Table of Contents
Primary mercury metal is produced commercially by heating mercury sulfide concentrate in furnaces to vaporize mercury metal, which is cooled and recovered in a condensing system. Potential health and safety problems exist because mercury vapors can escape from the furnace and condenser. The Bureau of Mines and McDermitt Mine, operated by Placer Amex Inc., the major producer of mercury in the United States, entered into a cooperative agreement, with the objective of investigating alternate processing techniques. Emphasis was placed on a hydrometallurgical technique for treating mercury concentrate to recover mercury metal.
Hydrometallurgical methods for recovering mercury from mercury ores or concentrates have been investigated. In one method, mercury was leached from ores and concentrates with a solution of sodium sulfide and sodium hydroxide. The mercury was recovered from solution by cementation with aluminum followed by retorting, or less successfully, by electrolysis. The sulfide-hydroxide solution did not solubilize elemental mercury contained in the ore or concentrate. Another proposed approach solubilized the combined and elemental mercury by leaching with chlorine or hypochlorite solutions. Mercury metal was recovered from solution by cementation with iron, or by adsorption on activated carbon and subsequent retorting. A hydrometallurgical method for producing mercury metal would eliminate the potentially hazardous operation of retorting mercury either from a concentrate or cementation product.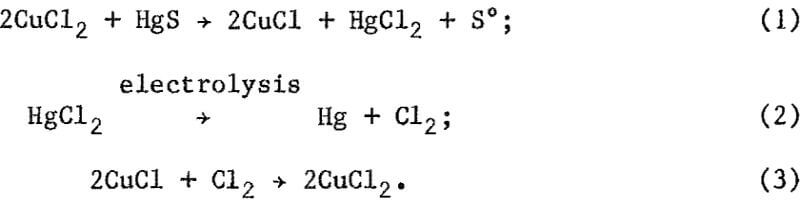
This report describes a preliminary, bench-scale investigation of a technique for leaching a mercury sulfide flotation concentrate and recovering metallic mercury by electrolyzing the pregnant solution. The two unit operations were combined and tested on a continuous basis. Mercury sulfide and elemental mercury were leached with cupric chloride solution according to equations 1 and 2:
2CuCl2 + HgS → 2CuCl + HgCl2 + S°………………………………………………………………………..(1)
2CuCl2 + Hg → 2CuCl + HgCl2…………………………………………………………………………………(2)
During electrolysis, mercury metal was produced at the cathode and chlorine at the anode according to equation 3:
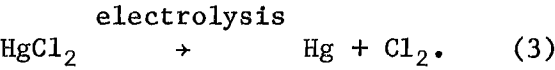
The chlorine reacted with the cuprous chloride in solution to regenerate cupric chloride according to equation 4:
2CuCl + Cl2 → 2CuCl2………………………………………………..(4)
Materials and Apparatus
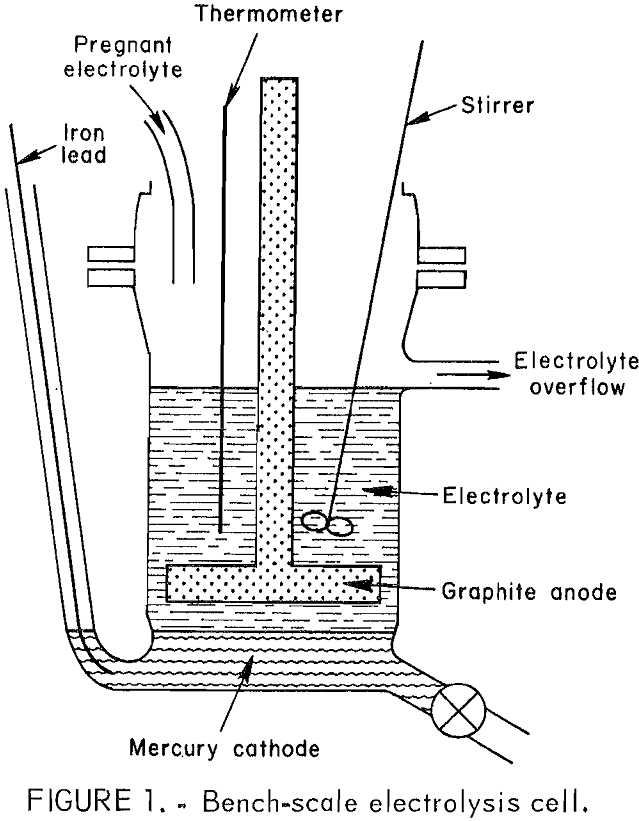
Mercury sulfide flotation concentrate supplied by the McDermitt Mine was used. An analysis of the concentrate is shown in table 1. Major minerals present were cinnabar (HgS) and corderoite (Hg3S2Cl2). Approximately 30 pct of the mercury in the concentrate was present as corderoite. Reagent-grade mercury sulfide was also leached. Reagent-grade chemicals were used to prepare the solutions.
Leaching reactors were 1-L glass resin kettles. The slurry was stirred with a Teflon paddle-type stirrer to insure that the solids were suspended in solution during leaching. The slurry was heated to the leaching temperature with an immersion heater housed in a fused-quartz sheath. The immersion heater was controlled with a thermocouple temperature controller. After leaching, the slurry was vacuum-filtered through Whatman No. 5 filter paper on a Buchner funnel.
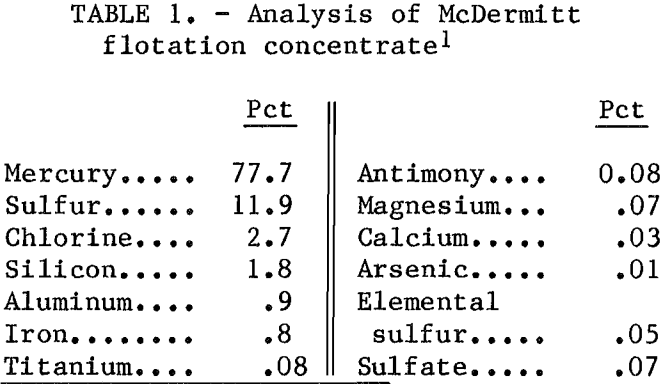
The electrolytic cell shown in figure 1 was a 500-mL glass resin kettle modified to include an overflow port, a valve for draining mercury, and a glass tube through which an iron rod was inserted for making direct electrical contact with the mercury pool cathode. Seven hundred grams of mercury metal served as the cathode. The anode was a horizontal graphite plate 3 in. in diameter by ½ in thick and was connected to a ½- in graphite rod extending through the lid. The electrolysis experiments were
made with an anode current density of approximately 100 amp/ft² and a cathode current density of approximately 80 amp/ft². Anode-cathode spacing was maintained at ½ in by periodically draining the mercury produced during electrolysis. A polyethylene stirrer and glass thermometer were inserted through the lid.
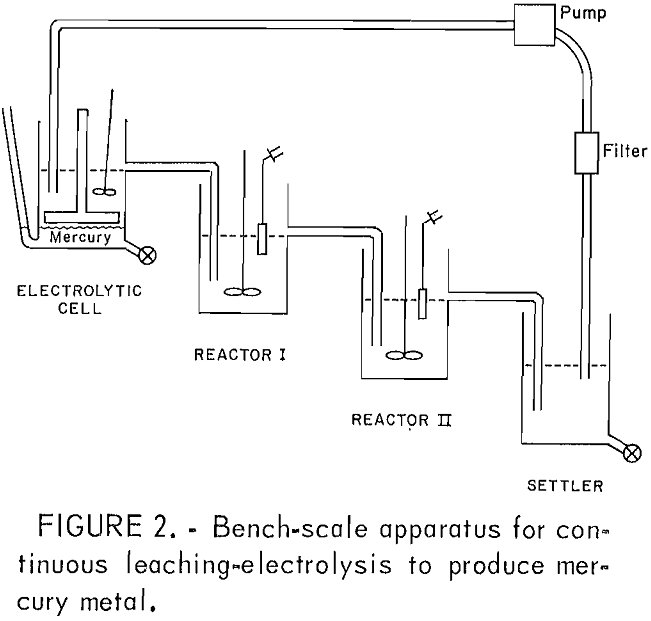
Figure 2 shows the bench-scale apparatus used for the continuous leaching-electrowinning tests. The leaching- electrolysis setup consisted of two leaching reactors, a 1-L glass resin kettle settler, and the electrolytic cell. The leaching reactors had glass tubing nipples for slurry overflow. The settler had a single valve to both drain the solution and remove the residue. The concentrate was added to reactor I, and the slurry overflowed into reactor II for additional leaching. The chances of concentrate short-circuiting the leaching section were decreased by using more than one reactor. Slurry from reactor II was a mixture of pregnant solution, insolubles, and elemental sulfur, and overflowed to a settling chamber. The clarified supernatant was pumped through a polishing filter before entering the electrolytic cell. The solids were periodically drained, filtered, and washed, and the solution was returned to the system.
Experimental Procedures and Results
Leaching
Ferric chloride has been shown to be an effective reagent for leaching metal sulfides (MS) according to equation 5:
2FeCl3 + MS → MCl2 + 2FeCl2 + S°………………………………………………..(5)
In preliminary tests, 40 g of mercury-concentrate, which was assumed to be mercury sulfide, was leached for 1-½ hr with 400 mL of 80° C solution containing 150 pct of the stoichiometrically required FeCl3 (72 g/L Fe) and 42 g/L HCl to prevent hydrolysis. All residues were displacement-washed with water. Less than 6 pct of the mercury was solubilized. Leaching of an equivalent amount of reagent-grade mercury sulfide solubilized less than 1 pct of the mercury.
Since mercury extraction was very limited with ferric chloride, cupric chloride was investigated for leaching mercury sulfide according to equation 1;
2CuCl2 + HgS → 2CuCl + HgCl2 + S°……………………………………………(1)
Forty grams of concentrate was leached for 1-½ hr with 400 mL of 80° C solution containing 300 pct of the stoichiometrically required CuCl2 (164 g/L Cu) and 42 g/L HCl to prevent hydrolysis. Ninety percent of the mercury was solubilized. Because of its low solubility, some cuprous chloride formed during leaching and remained in the residue.
To increase the solubility of cuprous chloride by the common ion effect, calcium chloride was added to the leaching solution. Calcium chloride also removed sulfate formed during leaching by precipitating calcium sulfate. Forty grams of concentrate was leached for 1-½ hr with 400 mL of 80° C solution containing 150 pct of the required cupric chloride. The solution contained, in grams per liter, 82 Cu, 72 Ca, and 42 HCl. More than 99 pct of the mercury was solubilized, and no copper reported to the washed residue. Sulfate levels were <0.08 g/L of the solution and 0.04 pct in the residue.
Experiments were conducted to determine if the concentrate could be leached with a ferric chloride solution containing a cupric chloride addition. The reason for leaching with a ferric chloride-cupric chloride solution was that the concentrate contained a small amount (0.8 pct) of iron. Since iron level will increase when the leaching solution is recycled, the leaching behavior of the mixed solution was of interest. Forty grams of concentrate was leached for 1-½ hr with 400 mL of 80° C solution containing 150 pct of the stoichiometrically required ferric chloride and 30 pct of stoichiometrically required cupric chloride. The solution contained, in grams per liter, 72 Fe, 16.4 Cu, 72 Ca, and 42 HCl. Seventy percent of the mercury was solubilized. Apparently, ferric chloride regenerated cupric chloride from cuprous chloride according to equation 6:
FeCl3 + CuCl → FeCl2 + CuCl2………………………………………………………(6)
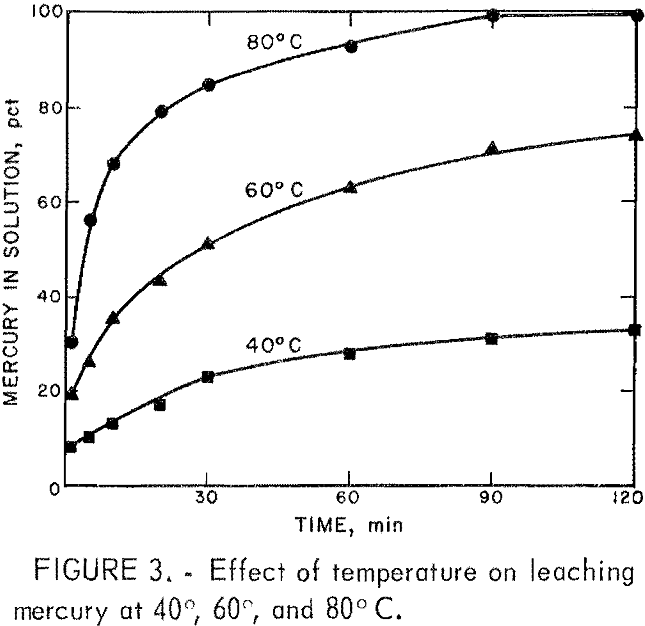
The effect of temperature on the mercury extraction rate was determined. A 40-g sample of the concentrate was leached with 400 mL of solution containing 150 pct of the stoichiometrically required cupric chloride and at 40°, 60°, and 80° C. The solution contained, in grams per liter, 82 Cu, 72 Fe, 72 Ca, and 42 HCl. Samples of the solution were removed at selected times and analyzed for mercury. The results presented in figure 3 show that increasing the temperature increased the completeness of the cupric chloride-mercury sulfide reaction.
Electrolysis
The leaching experiments demonstrated that mercury could be effectively leached from a sulfide concentrate. A method for recovering mercury metal from the leaching solution was necessary to complete the process. Electrowinning mercury metal from reagent-grade mercuric chloride
solution into a mercury pool cathode was investigated.
The electrolyte contained 100 g/L Hg, and mercuric chloride crystals were periodically added to the cell to maintain the mercury concentration. An anode current density of 100 amp/ft² was selected because commercial aqueous electrolysis is usually performed in this range. At 100 amp/ft², the cell operated at 5.0 amp and 2.8 v. Current efficiency was 81 pct and energy consumption was 0.6 kwhr/lb Hg.
The best leaching solution contained calcium, copper, and iron, in addition to mercury and hydrochloric acid. An experiment was run to determine if these impurities affected electrolysis of mercury or if they contaminated the electrowon mercury when the leaching solution was electrolyzed to recover mercury metal. A solution was made to have a similar composition to the pregnant leaching solution. The simulated leaching solution contained, in grams per liter of solution, 100 Hg, 70 Cu, 100 Ca, 30 Fe, and 100 HCl. During electrolysis, the mercury concentration decreased from 100 to 80 g/L. The electrowon mercury metal was not contaminated with calcium, copper, or iron. The cell operated at 3.0 v and 5.0 amp. Current efficiency was 45 pct and energy consumption was 0.8 kwhr/lb of mercury produced. An explanation for the decreased current efficiency with the simulated leaching solution is that the Cu²+ and Fe³+ can be reduced to Cu+ and Fe²+ at the cathode. The Cu+ and Fe²+ would then be oxidized to Cu²+ and Fe³+ by chlorine produced at the anode.
During electrolysis of solutions that had been used to leach concentrate, a scum formed on the mercury pool cathode. The scum was identified as mercurous chloride (Hg2Cl2) by X-ray diffraction. Since the scum could cause problems during extended cell operation, it was monitored during continuous leaching- electrolysis experiments.
Continuous Leaching-Electrolysis
The apparatus for continuous leaching-electrolysis was operated for a 3-day period and an 11-day period. The 11-day test provided operational data for cost evaluation and time for problems to develop.
During these experiments, reactors I and II where leaching occurred were maintained at 80° C. The solution was cooled by natural heat loss to about 35° C in the settler. No further temperature decrease occurred in the electrolytic cell because of the heat generated by electrolysis. The temperature of the electrolytic cell was important because decreasing the temperature could cause precipitation of calcium sulfate, which would be undesirable in the cell. Although it is known that calcium sulfate has reverse solubility in water, it was observed that in this complex solution, calcium sulfate precipitated when the solution temperature decreased. The solution was pumped from the settling container through a filter to the electrolytic cell at a rate of about 6 mL/min. The solution in the electrolytic cell was gently stirred to mix the incoming liquid with the electrolyte.
For the 3-day experiment, 3.5 L of starting solution was made by leaching mercury concentrate and contained, in grams per liter, 70 Cu, 66 Fe, 71 Ca, 82 Hg, and 42 HCl. A high initial iron concentration was investigated, because iron in the concentrate would be leached and could eventually increase to these levels when the solution was recycled. The solution was charged into the system and electrolysis was initiated. Leaching temperature was 80° C and retention time was 2 hr. Periodically, concentrate was fed into reactor I, residue was removed from the settling chamber, and mercury was tapped from the electrolytic cell.
During 3 days of operation, 680 g of concentrate was leached, 118 g (dry weight) of residue was removed, and 367 g of mercury metal was produced. Ninety-nine and five-tenths percent of the mercury in the concentrate was solubilized. The mercury that was leached and not electrowon increased the mercury concentration in solution. The electrolytic cell, operated at 4.5 amp and 3.5 v, had a current efficiency of 30 pct and an energy consumption of 1.4 kwhr/lb Hg produced. Because chlorine produced at the anode reacted efficiently with the solution, very little escaped. No additional reagents were added during the experiment, and the only reagent consumed was a small amount of calcium, which reacted with sulfate ion and precipitated as calcium sulfate. Sulfate was formed during leaching with regenerated solution. The calcium chloride controlled sulfate in solution to <0.3 g/L SO4. The last residue sample contained 10.0 pct SO4= and 3.8 pct Ca. No problems were encountered during the experiment.
The next campaign was conducted for 11 days with essentially the same conditions as reported in the preceding paragraph.
A total of 3.5 L of the solution was prepared by leaching flotation concentrate at 80° C for 2 hr. The solution contained, in grams per liter, 60 Cu, 30 Fe, 70 Ca, 80 Hg, and 42 HCl. Electrolysis was performed at 5.2 amp and 3.2 v. A total of 2,360 g of concentrate was leached, 428 g of dry residue was removed, and 1,647 g Hg was produced at an average current efficiency of 32 pct and an average energy consumption of 1.2 kwhr/lb Hg.
Leaching solubilized 99.5 pct of the mercury, although some short-circuiting of unleached concentrate was observed. As in the 3-day test, no additional reagents were added and the calcium chloride controlled sulfate formed during leaching. A possible explanation for sulfate formation during leaching with regenerated solution is that chlorine produced at the anode formed hypochlorous acid in addition to regenerating cupric chloride. Hypochlorous acid would react with the sulfide minerals to form sulfate rather than elemental sulfur. As the experiment proceeded, the residue and solution reached steady-state concentrations of 10 pct and <0.3 g/L SO4, respectively. Removing sulfate from solution prevented the formation of significant quantities of insoluble mercury sulfate.
The only significant problem observed during the 11-day experiment was the formation of mercurous chloride on the mercury cathode in the electrolytic cell. During the first 5 days of operation, the mercurous chloride remained approximately 1/16 in thick. During the sixth day, the layer increased over several hours to 3/8 in. Because the layer caused the cell voltage to increase and would eventually interfere with electrowinning, the cell was drained, cleaned, and recharged with clean mercury and the used electrolyte. The mercurous chloride quickly reappeared on the mercury surface and increased to a thickness of ½ in within several hours. During the next 2 days of operation, the thickness of the mercurous chloride decreased to less than 1/8 in and remained at this thickness for the final 3 days of operation.
The cause of the mercurous chloride formation and subsequent elimination is not fully understood, but it was probably dependent on the concentration of mercury in solution. Initially, the concentration was maintained at 70 to 90 g/L Hg. As the experiment proceeded, mercury concentrate was added to the leaching section faster than mercury was removed by electrowinning. The mercury in solution increased to about 145 g/L Hg. The higher mercury concentration favored the mercurous chloride formation. After the cell was drained and recharged, additions of concentrate were decreased to permit the mercury concentration in solution to decrease to about 40 g/L Hg. When the mercury concentration decreased, the amount of mercurous chloride decreased.
The concentration of mercury in solution also influenced energy consumption. With an average mercury concentration of 100 g/L Hg, energy consumption was 1.0 kwhr/lb of mercury produced. At an average mercury concentration of 40 g/L Hg, energy consumption was 1.7 kwhr/lb Hg. The surface of the mercury metal tapped from the cell remained bright and shiny for several months and indicated that the metal purity was high, >99.9 pct.
The leaching solution and residue were periodically removed from the settler. After filtering the residue from the solution, the filtrate was returned to the system and the residue was washed. Preliminary tests showed that iron cementation could be used to treat the wash water to recover copper and mercury for recycle. A sample of wash water was treated with an excess of powdered iron. Copper decreased from 5 g/L to <0.003 g/L, and mercury decreased from 5.3 g/L to 0.0017 g/L.
Economic Evaluation
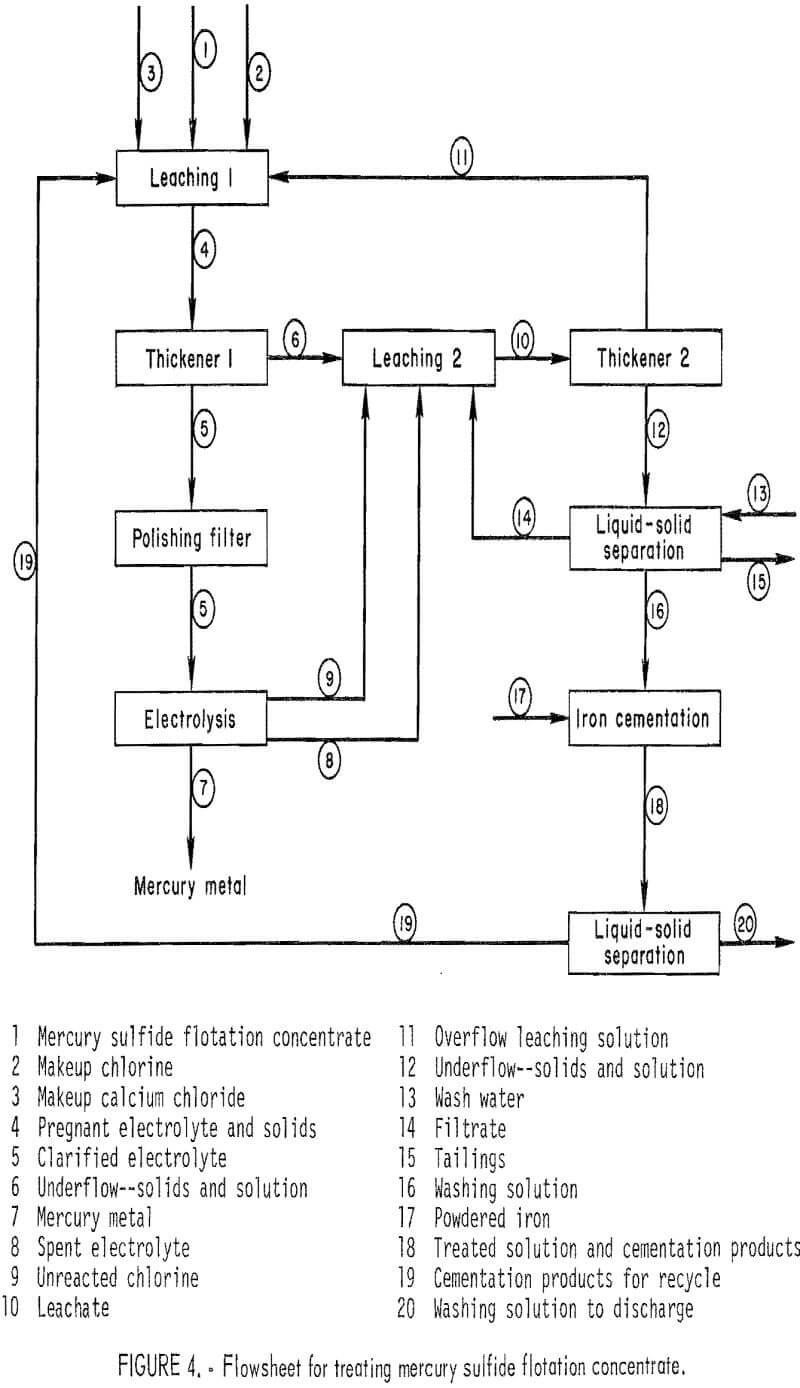
A preliminary economic evaluation of the process was performed by the Process Evaluation Staff of the Bureau of Mines. The flowsheet on which the evaluation was based is shown in figure 4. Mercury sulfide concentrate is leached in two stages with cupric chloride solution. The polished pregnant mercury-containing solution is electrolyzed to produce mercury metal and chlorine, which regenerates the cupric chloride. Wash water is treated with iron powder to remove the mercury and copper to very low levels, and the cement product is recycled to the leaching step. The plant was designed to operate 300 days per year and 24 hr per day. Production would be 105 flasks of mercury per day.
The capital cost was a “study estimate” used by Weaver and Bauman. Since research on the technique is very preliminary and has not been scaled up, the accuracy of the cost estimate may not be within the usual ±30 pct. Capital cost was $3.7 million, and cost per flask of mercury, excluding the cost of obtaining the concentrate, was $62 per flask.
Summary and Conclusions
Bench-scale tests showed that mercury was leached from a mercury sulfide flotation concentrate with a cupric chloride solution. Pure mercury metal was electrowon from the leaching solution in a monopolar horizontal plate electrowinning cell with a graphite anode and a mercury pool cathode. The leaching-electrolysis experiment was operated on a continuous basis for 11 days. During the continuous operation, concentrate containing 77.7 pct Hg was leached and 99.5 pct of the mercury was solubilized. Operation of an electrolysis cell produced mercury at an average current efficiency of 32 pct and an average energy consumption of 1.2 kwhr/lb Hg.
A preliminary economic evaluation of the method for a plant to produce 31,500 flasks of mercury per year indicated a capital cost of $3.7 million and an operation cost of $62 per flask of mercury.
