Table of Contents
- Laboratory Investigations
- Sandy Flint Clay A. P. G. 1
- Physical and Chemical Character
- Beneficiation
- Wet-Table and Wet-Cyclone Concentration
- Determination of Firing Properties
- Sandy Flint Clay H.-W. 2
- Physical and Chemical Character
- Beneficiation
- Wet-Table and Wet-Cyclone Concentration
- Determination of Firing Properties
- Sandy Plastic Clay A, P. G. 3
- Physical and Chemical Character
- Beneficiation
- Wet-Table and Wet-Cyclone Concentration
- Determination of Firing Properties
- Pyritic Plastic Clay A. P. G. 10
- Physical and Chemical Character
- Beneficiation
- Wet-Table and Wet-Cyclone Concentration
- Determination of Firing Properties
Sandy flint and pyritic plastic clays from east-central Missouri can be beneficiated to good firing quality by ore dressing methods. Quartz and pyrite were the chief contaminants removed by the two gravity concentration methods used in the studies. Most iron-oxide minerals in these clays occur as finely-divided, low-gravity, hydrous material, often in the form of stains or smears on other minerals, and are not removed by gravity concentration procedures. Coarse iron minerals are more detrimental to refractories; however, they can be removed by wet-tabling or wet-cycloning.
Alumina content of sandy flint clays was increased 2 to 6 percent by beneficiation, and the PCE was raised 1 to 3 cones. Similar treatment of pyritic plastic clays raised the alumina content 2 to 3 percent, and increased the PCE by one cone. Modified preliminary treatments of a sandy plastic clay followed by wet-tabling and wet-cycloning raised the alumina content by 8 and 13 percent and increased the PCE by 0 and 3 cones, respectively.
The purpose of this investigation was to develop economical mineral dressing processes for removing objectionable quantities of impurities, such as quartz and pyrite, from refractory clay deposits in east central Missouri. The presence of excessive quartz in these clays causes crazing. Pyrite causes bloating and unsightly dark blobs on the surface of fired refractory brick. The iron concentration in the proper state of oxidation may also act as nucleation centers for carbon crystal growth which can result in bursting of the refractory. Numerous deposits, representing a very large tonnage, have been examined and prospected by various refractories companies and found to contain impurities in objectionable quantities. Upgrading these clays would greatly extend our reserves of high-quality refractory clays.
Wet-tabling and wet-cyclone concentration were found to be effective for removal of impurities from clays. Of the two methods, wet-cyclone concentration would be cheaper and more effective. Although wet-tabling improved the refractory properties of each clay, beneficiation by wet-cyclone produced clays of superior quality. Clay recoveries by wet-cycloning ranged from 56.1 to 81.8 percent of the weight of the crude samples, and the beneficiated clays contained from 71.9 to 94.4 percent of the total alumina. The pyrometric cone equivalent (PCE) was increased from 1 to 3 cones by wet-cycloning. The beneficiated clays also met the reheat shrinkage requirements on refining to 1,400° C. Missouri is one of the major domestic sources of fire clay. In addition to deposits of high-grade refractory clays, there are many deposits that contain small percentages of impurities, which render the clays unfit for the production of high-heat-duty firebrick. Development of methods to beneficiate these submarginal clays to usable materials would greatly increase commercial fire clay resources.
This report covers the initial experimental research in a comprehensive mineral dressing investigation for removing impurities from clays occurring in east-central Missouri. Samples used in this investigation are identified as follows: Sandy flint clay A. P. G. 1, sandy plastic clay A. P. G. 3, and pyritic plastic clay A. P. G. 10 (all from the A. P. Green Fire Brick Co., Mexico, Mo.) and sandy flint clay H. W. 2 (from Harbison-Walker Refractories Co., Fulton, Mo.). These samples were considered typical of the submarginal clay deposits of the area, representing sufficient tonnage to warrant exploitation.
The wet-cyclone separation of quartz sand and clay has been applied commercially by the Owens-Illinois Glass Co. and Gladding, McBean & Co. near Ione, Calif.
In previous Bureau of Mines publications some work was reported on beneficiation of clay, in which classification, flotation, and sedimentation were employed to upgrade the raw clays. These methods have been applied commercially by Harris Clay Co., Spruce Pine, N. C. and others.
The connotation of the word quartz as used throughout this paper is that of chemically uncombined or non-silicate silica, rather than physically free silica only.
General Laboratory Procedure
The four samples contained different proportions of the contaminants, and the variations were sufficient to require modification of treatment methods among samples.
Physical and Chemical Character
Preliminary examination of the samples included a petrographic study and chemical analysis of each clay. Differential thermal analyses were made on representative flint and plastic type clays. A physical character study was made to determine the relative freeing-size range of each ore. In the latter procedure a portion of each sample was mixed to 50 percent solids with water and attrition scrubbed for 20 minutes in a Fagergren flotation cell; the slurry was screened on a 48-mesh sieve. The plus 48-mesh material was wet ground in an Abbe mill with a light load of pebbles to pass 48-mesh, after which the total minus 48-mesh material was wet-sized by screening and size analyzed.
Beneficiation
The following beneficiation methods were used, except where otherwise specified. Samples were blunged with water for 20 minutes and screened on a 48-mesh sieve to prepare the clays for separation. The plus 48-mesh material was wet-stage ground to pass 48-mesh. The two fractions were combined and treated without fractionation.
Two methods of concentration were used wet-tabling and wet-cyclone concentration. In wet-tabling the standard procedure was followed. In this report the term “table tailing” is used to designate the position on the table of the clay product and does not imply a waste product, the usual meaning of the term. In wet-cyclone concentration the samples were mixed with water to give a pulp with specific gravity of 1.07, and pressure at the intake orifice of the cyclone was maintained at 22.5 pounds per square inch. Impurities were removed in the cyclone underflow.
Determination of Firing Properties
Commercial use of a refractory clay is determined by such fired properties as color, hardness, water absorption, apparent porosity, shrinkage, and specific gravity. Fired properties were determined for all crude and beneficiated clays, using procedures outlined by the American Society for Testing Materials (ASTM). These include forming test bars in conformity with ASTM designation C179-44 (except for specimen size, 1-1/8 by 1 by 4 inches) and firing in accordance with schedule B; testing for reheat change on the same test bars in conformity with ASTM designation C113-46, using firing schedule B; and preparing pyrometric cones in conformity with ASTM designation C24-46, using firing schedule B.
Tests for general appraisal of the beneficiated clays were made on hand-pressed specimens made with workable mud. These were fired to cover the usual commercial ranges (1,800° to 2,400° F.) at intervals of 100° to 200° F., and the fired properties were determined in accordance with ASTM designation C20-46 (except for specimen size, 1 by 1-½ by 3/8 inches).
Laboratory Investigations
Sandy Flint Clay A. P. G. 1
Physical and Chemical Character
A petrographic study showed that the sample was essentially a very fine-grained, kaolin-type clay containing scattered quartz sand grains and thin seams or layers of brown iron oxide. Some of the material also was slightly stained with iron and manganese oxide.
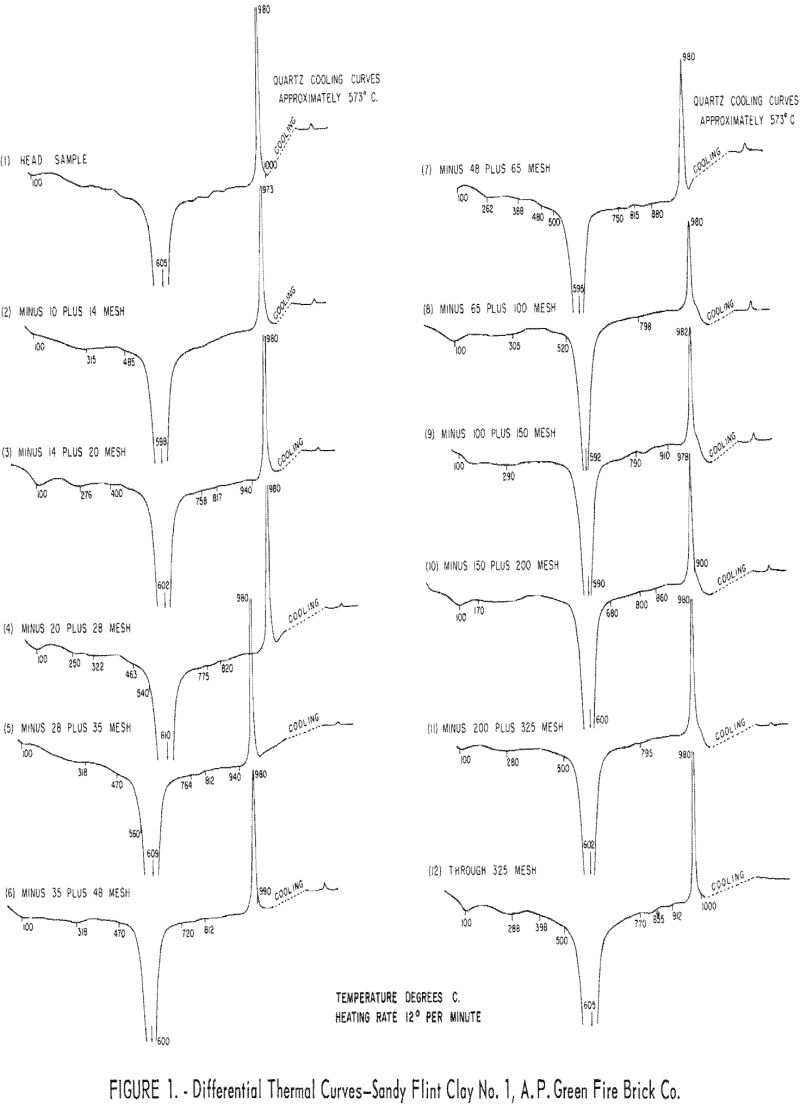
Differential thermal analyses were made on the crude sample and on the various size fractions obtained by wet-screening the clay. Curves derived from the analyses are shown in figure 1. The endothermic peak, about 600° C., is attributed to dehydration of kaolin, while the exothermic peak, about 980° C., indicates the formation of gamma alumina and amorphous silica. The large narrow endothermic peak, about 600° C., indicates a rather narrow range of particle size, and the sharp exothermic peaks indicate well developed crystal form.
During cooling, the height of the exothermic peak at 573° C., is usually indicative of the relative amount of quartz present in a sample. Differential thermal analyses curves, however, will not necessarily agree with chemical analyses for quartz content, because chalcedonic quartz, chert, and noncrystalline silica do not give similar thermal reactions. Extremely fine quartz is sometimes covered by a nonquartz silica layer showing no thermal effect.
Cooling curves Nos. 7 through 11 show that the quartz content detected by differential thermal analysis decreases progressively with the decrease in grain size of the ore, beginning with the minus 65- plus 100-mesh fraction. Curve No. 12 of the minus 325-mesh fraction indicates that no quartz is present. By chemical analysis this fraction contained 5.1 percent quartz. These results show that differential thermal analysis for quartz is not entirely reliable. This observation was also found to be true by other investigators. Chemical analysis of the clay showed 36.2 percent Al2O3 (alumina), 0.44 percent Fe2O3 (ferric oxide), and 49.1 percent SiO2 (silica), of which 11.1 percent was quartz.
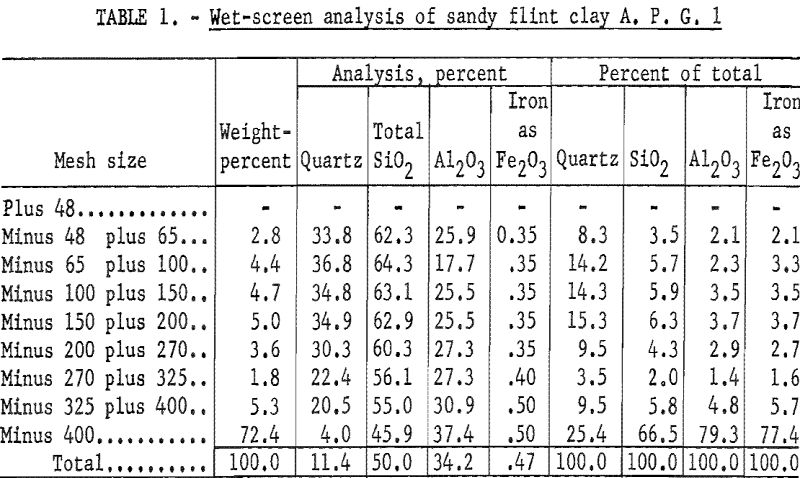
A portion of the blunged and ground minus 48-mesh sample was fractionated by screen sizing, described under general laboratory procedures. The results (table 1) show that 72.4 percent of the sample was in the minus 400-mesh fraction. Analysis of this fraction showed that most of the quartz, or uncombined silica, present in the crude clay was coarser than 400-mesh. From results of this analysis two possible means of beneficiation were suggested, wet-table and wet-cyclone concentration.
Beneficiation
Portions of the sample were prepared for and separated by wet-table and wet-cyclone concentration.
Wet-Table and Wet-Cyclone Concentration
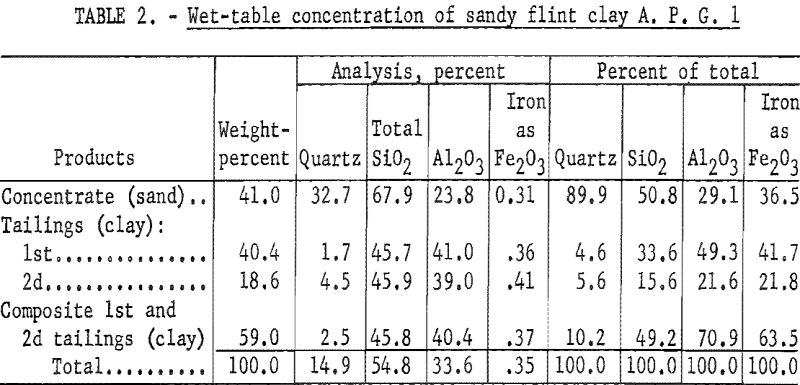
Results of wet-tabling the A. P. G. 1 sample (table 2) show that 59.0 percent of the sample, which contained 70.9 percent of the total alumina, was separated as clay concentrate or table tailing. This clay concentrate contained 40.4 percent Al2O3, 0.37 percent Fe2O3, and 45.8 percent SiO2, of which 2.5 percent was quartz.
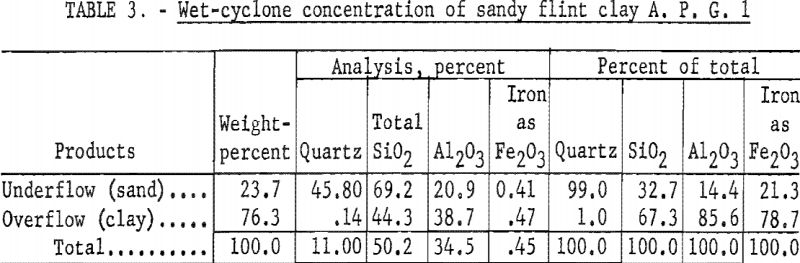
Results of wet-cycloning (table 3) show that a clay fraction was recovered representing 76.3 percent of the sample in a product containing 85.6 percent of the total alumina. This clay fraction analyzed 38.7 percent Al2O3, 0.47 percent Fe2O3, and 44.3 percent SiO2 of which 0.14 percent was quartz.
Determination of Firing Properties
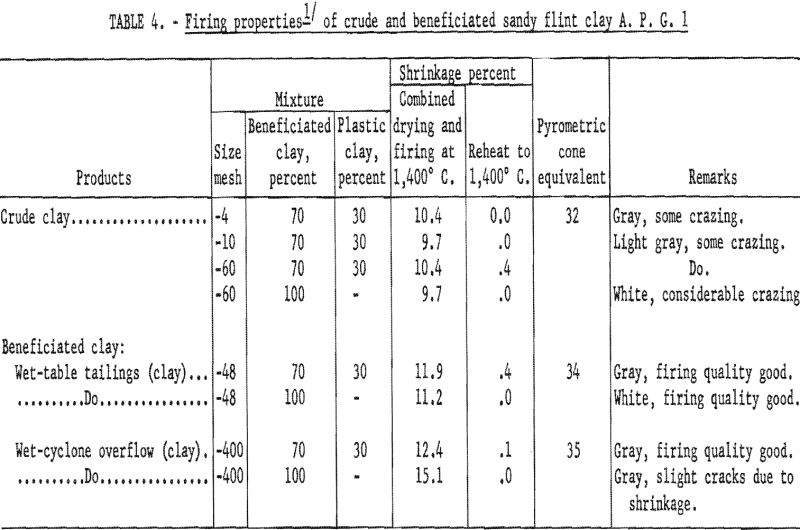
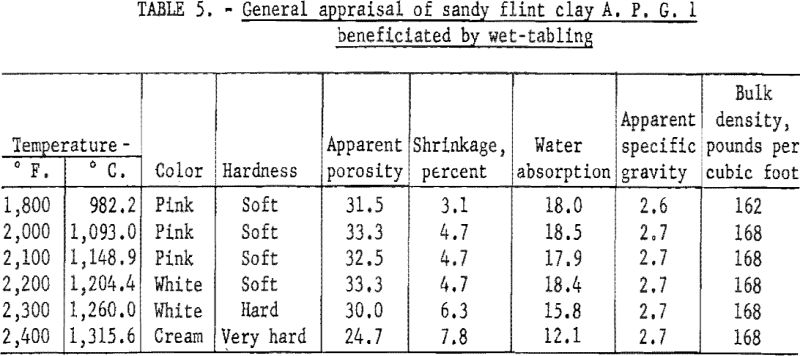
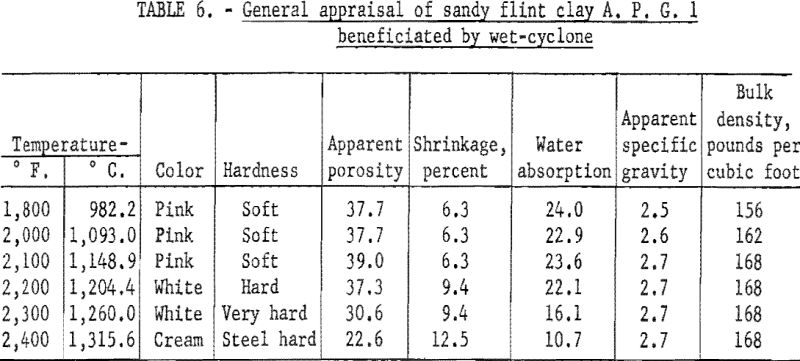
In determining the firing qualities of both crude and beneficiated clay, procedures were followed as previously outlined. The results (tables 4, 5, and 6) show that wet-tabling and wet-cyclone concentration improved the PCE from cone 32 of the crude clay to cones 34 and 35, respectively. Both beneficiated clay fractions when mixed with 30 percent plastic clay showed good firing qualities in respect to color, hardness, and percent shrinkage on reheat at 1,400° C. Test bars made with the beneficiated clay met the requirements for superduty refractory in respect to PCE and reheat shrinkage.
Sandy Flint Clay H.-W. 2
Physical and Chemical Character
The sample was a very fine-grained mixture of halloysite and kaolinite, containing scattered quartz sand grains and thin seams or layers of brown iron oxide. Some of the material was also stained with iron and manganese oxides. Examination of size fractions from wet-screening the minus-10-mesh material showed that most of the quartz grains were free at minus 200-mesh. The sample contained 35.4 percent Al2O3, 0.60 percent Fe2O3, and 47.8 percent SiO2, of which 9.2 percent was quartz.
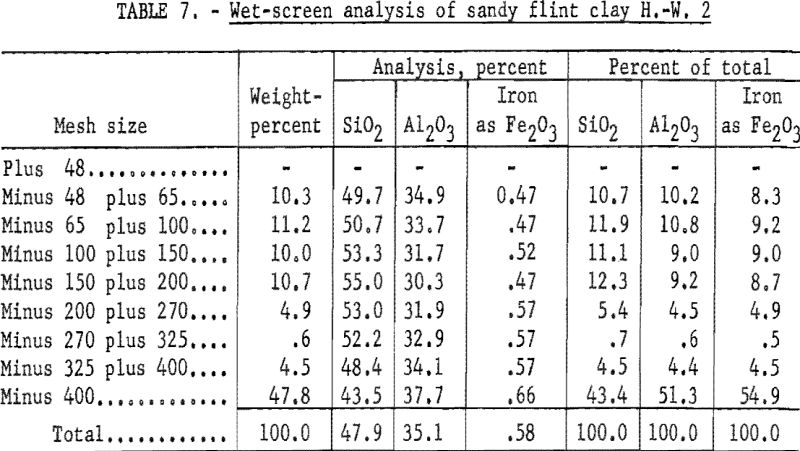
A portion of the sample was mixed to 50 percent solids with water, attrition scrubbed, and screened; the oversize was ground to minus 48-mesh. The minus 48-mesh material was size analyzed as described under general laboratory procedure. Results (table 7) show an appreciable decrease in the total silica in sizes finer than 325-mesh.
Beneficiation
The clay was blunged and ground to minus 48-mesh. Separate portions were beneficiated by wet-table and wet-cyclone concentration previously described.
Wet-Table and Wet-Cyclone Concentration
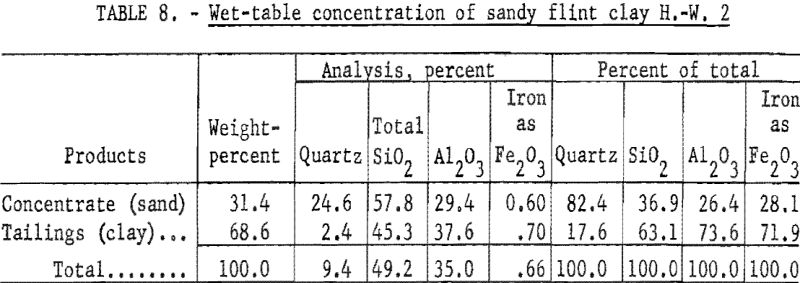
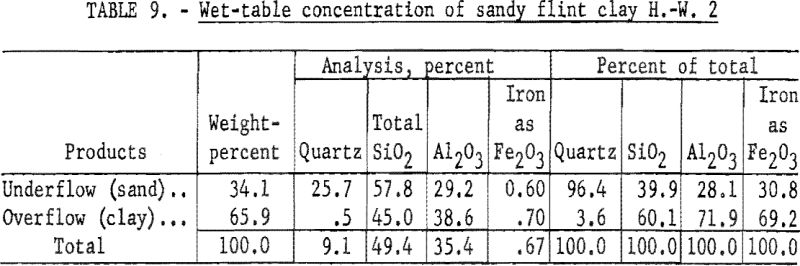
Results of wet-table concentration (table 8) show that 68.6 percent by weight of the sample was recovered in a clay product containing 73.6 percent alumina. This material analyzed 37.6 percent Al2O3, 0.70 percent Fe2O3, and 45.3 percent SiO2, of which 2.4 percent was quartz.
Results of wet-cyclone concentration (table 9) show that 65.9 weight- percent of the sample was recovered in a clay product containing 71.9 percent of the total alumina. This recovery was slightly lower than that made by tabling; however, the quality of the product was superior. The material contained 38.6 percent Al2O3, 0.70 percent Fe2O3, and 45.0 percent SiO2, of which 0.50 percent was quartz.
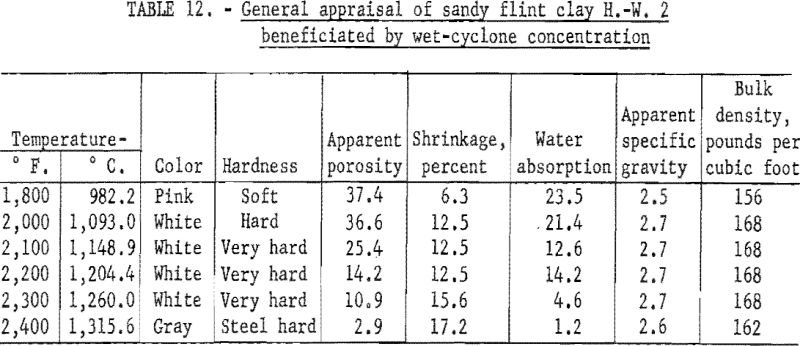
Determination of Firing Properties
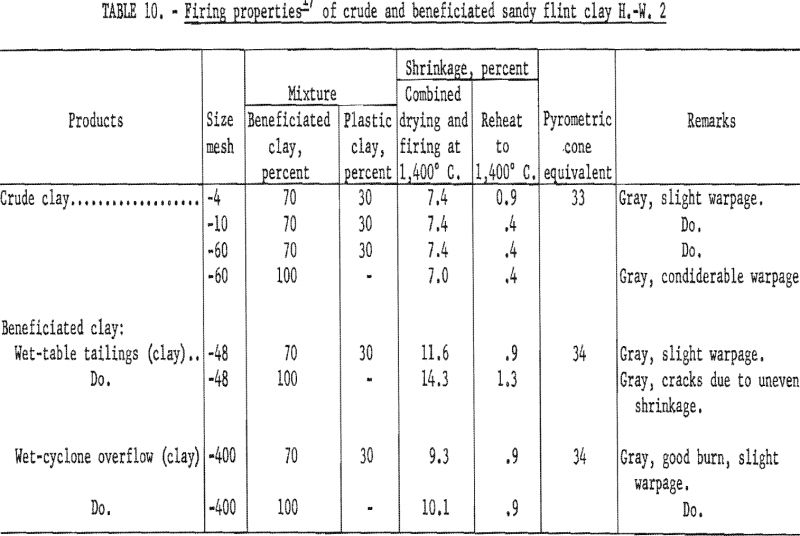
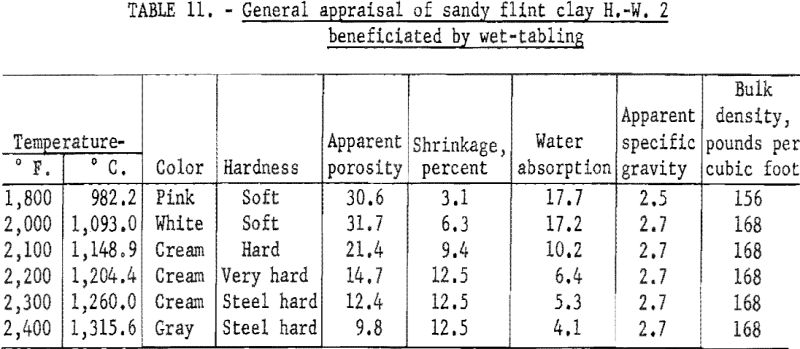
Firing qualities of the crude and beneficiated clays were determined. Detailed results of this evaluation are shown in tables 10 to 12. The PCE of the crude clay was 33, and that of the clay beneficiated by wet-tabling and by wet-cyclone concentration was 34. The firing quality of the beneficiated clay when mixed with 30 percent standard plastic clay was good, showing only slight warpage and less than one percent shrinkage on reheat firing of the specimens to 1,400° C. Both beneficiated clay products met the requirements for superduty clay in respect to PCE and reheat shrinkage.
Sandy Plastic Clay A, P. G. 3
Physical and Chemical Character
Petrographic examination showed that the sample consisted essentially of fine, crystalline, kaolin-type clay; considerable quartz sand; and traces of iron oxides, zircon, tourmaline, and ilmenite.
A portion of the minus 10-mesh sample was blunged with water for 2 hours and was screened on 65-, 200-, and 325-mesh sieves. The minus 325-mesh fraction was allowed to settle for one hour, and the settled portion was removed. Microscopic examinations of these fractions showed that the minus 65- plus 200-mesh fraction was mostly white quartz sand and hard clay, with some sand locked to minus 100-mesh; the minus 200- plus 325-mesh portion was mostly white quartz sand with little clay. The minus 325-mesh portion that settled in one hour contained considerable fine quartz sand and some clay; that material which remained suspended after one hour contained virtually no quartz.
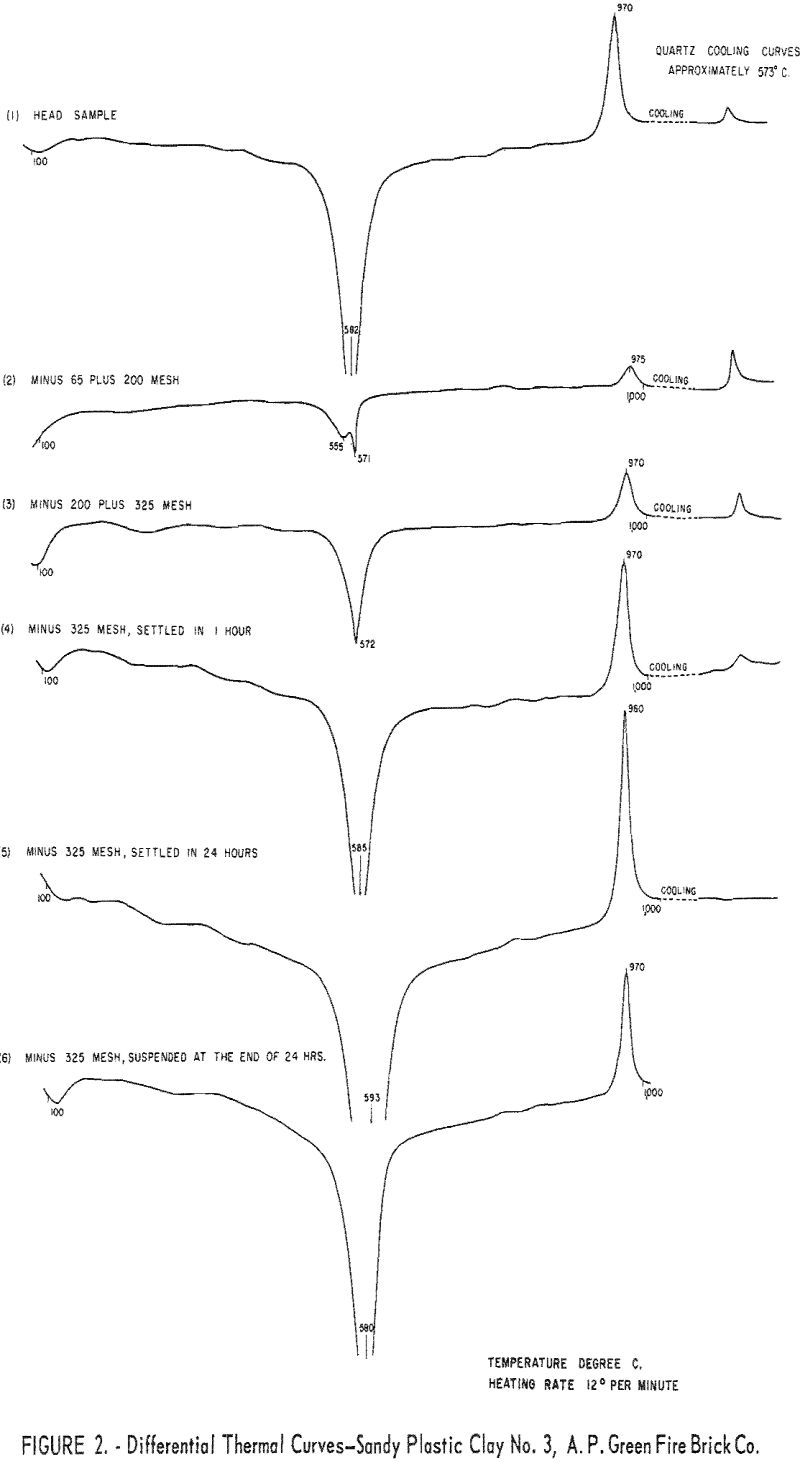
Differential thermal analyses were made on the head sample and on some of the sized fractions. Curves derived from these analyses are shown in figure 2.
The curve of the head sample (figure 2) shows a large endothermic peak at 582° C. and a sharp exothermic peak at 970° C., upon heating, and a small exothermic peak at 573° C., upon cooling. The endothermic peak, 582° C., is attributed to dehydration of the kaolin minerals combined with endothermic inversion of quartz. The effect of quartz inversion is small, shown by the reverse inversion peak obtained upon cooling. The exothermic peak, 970° C., is caused by changing the dehydrated clay to gamma alumina and amorphous silica.
Curve 2, figure 2, shows the differential thermal analysis curve obtained on a minus 65- plus 200-mesh fraction of the clay. The double endothermic peak at 565° C, and 571° C., the small exothermic peak at 975° C., and the fairly large exothermic quartz reverse-inversion peak, indicate that quartz is the predominant constituent of this fraction. Curves 3 through 6 show a progressive decrease in quartz content. The crude sample contained 22.2 percent Al2O3, 0.70 percent Fe2O3, and 67.6 percent SiO2 of which 44.9 percent was quartz.
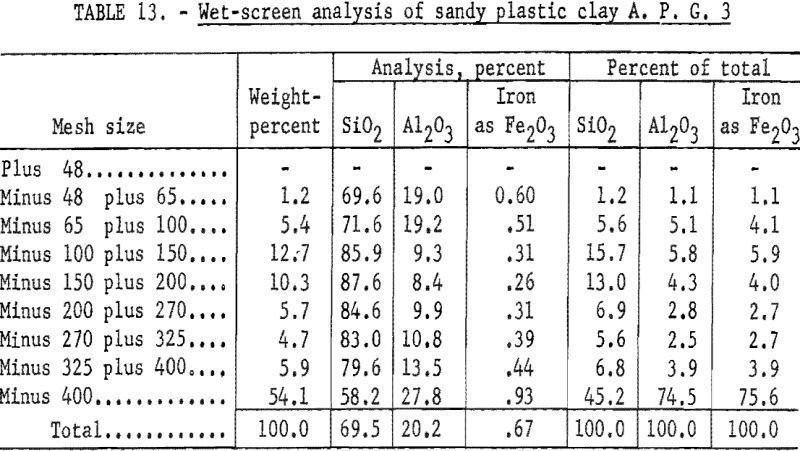
Part of the sample was mixed with water and was attrition scrubbed for 20 minutes in a Fagergren flotation cell; the slurry was screened on a 48-mesh sieve. The plus 48-mesh material was wet-ground with a light load of pebbles to pass 48-mesh in an Abbe mill. The total minus 48-mesh product was wet-screened into fractions. Results (table 13) indicate that 54.1 percent of the clay was in the minus 400-mesh fraction. Most of the quartz, or free silica, present in the sample was coarser than 400-mesh.
Beneficiation
Sample A. P. G. 3 was prepared and tested in the manner described under general laboratory procedure.
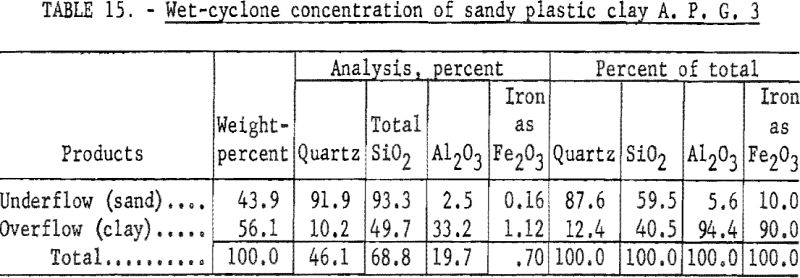
Wet-Table and Wet-Cyclone Concentration
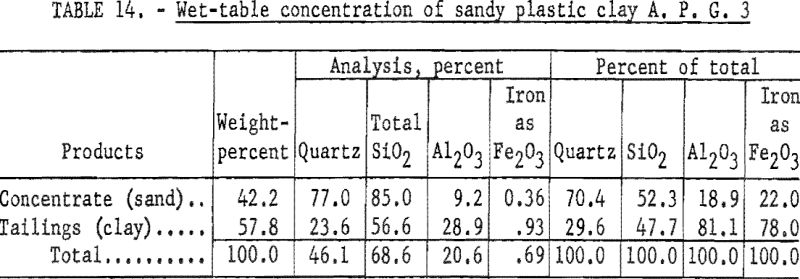
Wet-tabling the minus 48-mesh material (table 14) resulted in insufficient rejection of quartz. Wet-cyclone concentration (table 15) recovered 56.1 percent of the feed in a product containing 94.4 percent of the total alumina. This material when analyzed, contained 33.2 percent Al2O3, 1.12 percent Fe2O3, and 49.7 percent SiO2, of which 10.2 percent was quartz.
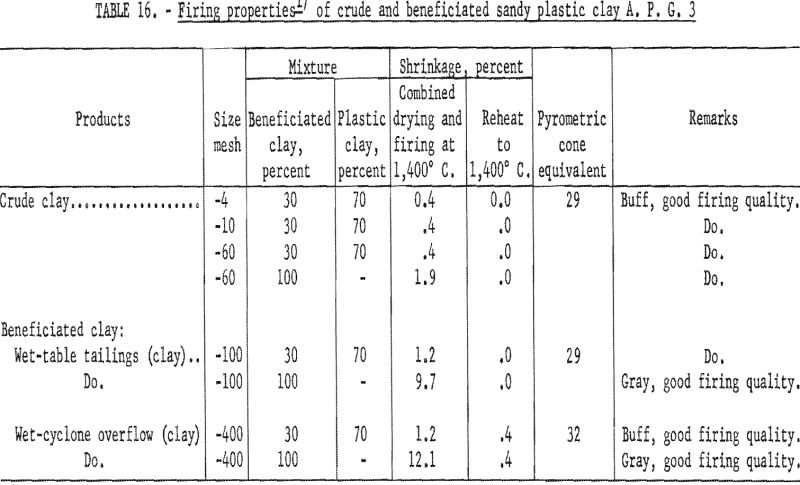
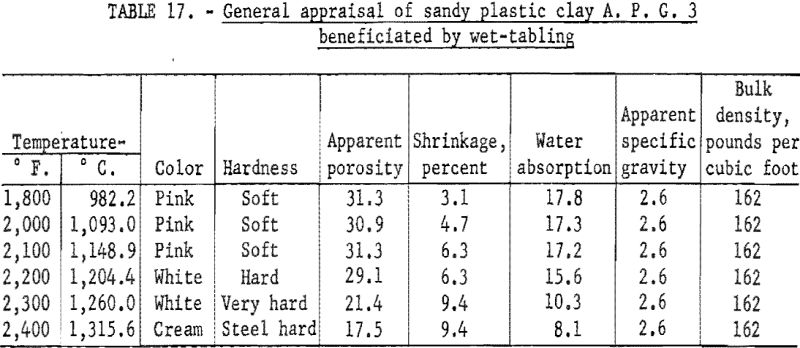
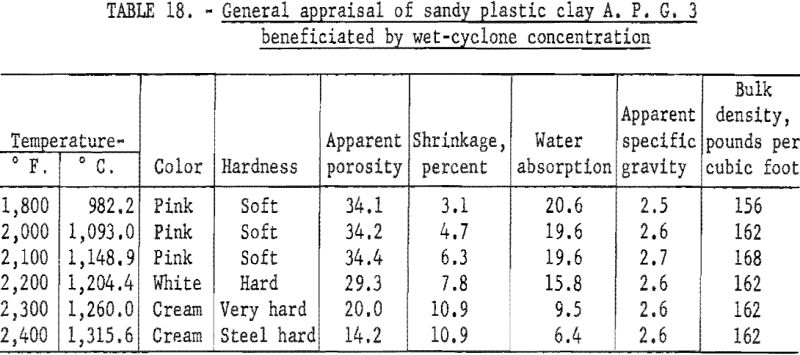
Determination of Firing Properties
Firing qualities of the crude and beneficiated clay fractions were determined. Detailed results of this evaluation are shown in tables 16, 17, and 18. Clay beneficiated by tabling had a PCE of 29 the same as the crude clay. Clay beneficiated by wet-cyclone concentration had a PCE of 32. High-duty refractory clay must have a minimum PCE of 31 to 32. The clay beneficiated by wet-tabling did not meet this requirement, while the clay beneficiated by wet-cyclone concentration did. When mixed with grog, the wet-cyclone product met the requirements for high-duty fire clay in respect to reheat shrinkage on refiring to 1,400° C.
Pyritic Plastic Clay A. P. G. 10
Physical and Chemical Character
Petrographic examination showed that the sample consisted of a light to dark gray clay with small amounts of pyrite, quartz sand, and black carbonaceous material. The clay fraction, by differential thermal analysis, was a mixture of halloysite and kaolinite. The sample contained 30.6 percent Al2O3 2.26 percent Fe2O3, 0.76 percent S, and 52.3 percent SiO2 by chemical analysis.
As in previous preliminary examinations, this clay was attrition scrubbed, and the wet slurry was ground to minus 48-mesh, screen sized, and analyzed. Results (table 19) revealed 60.8 percent iron in the minus 400-mesh screen fraction, indicating that the treating process used for sandy clays would not improve the quality of this plastic clay enough to meet requirements for use in superduty firebrick.
Beneficiation
The previously described procedure of sample preparation before clay concentration was not satisfactory for this material. A combination of blunging, screening on 10-mesh to remove the coarse pyrite, and stage grinding the minus 10-mesh fraction to pass 65-mesh was the method used to prepare the sample for subsequent wet-table and wet-cyclone separation.
The plus 10-mesh fraction screened from blunged material was 0.6 percent of the sample weight and contained 15.5 percent of the total iron and 32.7 percent of the total sulfur in the ore.
Wet-Table and Wet-Cyclone Concentration
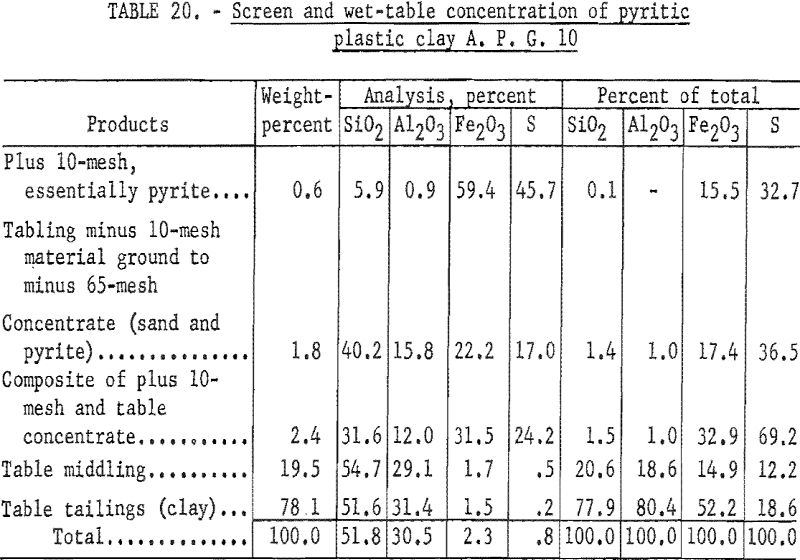
Wet tabling results (table 20) show that 78.1 percent of the sample was recovered in a clay product containing 80.4 percent of the total alumina; the analysis of this product was 31.4 percent Al2O3, 1.5 percent Fe2O3, 0.2 percent S, and 51.6 percent SiO2.
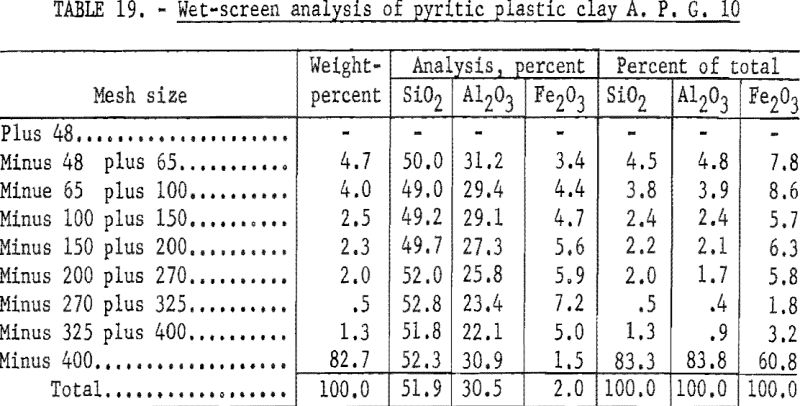
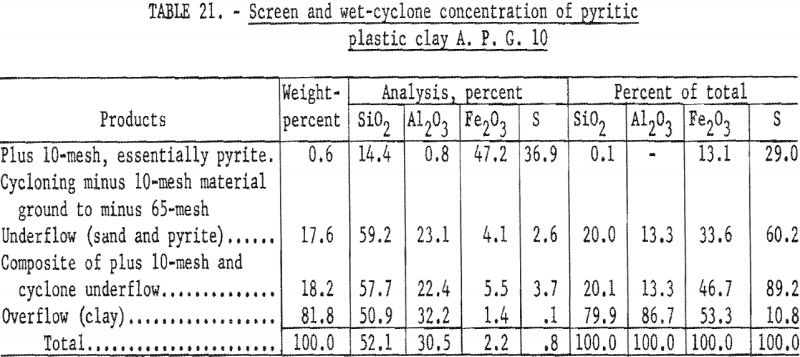
Wet-cyclone concentration (table 21) recovered 81.8 percent by weight of the sample in the clay fraction. This material represented a recovery of 86.7 percent of the alumina in a product that contained 32.3 percent Al2O3, 1.4 percent Fe2O3 0.1 percent S, and 50.9 percent SiO2.
Determination of Firing Properties
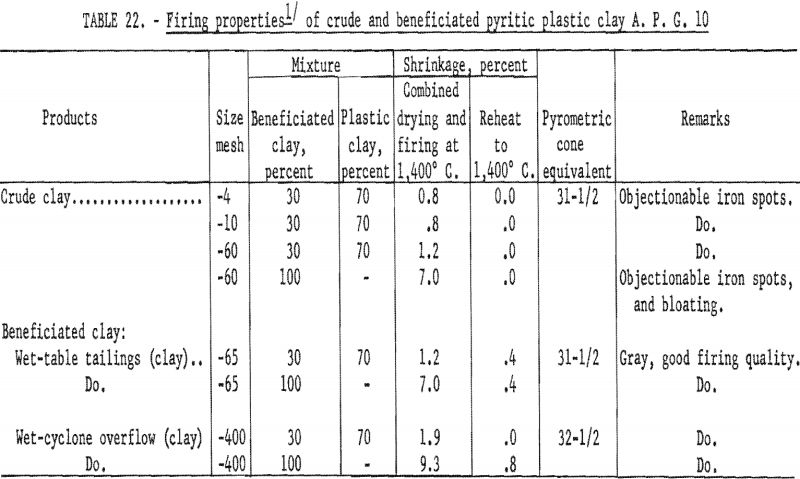
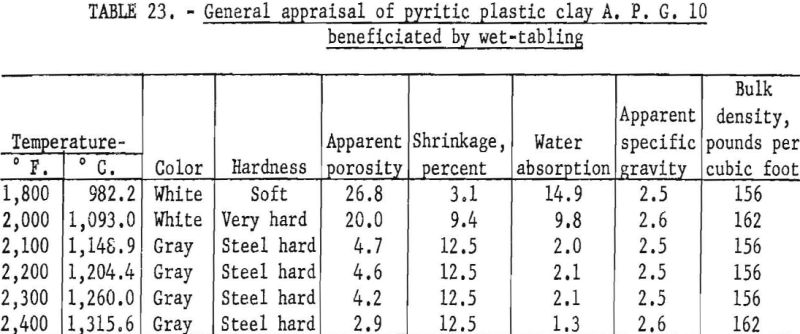
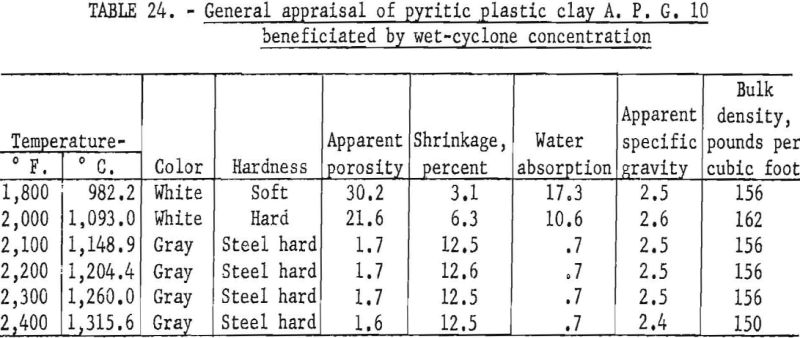
Firing qualities of the crude and beneficiated clays were determined as previously outlined. Detailed results of this evaluation are shown in tables 22 to 24. The clay beneficiated by wet-table concentration had a PCE of 31-½, the same as that of the crude sample. The clay beneficiated by wet-cyclone concentration had a PCE of 32-½, one cone more than the crude clay. The wet-cyclone clay product had good firing qualities, both alone and when mixed with 70 percent of grog, and could be classed as a high-duty fire clay product.
contact us here
