Table of Contents
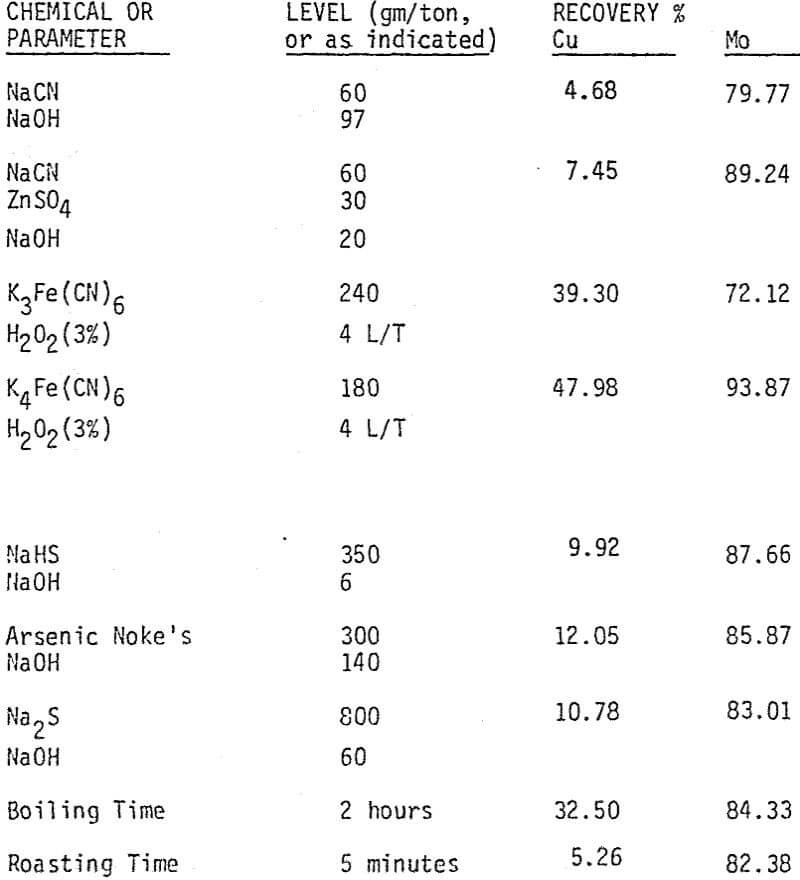
Bulk and differential laboratory flotation tests have been carried out using a synthetic mixture of chalcopyrite and molybdenum ore. In addition to boiling and roasting, six different depressants are tested so that the best conditions of separation can be identified. These reagents include sodium cyanide with and without zinc sulfate, potassium ferrocyanide, sodium hydrosulfide, arsenic Noke’s reagent, and sodium sulfide. The data indicate that most of these reagents are effective chalcopyrite depressants.
Experimental
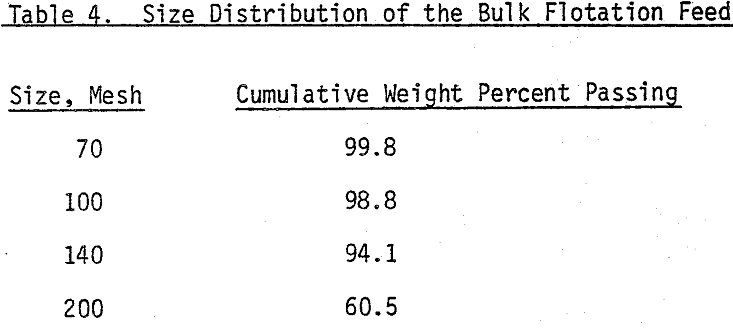
The mineral samples in this investigation, Molybedenite ore (0.35% MOS2) from the Climax Mine, and Chalcopyrite (1.4% copper) mineral from Messina ore were used in this study.
The chemical depressants include sodium cyanide (NaCN) and sodium hydro-sulfide (NaHS) purchased from MCB. Other chemicals such as zinc sulfate, potassium ferric cyanide, potassium ferro cyanide, arsenic oxide and sodium sulfide are supplied by T. J. Baker Chemical Company. Sodium isopropyle xanthate (NaIPX) of Dow Chemicals (Z-11) is used as bulk flotation collector. The frother in all flotation experiments is pine oil. Lime is used for pH adjustments. All the chemicals are of analytical grade except NaHS which is technical grade. Deionized distilled water is used in all tests.
Results and Discussion
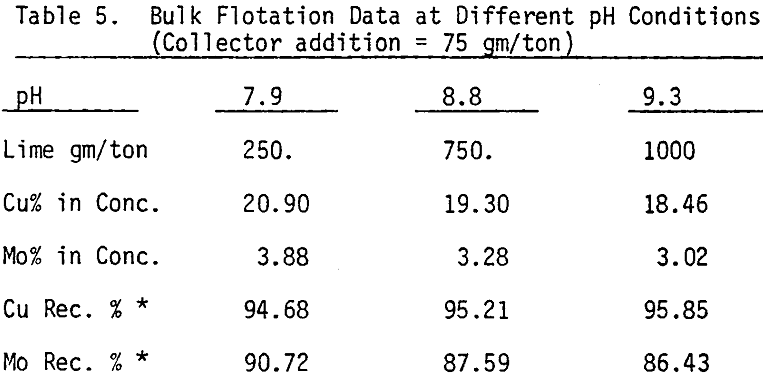
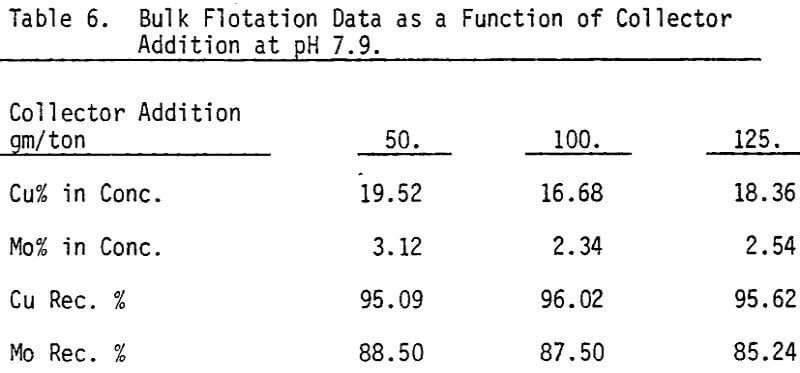
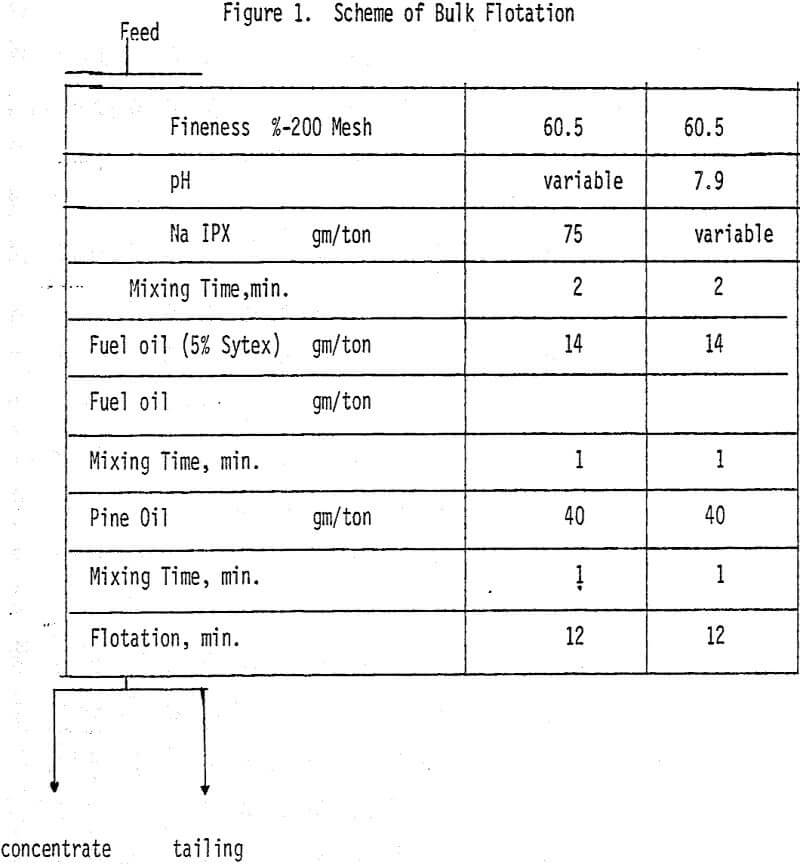
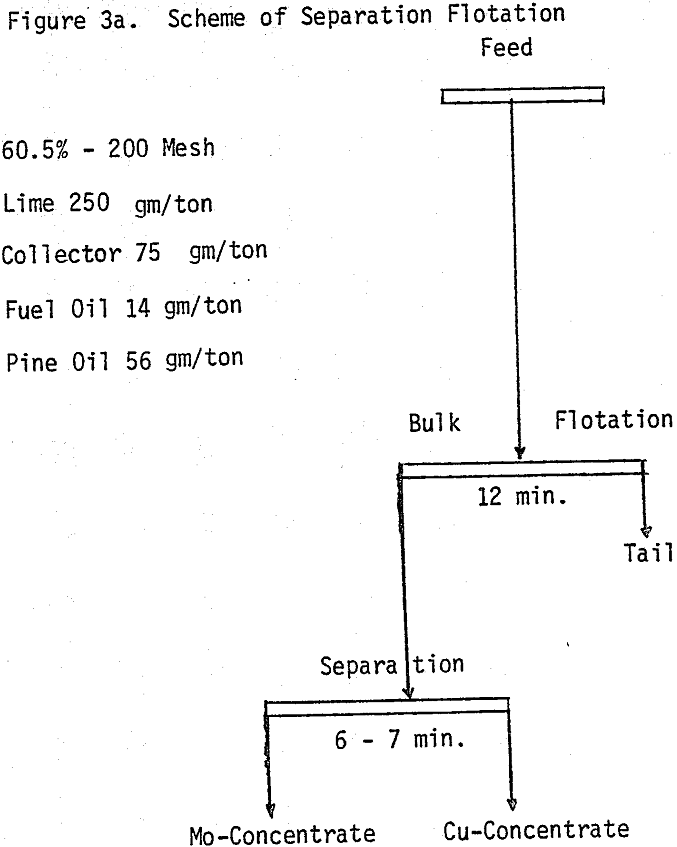
Bulk flotation tests are conducted at different pH values, using 75 gm/ton sodium isopropyle xanthate as a collector. In order to determine the optimum amount of collector, flotation tests are carried out at pH 7.9 using different levels of collector.
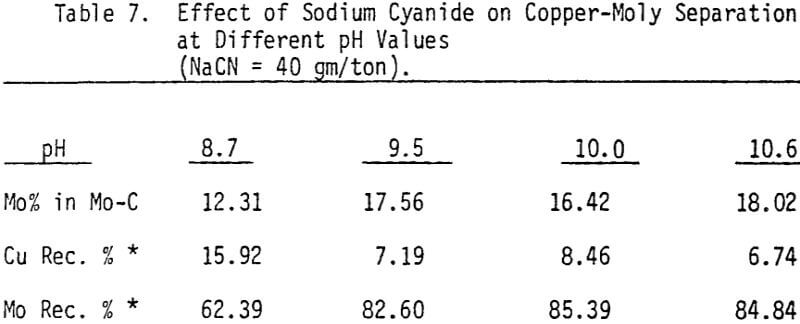
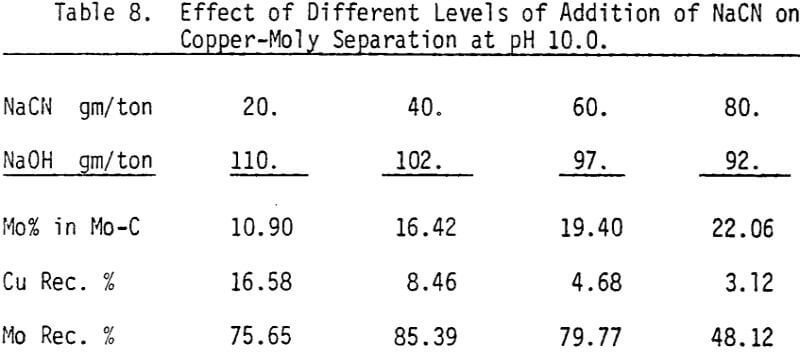
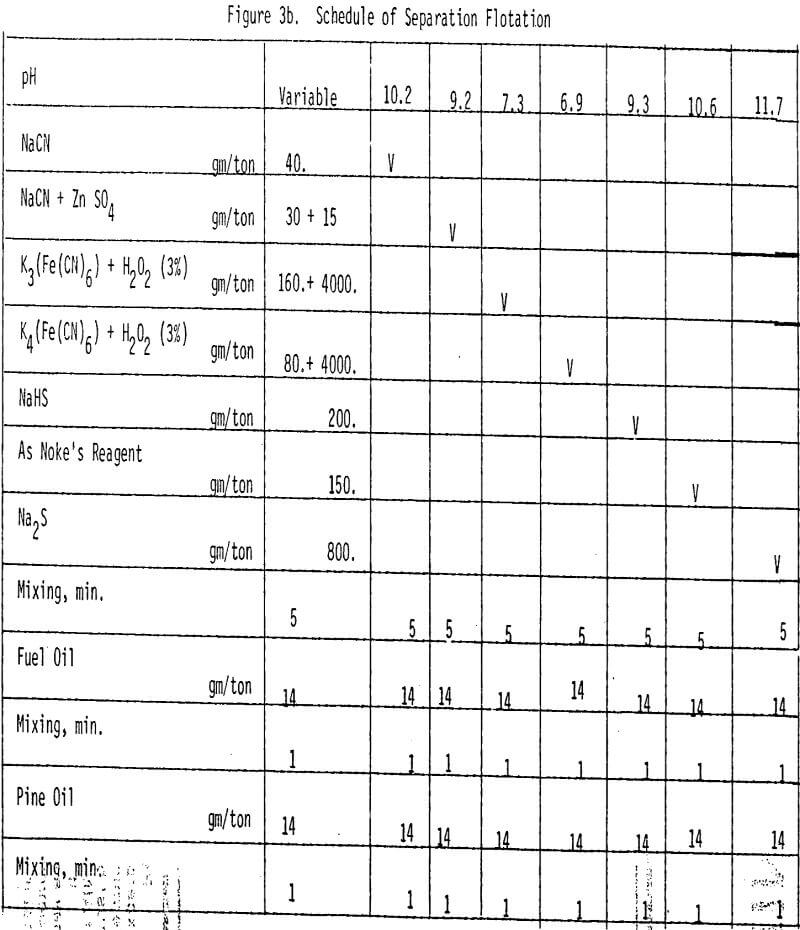
Depression of chalcopyrite from the bulk concentrate is accomplished by adding 40 gm/ton NaCN to the separation flotation cell at different pH values.
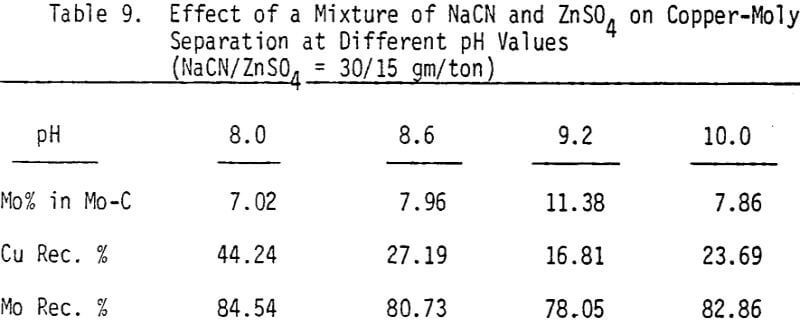
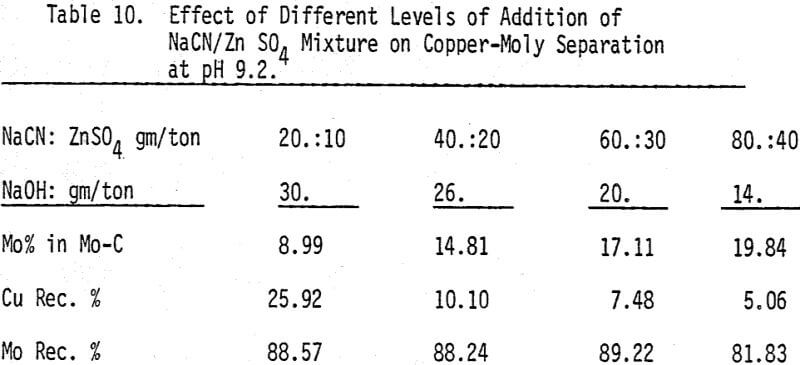
A mixture of sodium cyanide and zinc sulfate is used as a depressant for chalcopyrite. A moly concentrate (89% recovery) of 17% MoS2 can be obtained. Higher and lower levels of addition have produced lower molybdenite recoveries.
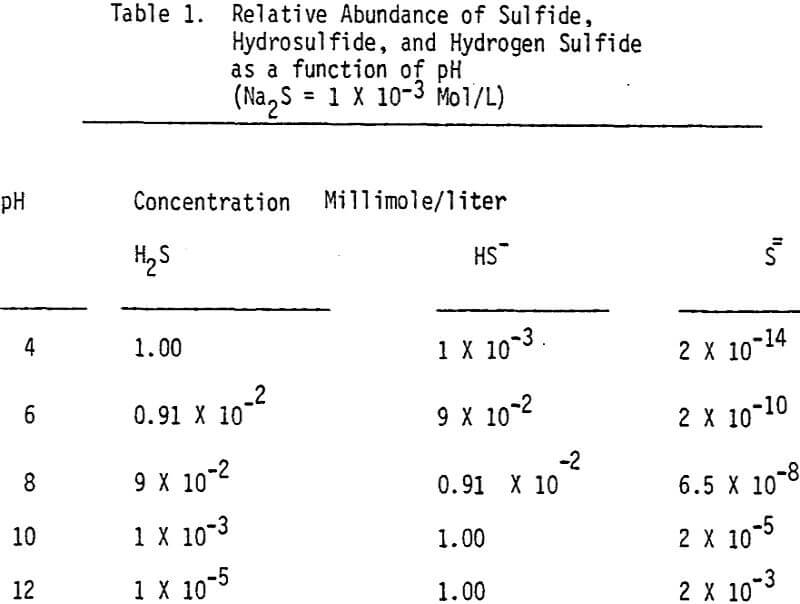
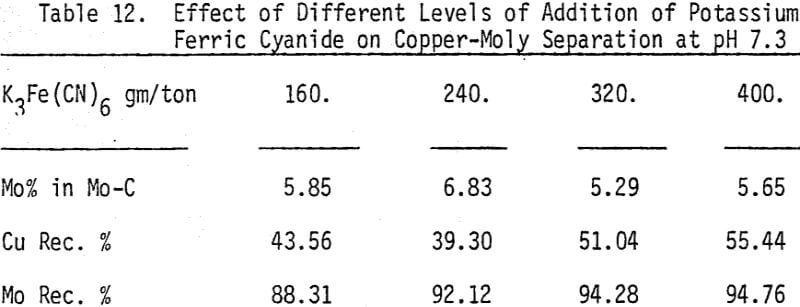
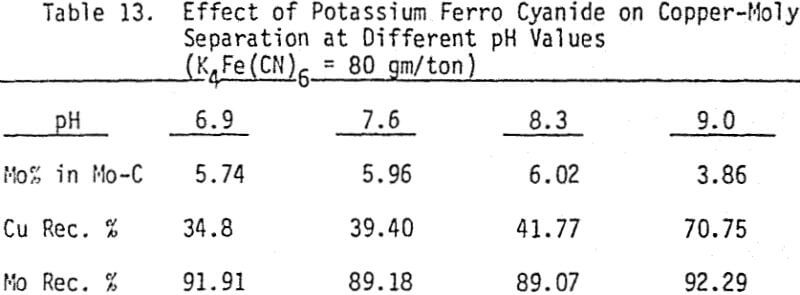
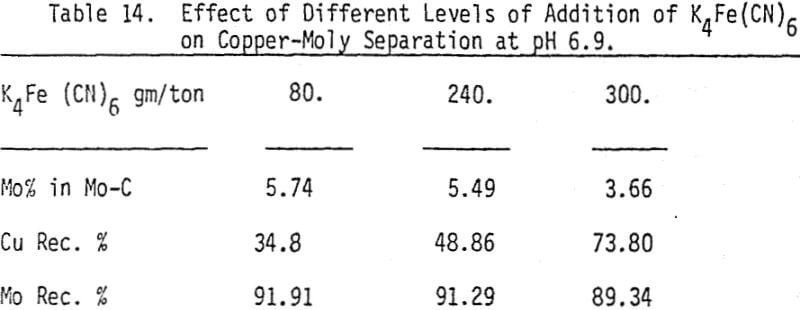
The effectiveness of these two reagents in chalcopyrite depression is tested by conducting several flotation tests at different pH values and various reagent dosages. It should be mentioned that hydrogen peroxide is added in order to destroy the adsorbed xanthate on chalcopyrite surface.
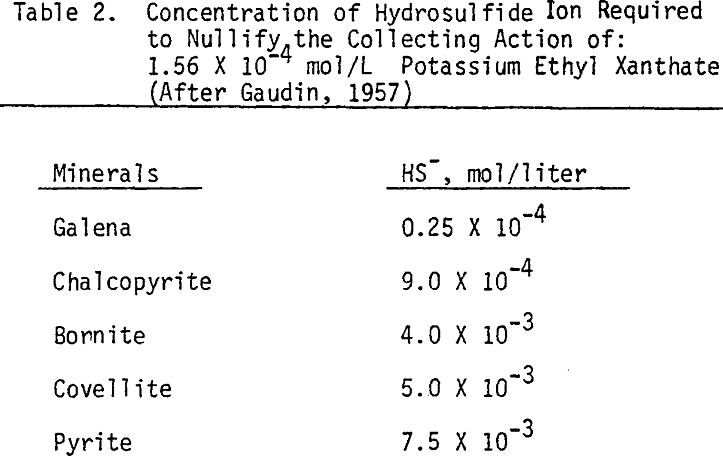
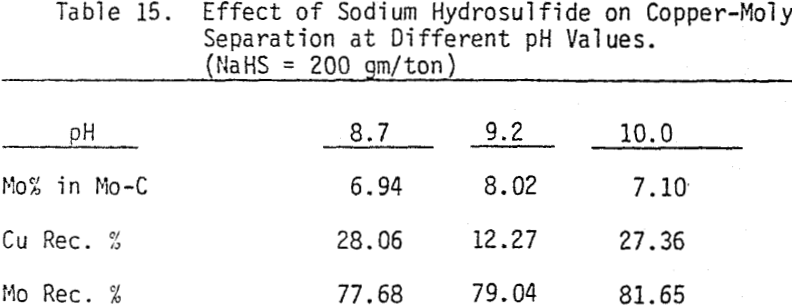
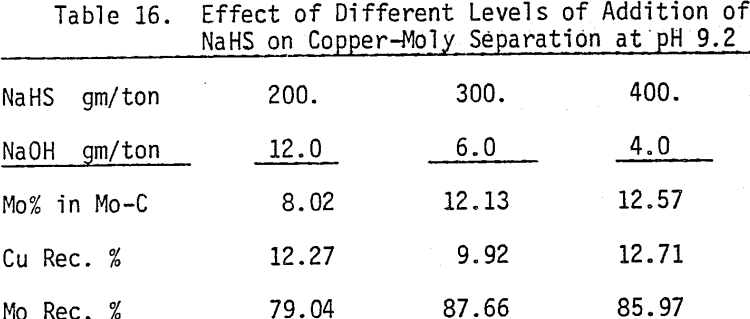
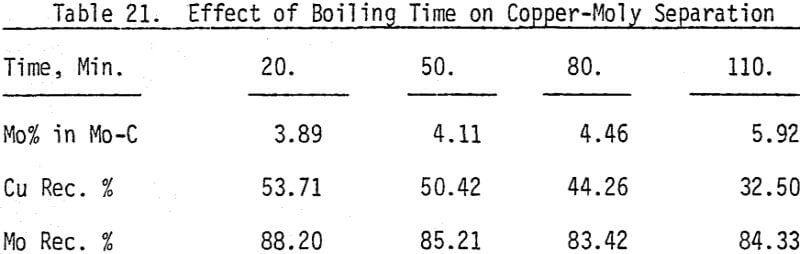
In order to determine the levels of addition and pH conditions needed to obtain optimum separation, several flotation tests are carried out using different dosages of NaHS at different pH values.
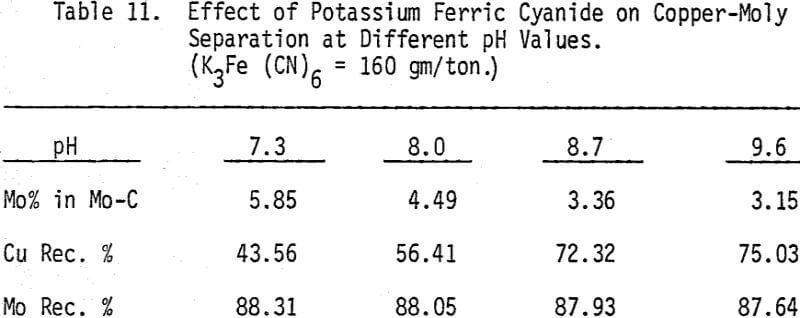
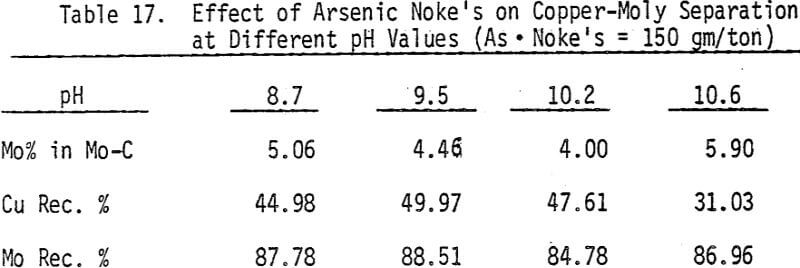
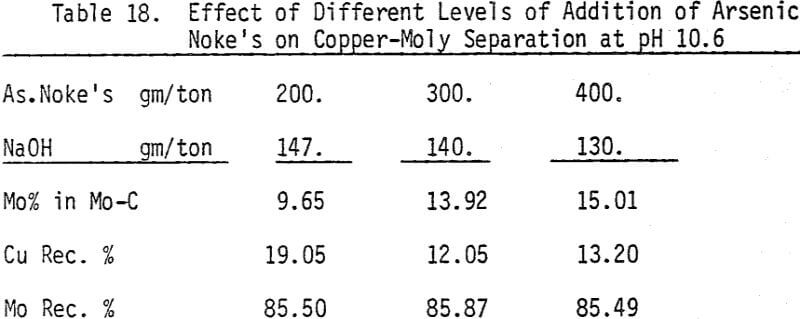
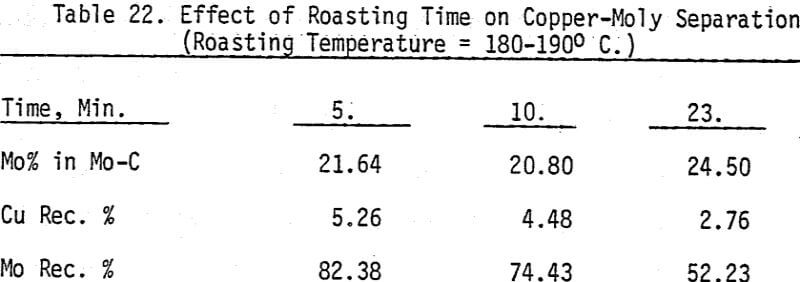
As mentioned earlier, industrial experience has proved that these reagents can be used to depress chalcopyrite in flotation circuits of complex sulfide ores. The results obtained for the effect of arsenic Nokes on copper-moly separation confirm that these compounds are effective depressants for chalcopyrite. However, sodium hydrosulfide seems to be more efficient.
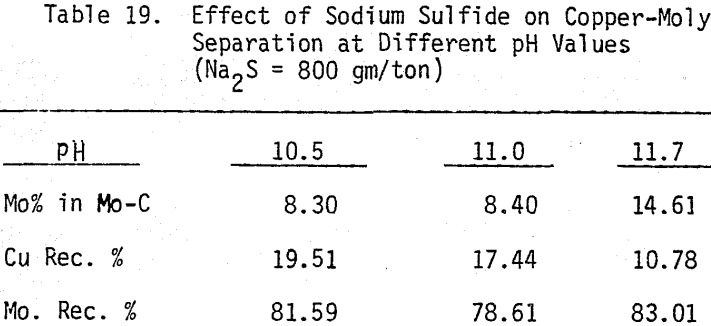
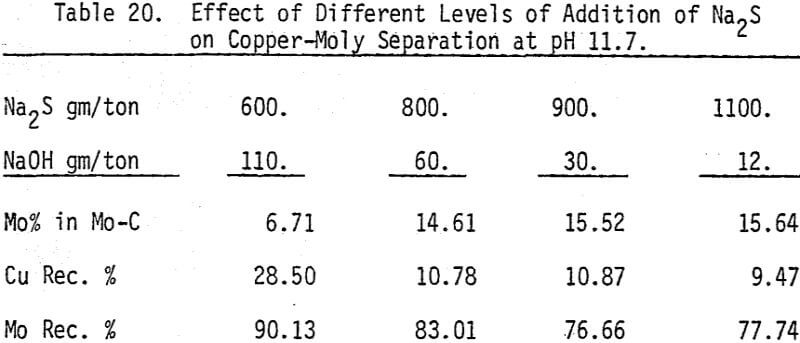
The influence of Na2S on molybdenite recovery from the bulk flotation concentrate is studied by conducting flotation tests at different pH conditions and various levels of additions. It is clearly shown that large dosage of sodium sulfide is needed to depress the chalcopyrite at highly alkaline conditions.
Summary and Conclusions
The results of separation flotation tests conducted in this investigation indicate that several depressants can be used effectively in separating moly from chalcopyrite-molybdenite ores.