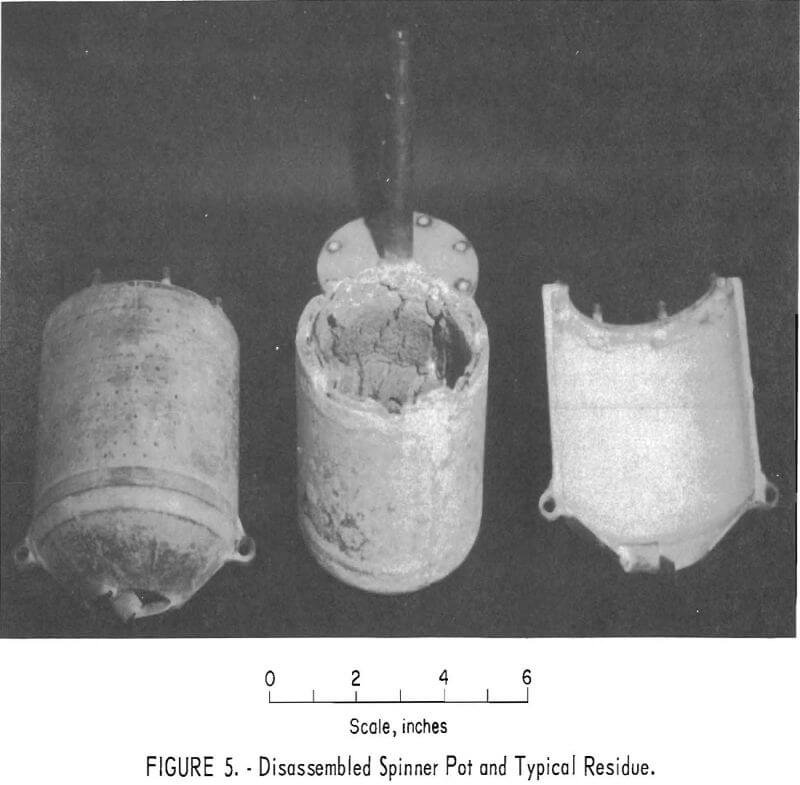
The centrifuge bowl (figs. 4 and 5) was hollow; the upper part consisted of a perforated cylinder, and the lower section was an inverted truncated cone. Two spiral-shaped lifting flights were fitted into the bottom of the cone originally, but they proved
inadequate. After returning to pulverized cork-water experiments for testing other designs of lifting flights, a design was evolved comprising a four-bladed propeller positioned inside the bottom of the bowl. A shaft was bolted to the top of the bowl so that it could be held and spun by the drill-press chuck.
Temperature of the melt was measured by a thermocouple and indicating pyrometer. The crucible cover had a sight glass and the crucible interior was illuminated to facilitate observation of the progress of the centrifugation.
In operation, the furnace and contained melting crucible were raised by elevating the drill-press table until the bottom of the spinning bowl just touched the surface of the melt containing floating solids. In contact with the surface, the rotating blades pumped the melt upward and outward along the interior surface of the conical section. Upon reaching the upper cylindrical section, molten zinc was thrown out through the perforations while the solid intermetallic crystals were retained within the bowl.
After separation was completed, the furnace was lowered so that the bowl no longer contacted the melt and the contents were spun dry. The bowl was then disassembled into two halves to allow the solid cake to be removed, as shown in figure 5.
Initial experiments were conducted with the contents of the melting crucible exposed to the atmosphere. However, it soon became apparent that an inert atmosphere was required. The extremely fine droplets of molten zinc thrown out from the centrifuge bowl would not coalesce and flow back down to the melt. This behavior was attributed to the formation of a thin film of oxide on the surface of the droplets. To prevent this oxidation, a cover, provided with a nitrogen inlet, was built over the melting crucible. To prevent chilling of the melt, the nitrogen was passed through a tubular pre-heating coil located inside a gas-fired furnace. Temperature of the heated nitrogen was measured by a thermocouple and indicating pyrometer. The thermocouple was housed in a T-joint situated in the line between the heating coil and the inlet to the melting crucible.
As experimental work proceeded, minor modifications were made in the apparatus. The inside of the centrifuge bowl was lined with a woven glass fabric to provide a more efficient filtering medium for the finer metallic crystals. A variable-speed transmission was added so that the bowl could be spun at infinitely variable speeds between the limits of 100 to 1,200 rpm. Finally, an additional heavy-duty bearing was installed to give support to the bowl drive-shaft and reduce vibration.
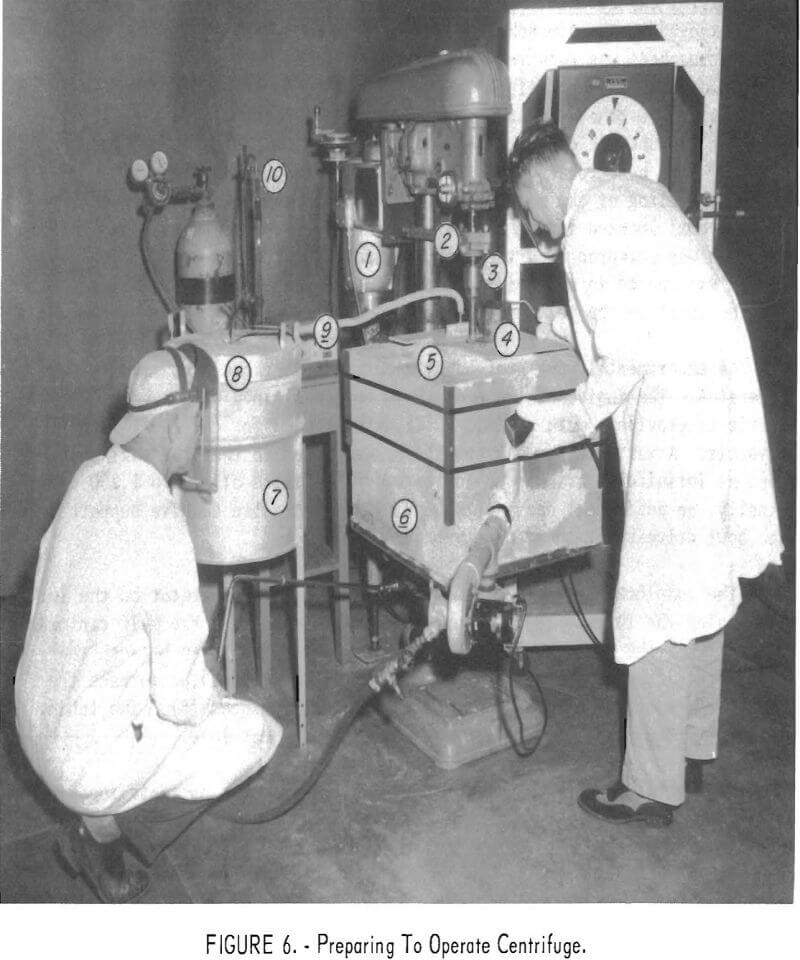
The complete apparatus is shown in figure 6. The operator on the left is raising the furnace and contained crucible so that the melt can make contact with the centrifuge bowl impeller. To his right can be seen the gas-fired nitrogen preheating furnace. At the top of the furnace the inlet and exit of the copper heat exchanger tube is shown. The tubing leading to the centrifuge is insulated to prevent heat loss.
The operator on the right is observing the progress of centrifugation by looking through the sight glass in the crucible cover. In his left hand he holds the safety cutoff switch for the drive motor. Just above the drill-press chuck can be seen the additional support and bearing that were added to strengthen the centrifuge bowl drive. Directly in line behind the chuck and the vertical tubular column supporting the table and drive mechanism, is the variable-speed transmission. The burner for the melting furnace is aimed tangentially at the annular space between the crucible and furnace walls. The hot gases swirl around the crucible in a counterclockwise direction and leave from the opening seen on the top left of the furnace.
Experimental Procedure
When the modifications to the centrifuge apparatus were completed a typical test run would proceed as follows:
A charge of 40 pounds of iron-contaminated zinc was weighed. Approximately 200 grams of this charge was broken off and melted in a small crucible furnace with 84.75 grams of pure aluminum for the low-iron (0.37 percent Fe) zinc tests. In tests involving the high-iron (0.56 percent Fe) zinc, 123.0 grams of aluminum were melted with about 300 grams of the initial zinc charge. After melting, the aluminum-zinc mix was allowed to cool to an ingot.
The remainder of the 40 pounds of zinc was placed in the centrifuge apparatus crucible and melted. When steady-temperature conditions were attained, the aluminum-zinc ingot was added and slowly stirred in. A graphite shaft and impeller, actuated by the same drive mechanism used later in the process to spin the centrifuge bowl, was utilized. It usually took approximately 10 minutes for the ingot to melt and blend in with the molten zinc charge. When everything was molten, the time interval designated “Alloying time” in table 3 was begun.
After holding at temperature for the required time, the graphite stirrer was removed from the drive-shaft chuck and replaced with the centrifuge bowl. The latter was heated for about 5 minutes with a propane torch to prevent chilling of the molten metal during the first few moments of centrifuging. The crucible cover was then put on. The nitrogen heating coil was placed in the gas preheating furnace which had been heated for 30 minutes. The coil was connected with the nitrogen tank, and gas began to flow into the crucible at 35 liters per minute and 500° C.
The centrifuge bowl drive was turned on, and the speed set at 575 rpm. The furnace was elevated until the bowl impeller started pumping molten metal into the centrifuge. As the center of rotation of the bowl was offset from the center of the crucible, the action of the impeller caused the surface of the melt to describe a circular motion, continually exposing fresh areas of the surface to be drawn up into the interior of the bowl. The filtered molten metal thrown out through the perforations in the bowl struck the crucible wall and flowed down to rejoin the remainder of the molten metal. The solid aluminum-iron intermetallic compound, along with some entrained zinc, remained inside the bowl.
After the molten metal was recirculated through the bowl for a predetermined time (6, 8, 10, or 20 minutes), the furnace was lowered until the spinner was free from the melt. The contents of the bowl were spun dry by increasing the speed of rotation for a definite time interval (1, 2, or 5 minutes). A speed of either 875 or 1,000 rpm was chosen for this cycle. In a few of the earlier tests, the speed of rotation during the spin-dry cycle was identical with the 575 rpm pumping speed. Then the centrifuge motor was turned off, the crucible cover was removed, the nitrogen flow was shut off, and the centrifuge bowl was removed from the chuck. The refined metal was ladled out from the
crucible into a mold. After cooling, the centrifuge bowl was taken apart and the residue was removed. Sample drillings were taken from both the residue and the refined metal.
Results of Centrifugation Tests
During the entire course of this work, design changes in the centrifuge and modifications in testing procedures were being made. Table 3 presents results of 11 tests from the time when it first appeared that the method was being successfully developed.
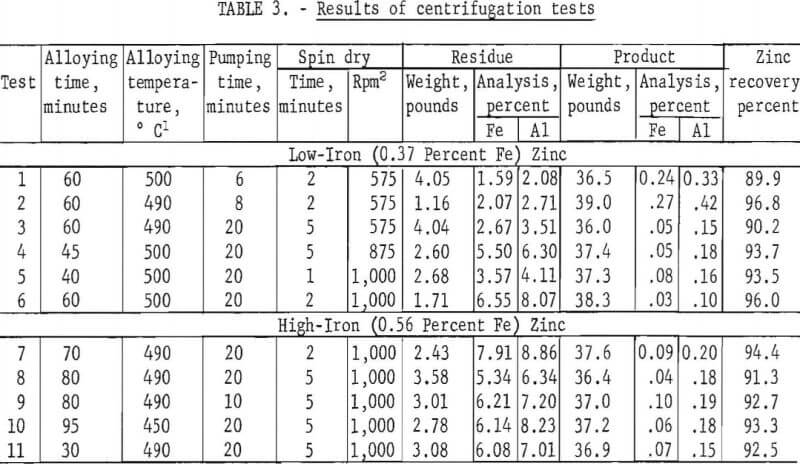
Tests 1 to 6 used low-iron (0.37 percent Fe) zinc. In test 1, the only filtering medium was the perforated bowl itself and a high-iron content in the filtrate resulted. To make matters worse, no attempt was made to shield the centrifugation operation from the atmosphere and this brought about surface oxidation of the fine metal droplets spun out from the bowl. As a consequence, considerable quantities of high-viscosity dross formed which upon being pulled back into the centrifuge bowl became trapped there and caused an excessive zinc loss. The next test was performed under an inert atmosphere of nitrogen and resulted in greatly improved recovery. However, the iron content of the filtrate was again high because the perforated wall of the bowl was the only filtering medium.
In test 3, a glass cloth lining was fitted inside of the centrifuge bowl and the nitrogen atmosphere was used again. The result was a significant improvement in metal purity. All subsequent tests were made with the glass cloth filter medium under a nitrogen atmosphere.
Increasing the spin-dry speed in test 4 from 575 rpm to 875 rpm resulted in an increased recovery with no sacrifice in purity. Installation of a heavy-duty bearing allowed this speed to be increased further to 1,000 rpm in test 5. The following test showed that lengthening the spin-dry cycle from 1 to 2 minutes permitted even better recoveries.
In tests 7 to 11 the high-iron (0.56 percent Fe) zinc was used as charge metal. The greater amount of Fe2Al5 solids retained by the centrifuge in the remainder of the tests also held up larger amounts of liquid zinc, causing slightly greater metal losses. These five tests were intended to illustrate the effects of alloying time, alloying temperature, and pumping time on recovery and purity. In the ranges tested, none of these variables appeared to have any significant effect.
Removal of Residual Aluminum and Recovery of Zinc from Residues
Refined products from both filtration and centrifugation tests are seen to contain about 0.2 percent aluminum. If the metal were redistilled, this amount should not be objectionable. If a marketable metal were desired without redistilling, this amount can easily be eliminated by a simple fluxing treatment using zinc chloride. Aluminum reacts with this flux and volatilizes as aluminum chloride, leaving a purified metal containing less than 0.01 percent aluminum.
Filtration residues would still contain from 80 to 90 percent zinc. This would have to be recovered for the process to be economically attractive. Normal distillation practice would probably be the most practical method for recovering the metal. Small-scale vacuum distillation tests from previous work suggest that the zinc distills from a relatively pure liquid phase at 500° C until the distillation is complete. The solubility of the solid Fe2Al5 might be sufficiently low in the liquid zinc phase, even at the higher temperatures required for distillation at atmospheric pressure, that the vapor pressure of zinc would be substantially the same as that for the pure zinc metal. However, some additional experimental work would be required to verify this.
Related: 3 Best Drill Press for Metal
