The Betts electrolytic process was for many years the only method available for debismuthizing lead Kroll suggested the use of calcium to form a high-melting-point Ca-Bi intermetallic compound that could be separated from molten lead. This process, however, was not used industrially. About a decade later, Betterton developed a commercially feasible process using a combination of calcium arid magnesium for the separation. Magnesium is added to the molten bullion in the form of cylindrical castings, and calcium is added as a Ca-Pb alloy containing 3 to 5 percent Ca. The use of the two reagents in combination gave better separation. Apparently, small amounts of both reagents in solution result in greater insolubility of the compounds Ca3Bi2 and Mg3Bi2 which appear in the dross.
Present practice in the industry is to hand-skim off these drosses, which contain considerable amounts of entrapped lead along with the intermetallic

compounds. In some cases the drosses are then placed in a hydraulic press to squeeze out some of the excess lead. Centrifuging could be a faster, more efficient technique than hand skimming and hydraulic pressing. This technique was successfully utilized for removing iron from zinc. The objective of this Bureau of Mines study is to demonstrate the applicability of this approach to the Kroll-Betterton process and to show that centrifugation is faster and more efficient than hand skimming.
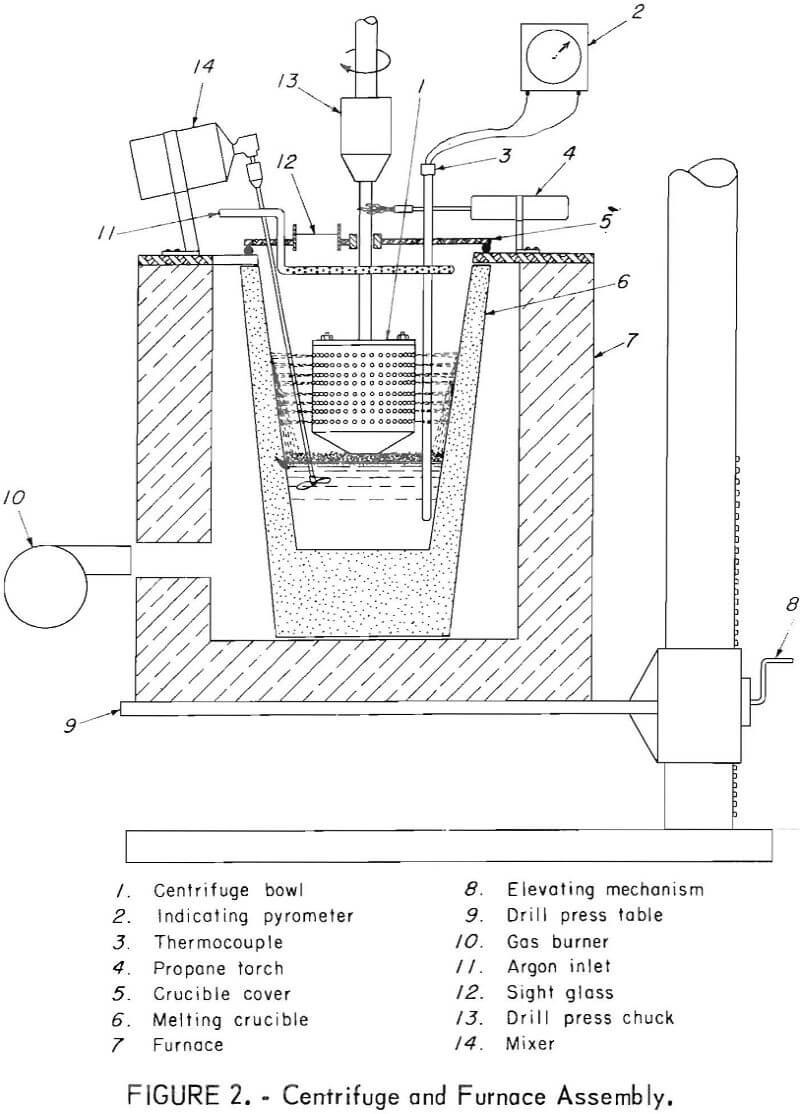
Description and Operation of the Centrifuge
The melting furnace, melting crucible, and centrifuge bowl are shown in figures 1 and 2. A medium-sized drill press performed the dual function of spinning the centrifuge bowl and supporting the gas-fired melting furnace. The furnace was constructed of insulated firebrick and rested directly on the drill press table. The furnace could be raised and lowered to permit proper contact between the bottom of the centrifuge bowl and the top of the molten charge in the crucible. A standard No. 4 clay-graphite crucible was used to hold the charge. The crucible had a flat false bottom of graphite cemented to the inside to raise the melt to the appropriate level for pumping into the spinning centrifuge bowl. The temperature of the melt was measured by a thermocouple and indicating pyrometer. The crucible cover had a sight glass, and the crucible interior was illuminated so that centrifuge operation could be observed.
The centrifuge bowl, figures 3 and 4, was hollow; the upper part consisted of a perforated cylinder, and the lower section was an inverted truncated cone. A four-bladed impeller was fitted into the open bottom of the cone, and a pair of small scoops, located adjacent to the impeller, aided in skimming the solids from the top of the melt. In all tests except one, the
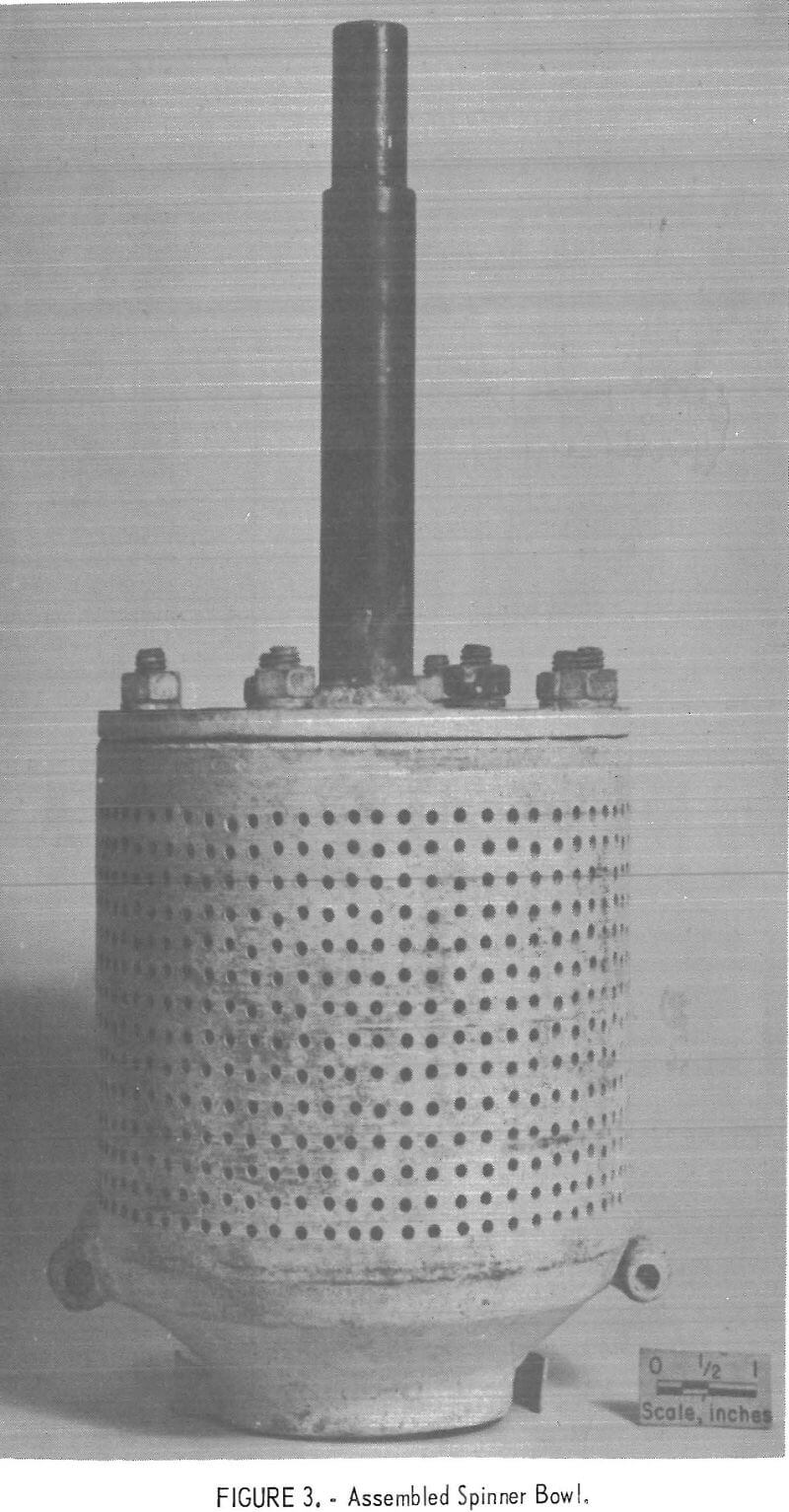
interior of the centrifuge was lined with a woven glass fabric which served as a filter medium. The centrifuge bowl, shown in figure 3, was used in the last three experiments reported in the table. It had 912 holes, each 0.109 inch in diameter. The other experiments utilized a centrifuge that had 430 holes, each 0.063 inch in diameter. In both cases the bowl was held and spun by the drill press chuck by means of a shaft bolted to the top.
The centrifuge was operated in an atmosphere of argon introduced into the crucible at 8 liters per minute. The argon was introduced at several points above the melt near the top of the crucible cover by means of a ring-shaped manifold. A propane torch heated the centrifuge bowl shaft where it emerged from the crucible cover to prevent loss of heat.
Both the furnace and melting crucible were raised during a test by elevating the drill press table until the bottom of the spinning bowl just touched the surface of the melt containing floating solids. The centrifuge was spun, and the action of the rotating blades and the small scoops caused the melt to be pumped upward through the bottom opening and outward along the interior surface of the conical section. Molten metal was thrown out through the perforations in the upper cylindrical wall of the bowl, and the solid inter¬metallic crystals were retained on the glass cloth filter surface.
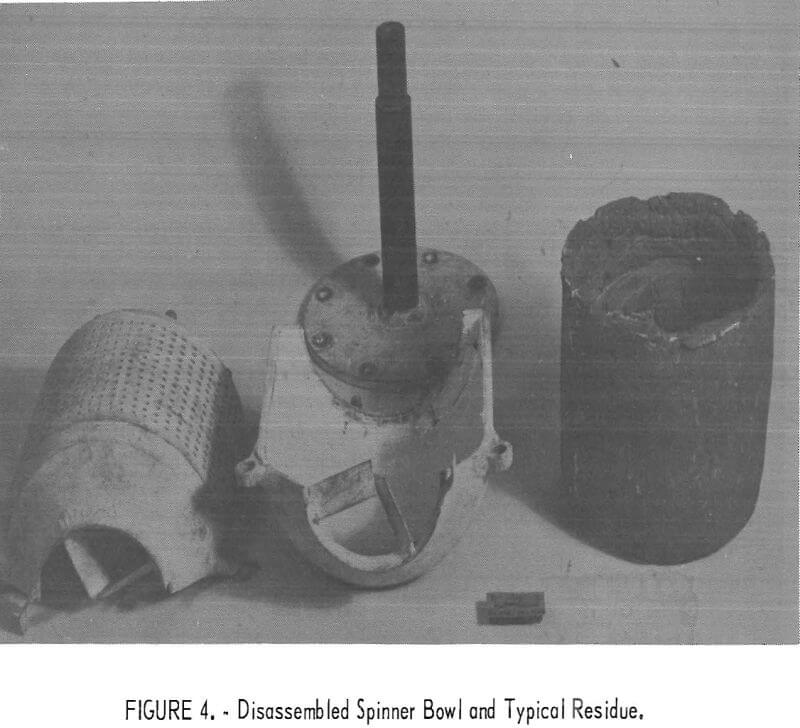
After separation was completed, the furnace and melting crucible were lowered so that the bowl no longer touched the melt, and the contents were spun dry to remove the trapped lead. The bowl was then disassembled to allow the solid cake to be removed, as shown in figure 4.
Experimental Procedure
A 60-pound charge of lead containing 0,5 percent bismuth was used as the starting material in a typical test run. The alloy was prepared by placing 59.7 pounds of “corroding grade” lead in the crucible, heating to the melting temperature, and adding 0.3 pound of pure bismuth metal. Argon was introduced into the chamber through the argon inlet tube before the lead melted. An inert atmosphere was normally maintained throughout the test. Bismuth was added through the opening in the crucible cover by sliding the sight glass to one side. After melting was complete, a motor-driven stainless steel impeller stirred the alloy while the burner was adjusted to give a steady furnace temperature of 350° C. The stirring was continued for 30 minutes, and during this period, two samples were taken of the alloy with the aid of a Pyrex glass tube immersed below the surface of the melt. One sample was removed after 15 minutes of mixing and the second at the end of the 30-minute period.
Intermetallic compounds were formed by adding metallic magnesium stick and a lead-3.6 percent calcium alloy to the melt. Exactly 0.065 pound of magnesium and 1.8 pounds of the lead-calcium alloy were added to the molten charge. The amount of calcium in the lead-calcium alloy was 0.065 pound. These proportions are used in commercial debismuthizing operations, and a 30-minute reaction with calcium and magnesium is sufficient time for bismuthides to form in our tests, the best results were obtained when 3 hours elapsed between the calcium and magnesium additions and removal of bismuthides. When magnesium was added, it took approximately 15 minutes for the sticks to dissolve in the lead. The lead-calcium alloy dissolved almost instantly. Stirring continued throughout the 3-hour reaction period.
In tests 1 and 2 (table 1), the intermetallic compounds that formed were skimmed off from the surface of the melt with a flat scoop. It was difficult to determine exactly at what point solids were removed. As the bismuthides were being skimmed off from the surface of the molten metal, the liquid lead that was exposed to the atmosphere quickly chilled, forming a mushy semi-solid which was inseparable from the solid bismuthides. As a result, excess lead was removed along with the solids. The refined lead was ladled into a suitable mold, and after cooling, samples were taken from the skimmed solids and the refined lead for chemical analysis.
Tests 3 through 12 were performed with the centrifuge bowl in position for immediate centrifugation. Stirring was discontinued, and the stainless impeller was removed from the crucible. The centrifuge was then turned on, and the bowl was driven at a speed of 575 rpm. The furnace and melting crucible were raised until the centrifuge impeller contacted the surface of the melt and began pumping molten metal into the bowl. The filtered molten metal was thrown out through the perforations in the bowl, against the crucible wall, and flowed down to rejoin the melt. The solid Ca-Mg-Bi intermetallic compounds, along with some trapped lead, remained inside the bowl. The molten metal was circulated through the bowl for a predetermined time (5, 10, and 20 minutes). The furnace and melting crucible were lowered until the impeller was free from the melt. The contents of the bowl were spun to remove trapped lead by increasing the speed of rotation to 900 rpm for a 2-minute period. The centrifuge was then turned off, and the centrifuge bowl was removed from the furnace. The melt was ladled into a mold, and the gas burner was shut off. After the residue in the centrifuge bowl was cool, the centrifuge was disassembled and the residue was removed. Drillings were taken from both the residue and the refined metal for chemical analysis.
Three experiments were performed under identical conditions as in previous tests except for a change in the time duration in which reaction of calcium and magnesium and bismuthides was allowed to proceed. One test was performed for 1 hour, a second test for 2 hours, and the third test for 3 hours. A centrifuge bowl that had 912 holes was used in all three tests for making the dross separation.
Results
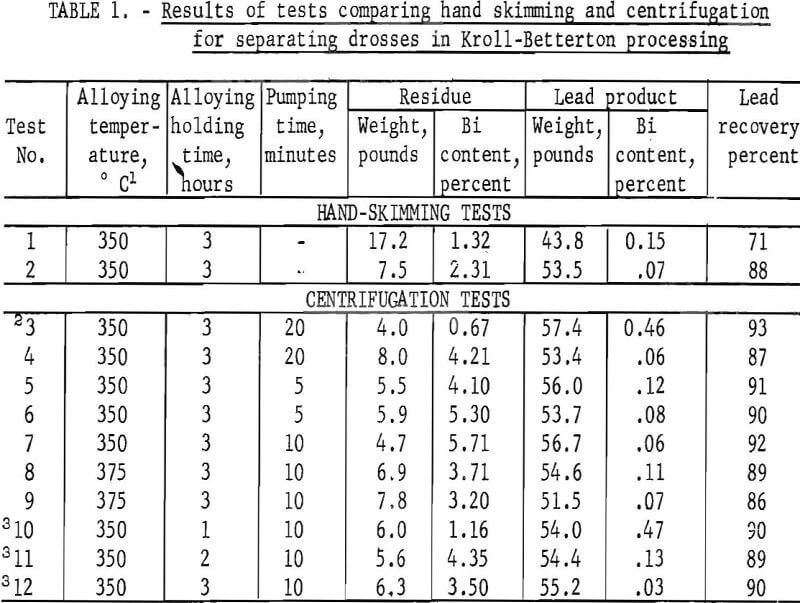
Twelve experiments were conducted utilizing the Kroll-Betterton process for debismuthizing lead. Results of tests on removing drosses by hand skimming and by centrifugation are given in table 1. The hand-skimming experiments were conducted for two reasons: (1) To vertify the formation of the bismuthides, and (2) to compare results with those obtained using the centrifuge.
In the first test, excess lead was removed with the solids. The high bismuth content in the lead product was due to agitation taking place during hand skimming, which caused some dross to mix with the molten lead and remain in the melt. Evaluation of the bismuth content in the products of the first test, calculated on the basis of the material balance, indicated that reaction of calcium and magnesium with bismuth was complete. Improved skimming operation resulted with an increase in metal recovery noticed in the results of test 2.
Results of test 3 showed that a filter medium is required in order to make the separation with the centrifuge. Even though a 20-minute pumping period was maintained, without the use of a glass cloth liner in the centrifuge bowl, dross removal was unachievable.
There is good agreement of the results from tests 2 and 4. However, the bismuth in the residue of test 4 is nearly double that found in the skimmed dross of test 2. One reason for such a difference is that oxidation took place during the skimming operation. This was substantiated by the removal of 1 pound of oxides and other solids from the bottom of the crucible in test 2 after ladling off the lead. In this test, the argon was shut off during the skimming and ladling process. In the tests using the dipping centrifuge, the crucible cover remained in place and the inert atmosphere was not disturbed until centrifuging was completed.
Results of the last three tests reported in the table show that a 3-hour reaction of calcium and magnesium with bismuth is required in order to effect maximum removal of bismuth. The 1-hour and 2-hour tests resulted in a lead bullion containing 0.47 percent bismuth and 0.13 percent bismuth, respectively, while in the 3-hour test, bismuth was decreased to 0.03 percent. The amount of lead recovered was comparable for all three tests.
The centrifuge was operated for times of 5, 10, and 20 minutes. A reaction of 3 hours, a pumping time of 10 minutes, and use of the centrifuge bowl having 912 holes gave best results. Two tests were run at a slightly elevated temperature of 375° C, but this increase in temperature did not improve lead recovery nor the amount of bismuth removed in the lead. The lead recovered in test 12 approaches commercial specifications for debismuthized lead. The filtered product contains 0.03 percent bismuth, and lead recovery is 90 percent. Calcium and magnesium contamination in the lead was less than 0.05 percent. In a commercial debismuthizing process where solids are skimmed off, the refined lead contains 0.02 percent bismuth. Evers has shown that to obtain maximum separation of the fine crystallites of bismuthides, the molten metal must be cooled very slowly over a period of 6 to 10 hours to temperatures close to the freezing point of the molten lead. Further refinement in the technique should show that centrifugation could produce a lead bullion equal to or exceeding commercial specifications.
Conclusions
The prospect of adapting centrifugation to commercial application at the present time is limited, but it is possible that this mode of separating dross from debismuthized lead in the Kroll-Betterton process can be perfected through further testing including multibatch processing.
Separating solids with the dipping centrifuge is particularly effective in a process where a small amount of dross is formed with a large quantity of molten metal. In this process, a large amount of impure lead can be treated before it is necessary to empty the centrifuge.
Centrifugation provides better control in removing drosses, faster operation with less oxidation and relatively little fuming, and recovery of a lead bullion that approaches commercial specifications. In a one-step operation, centrifugation can produce refined lead of satisfactory purity and a residue in which the bismuth concentration is increased and the amount of residue decreased, compared with the residue removed by hand skimming.
